Abstract
Aims: The GLOBAL LEADERS trial is a superiority study in patients undergoing percutaneous coronary intervention, with a uniform use of Biolimus A9-eluting stents (BES) and bivalirudin. GLOBAL LEADERS was designed to assess whether a 24-month antithrombotic regimen with ticagrelor and one month of acetylsalicylic acid (ASA), compared to conventional dual antiplatelet therapy (DAPT), improves outcomes.
Methods and results: Patients (n >16,000) are randomised (1:1 ratio) to ticagrelor 90 mg twice daily for 24 months plus ASA ≤100 mg for one month versus DAPT with either ticagrelor (acute coronary syndrome) or clopidogrel (stable coronary artery disease) for 12 months plus ASA ≤100 mg for 24 months. The primary outcome is a composite of all-cause mortality or non-fatal, new Q-wave myocardial infarction at 24 months. The key safety endpoint is investigator-reported class 3 or 5 bleeding according to the Bleeding Academic Research Consortium (BARC) definitions. Sensitivity analysis will be carried out to explore potential differences in outcome across geographic regions and according to specific angiographic and clinical risk estimates.
Conclusions: The GLOBAL LEADERS trial aims to assess the role of ticagrelor as a single antiplatelet agent after a short course of DAPT for the long-term prevention of cardiac adverse events, across a wide spectrum of patients, following BES implantation.
Background
The optimum duration of dual antiplatelet therapy (DAPT) after coronary drug-eluting stent placement remains uncertain. Practice guidelines recommend DAPT for at least 12 months following drug-eluting stent (DES) implantation in patients with acute coronary syndromes (ACS), and for at least six months in patients with stable coronary artery disease (SCAD) in Europe1,2. The evidence base supporting the “at least 12 months recommendation” in ACS patients is weak, and some randomised controlled trials actually provide evidence suggesting that the risks of prolonging DAPT beyond six to 12 months outweigh the potential benefits3-6.
Conversely, recent trials on DAPT duration have shown a clear reduction in rates of stent thrombosis and major adverse cardiovascular events in patients free from bleeding or ischaemic events at 12 months who continued the DAPT regimen up to 30 months. While an increase in bleeding risk was a potentially anticipated finding, the observation of a possible increase of non-cardiovascular mortality in patients prolonging DAPT beyond 12 months remains a matter of concern7-9. For more than a decade, the mainstay of DAPT has been the combination of the weak cyclo-oxygenase-1 inhibitor acetylsalicylic acid (ASA), and clopidogrel. However, the inherent limitations of clopidogrel are well recognised, including a slow onset of action and a variable response10-13. Prasugrel and ticagrelor provide superior protection against ischaemic adverse events compared with clopidogrel in patients following an ACS undergoing percutaneous coronary intervention (PCI)14-16. Notwithstanding this, both drugs incur an increased risk of spontaneous bleeding. As of today, these drugs have not been tested in patients undergoing elective PCI for SCAD, or as monotherapy (i.e., without ASA).
Ticagrelor is a reversible oral P2Y12 receptor antagonist that does not require metabolic activation17. The inter-individual variability of platelet inhibition is low17. In the Platelet Inhibition and Patient Outcomes (PLATO) trial there was a hint of an attenuation of the treatment effect of ticagrelor by an ASA maintenance dose above 150 mg once daily18,19. Given the fact that strong P2Y12 receptor inhibition may also provide inhibition of the COX-1 pathway, it remains possible that ticagrelor monotherapy maintains efficacy compared to the combined use of aspirin and ticagrelor, while improving safety (i.e., a reduction of spontaneous/gastrointestinal bleeding)20.
Finally, during long-term follow-up, optimisation of secondary prevention and overall medical management are at least as important as the selection of which stent to use21-24. A potent consistent and reversible P2Y12 receptor antagonist such as ticagrelor may provide superior efficacy with similar or improved safety compared to the current standard regimen24.
Study objectives
GLOBAL LEADERS (ClinicalTrials.gov, NCT01813435) was designed to determine the benefits and risks of an antithrombotic regimen using ticagrelor combined with acetylsalicylic acid (ASA) for one month and alone for 23 months, compared to conventional DAPT in patients on bivalirudin undergoing biolimus-eluting stent implantation.
Substudies are planned to enhance understanding of the treatment effects and refine modelling of outcomes (Online Appendix 1). The SYNTAX score (SX score) for each patient will be calculated by scoring all coronary lesions with a DS ≥50%, in vessels ≥1.5 mm, using the SX score algorithm (available at www.syntaxscore.com)25. The GRACE risk score at index admission will be calculated for all patients suffering an acute coronary syndrome (ACS)26.
Study oversight
GLOBAL LEADERS is an investigator-initiated and scientifically driven trial sponsored by the European Clinical Research Institute (www.ECRI-trials.com), a non-profit organisation which received funding from Biosensors (Morges, Switzerland), AstraZeneca (Mölndal, Sweden), and The Medicines Company (Parsippany, NJ, USA) for the study conduct (Online Appendix 2).
The academic members of the steering committee designed the trial in collaboration with an independent statistician (Peter Jüni, Bern, Switzerland). The steering committee, together with an operations committee and academic representatives from ECRI, will oversee the medical, scientific, and operational conduct of the study. The steering committee members are responsible for the reporting of the results and the drafting and editing of this and forthcoming manuscripts.
An independent data and safety monitoring board (DSMB) monitors patient safety on an ongoing basis and has access to unblinded data.
Site management and monitoring is performed by Cardialysis in Europe and Theorem in Brazil, Canada, and Singapore. Angiographic data (SYNTAX score) and 12-lead ECGs (post-index PCI before discharge, three-month and two-year follow-up) will be analysed off-line by an independent core laboratory (Cardialysis, Rotterdam, The Netherlands).
Data cleaning and preparation will be performed by the Cardialysis data management group. After database lock, the database will be housed for statistical analysis at the Clinical Trials Unit (Department of Clinical Research, University of Bern, Bern, Switzerland).
The study adheres to the ethical principles of the Declaration of Helsinki, to specifications of the International Conference of Harmonization, and to Good Clinical Practice. The study underwent approval by an ethics committee or institutional review board at each site.
Study design
The GLOBAL LEADERS trial is designed as a multicentre, multinational, open-label superiority randomised clinical trial. Patients will be randomised before intervention to the experimental or reference DAPT antithrombotic strategy (Figure 1) in a 1:1 ratio using a web-based system with random, permuted blocks and stratified by site and clinical presentation (SCAD versus ACS). Coronary anatomy is to be known in order to assess eligibility for PCI before randomisation in all patients. Multiple target vessel interventions are allowed either within the index procedure or as a pre-specified, staged (within three months following the index PCI) procedure. Randomised treatment continues for a minimum of two years post index PCI procedure in all patients (in case of a staged procedure, for up to two years after the initial stage of the index procedure). Outpatient study visits are scheduled at one, three (safety visit), six, 12, and 18 months after the index PCI procedure, with an end-of-treatment visit at 24 months.
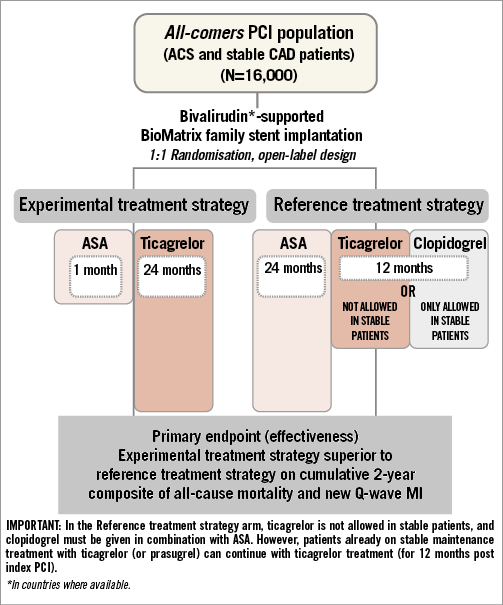
Figure 1. GLOBAL LEADERS study design diagram.
Patient population
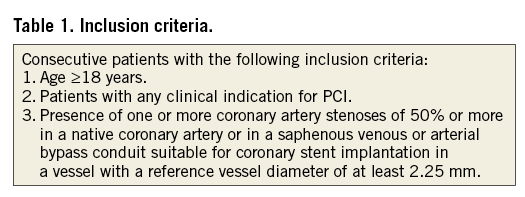
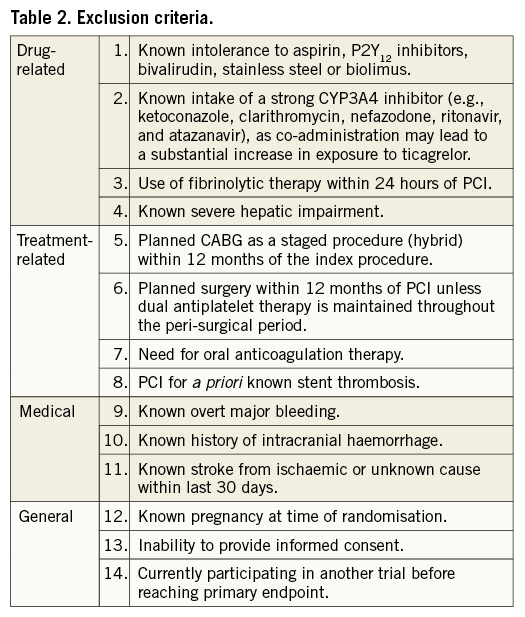
The GLOBAL LEADERS trial will enrol approximately 16,000 patients in more than 100 interventional cardiology sites in Europe, Asia, Brazil, Australia and Canada during a period of approximately 16-24 months. Inclusion and major exclusion criteria are shown in Table 1 and Table 2, respectively. According to the “all-comers” concept, there are no restrictions as to the number, severity, or location of lesions, or the number of stents used. Key exclusion criteria are limited to study medication safety/intolerance, stent component allergies, or planned need for surgery within the six months after the index procedure. Patients with ST-elevation ACS treated with fibrinolysis or patients in need of chronic anticoagulation are deemed not eligible.
Treatment regimens and concomitant medications
Patients are randomly assigned to either ticagrelor combined with ASA ≤100 mg for one month followed by monotherapy for 23 months or standard DAPT with clopidogrel for 12 months plus ASA up to 100 mg (SCAD patients), or ticagrelor standard treatment for 12 months plus ASA ≤100 mg (ACS patients) followed by ASA monotherapy for the study duration (Figure 1). The use of clopidogrel is restricted to patients undergoing elective PCI for stable lesions (cardiac biomarker negative, no clinical signs and/or symptoms of ongoing myocardial ischaemia lasting >20 minutes).
DAPT is started as early as possible, and no later than two hours after the index procedure. Patients receiving at least one maintenance dose of ticagrelor or prasugrel will be allocated to a ticagrelor arm (either intervention or reference) upon randomisation. In the case of staged PCI or in case of unplanned reintervention (other than for definite stent thrombosis or ST-segment elevation myocardial infarction) in the study treatment arm, the 30-day treatment period with ASA will restart at the time of the staged procedure or reintervention.
Patients assigned to a ticagrelor arm who have not yet received a loading dose of ticagrelor or prasugrel, or who have received a prasugrel 60 mg loading dose within five days prior to randomisation, but not continued thereafter with a daily 10 or 5 mg maintenance regimen without interruption, will receive a loading dose of ticagrelor of 180 mg (two 90 mg tablets). Otherwise, a dose of ticagrelor of 90 mg will be administered as the first dose. Patients assigned to clopidogrel who have not yet received a loading dose of clopidogrel or have not been taking clopidogrel or ticlopidine for ≥5 days before randomisation will receive a 600 mg loading dose and, otherwise, a maintenance dose of clopidogrel study drug as their first dose. For patients not previously having received ASA, a loading dose of 325 mg is preferred (160-500 mg allowed).
In case of ticagrelor discontinuation due to adverse effects other than bleeding (i.e., atrioventricular block, dyspnoea), patients should be put on a standard dose of prasugrel in both study arms.
In case of definite stent thrombosis or ST-segment elevation myocardial infarction (STEMI), patients will no longer need to adhere to the study protocol-mandated medication regimen and will be treated according to best clinical practice.
Patients who require oral anticoagulation after randomisation should be treated according to local guidelines. Triple therapy should only be prescribed for the shortest necessary duration with frequent INR measurement (target INR 2–2.5), with clopidogrel becoming the default P2Y12 inhibitor.
Concomitant medication/stent
The GLOBAL LEADERS trial demands uniform anticoagulation during the procedure and uniform stent platform use during the index procedure (including staged procedures) and any unplanned or intercurrent re-PCI.
In all patients, anticoagulation during the procedure will be accomplished with bivalirudin (Angiox® or Angiomax®; The Medicines Company, Parsippany, NJ, USA in the countries where the drug is approved) given as a bolus of 0.75 mg/kg followed immediately by an infusion of 1.75 mg/kg/hr for at least the duration of the PCI, and may be continued for up to four hours post PCI, at which time the infusion should be either stopped or reduced to a dose of 0.25 mg/kg/hr for up to 12 hours at the discretion of the investigator. Dose adjustments may be required according to drug labelling (i.e., renal dysfunction). Bivalirudin, when used instead of heparin with optional glycoprotein IIb/IIIa (GP IIb/IIIa) inhibitors, has consistently demonstrated a reduction in protocol-defined major and minor bleeding27. The use of GP IIb/IIIa inhibitors in the GLOBAL LEADERS trial should be limited to bail-out situations only (i.e., no-reflow phenomena, giant thrombus). The use of unfractionated heparin (up to an arbitrary set maximum of 4,000 IU) during the index diagnostic angiogram is left to the discretion of the investigator.
The stent device(s) that will be used during the index procedure (including staging) belong to the BioMatrix™ drug-eluting stent family (BES; Biosensors Europe SA, Morges, Switzerland) and are CE-marked and commercially available. The Biolimus A9™/BA9™-eluting stents with biodegradable polymer represent a safe and effective alternative to the sirolimus-eluting stents with durable polymer in patients with on- and off-label indications28.
In the event of an intercurrent repeat intervention, for reasons beyond definite stent thrombosis and STEMI, the physician should adhere to the study stent and treatment strategy including one month of concomitant ASA ≤100 mg post PCI (including the repeat and unplanned procedures) in the ticagrelor monotherapy arm.
Study endpoints
The trial endpoints are listed in Table 3. The primary outcome is the composite of investigator-reported all-cause mortality or non-fatal, new Q-wave myocardial infarction (MI) identified by an independent ECG core laboratory (Cardialysis, Rotterdam, The Netherlands) at two years post randomisation. The rationale for choosing this endpoint is to have an unbiased estimate of the effect regarding irreversible organ damage. All-cause mortality is an objective endpoint and can be readily ascertained with minimal bias or need for adjudication. Similarly, the appearance of new Q-waves on 12-lead electrocardiography (Minnesota Code) following PCI and myocardial infarction is an objective and independent predictor of future mortality29. A new left bundle branch block associated with an MI will be considered equivalent to a new Q-wave.
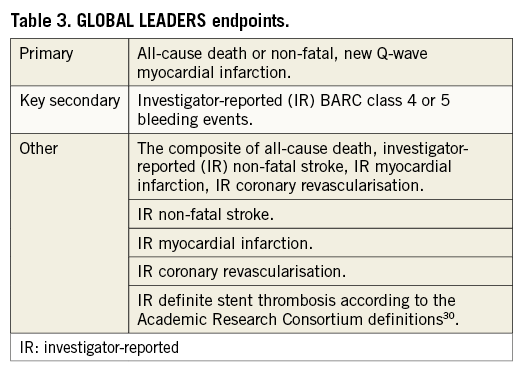
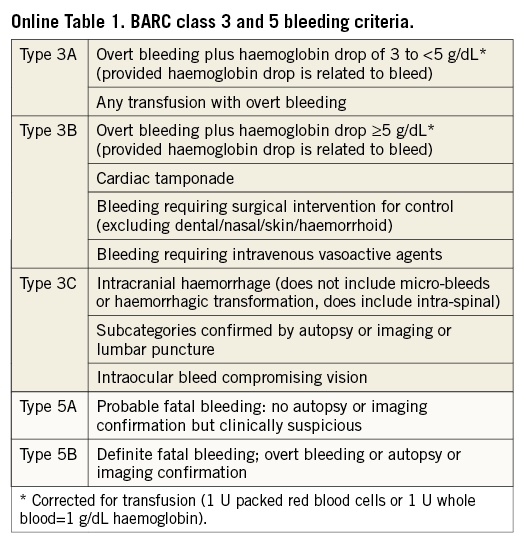
The key safety variable is any investigator-reported Bleeding Academic Research Consortium (BARC) class 3 or 5 bleeding (Online Table 1)31. BARC consensus definitions have recently been validated post PCI. BARC class 3 or 5 events are independently associated with increased one-year mortality to an extent comparable to that provided by the TIMI criteria32. An independent medical reviewer will check the reported endpoint events for validity.
Statistics
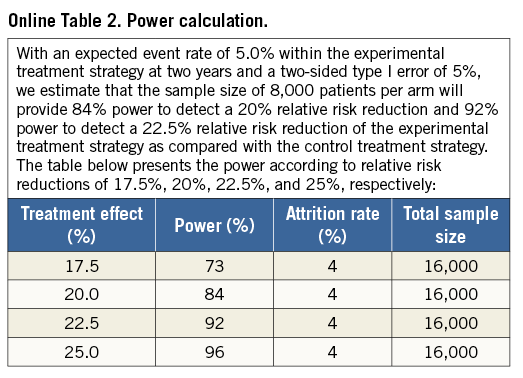
The assumptions regarding event rate for the composite of all-cause mortality and Q-wave MI in the reference strategy arm are based on actual data from the BES arm in the LEADERS trial at two years after the index procedure (Online Table 2). With an expected event rate of 5.0% within the reference treatment strategy arm at two years and a two-sided type I error of 5%, we estimate that a sample size of 8,000 patients per arm will provide 84% power to detect a 20% relative risk reduction and 92% power to detect a 22.5% relative risk reduction of the experimental treatment strategy compared with the reference treatment strategy.
Analysis of the primary composite and secondary endpoints will be performed according to the intention-to-treat principle of all randomised patients as time-to-first-event. The primary and all secondary endpoints will be analysed using the Mantel-Cox method accompanied by the log-rank test to calculate corresponding p-values. Corresponding survival curves will be constructed using Kaplan-Meier estimates. Patients start being at risk on the day of their index PCI, or, if no PCI was performed, on the day of randomisation. Per-protocol analyses of the primary composite effectiveness endpoint and secondary safety endpoint will be performed as sensitivity analyses. To evaluate for consistency of results among subgroups of interest, exploratory subgroup analyses are pre-specified (Online Appendix 1).
Landmark analyses will be performed according to two pre-specified landmark points at 30 days and at one year (365 days), with RR calculated separately for events up to the landmark point and for events occurring after the landmark point up to two years. For each type of event, patients will be censored at the time of the first event: a patient who experienced an event contributing to the primary composite endpoint during the first year, for example, will be censored at the time of the event and excluded from the analysis of subsequent years after the landmark point. Landmark analyses will be accompanied by a test for interaction between treatment effect and time (before versus after the landmark point).
Demographics, baseline (including prior and current medications) and procedural characteristics will be described using counts and percentages if categorical, and means and SD, if continuous. P-values for procedural characteristics on patient-level data will be computed using chi-square and Fisher’s exact tests for categorical variables, t-tests for continuous variables, and Wilcoxon’s Mann-Whitney U tests for non-symmetrically distributed continuous variables. P-values for procedural characteristics on lesion-level data will be computed using generalised linear mixed models. In each model a random effect of patient identifier will account for multiple lesions measured within patients.
Present status
The first patient in the GLOBAL LEADERS trial was enrolled in June 2013. By March 1, 2015, 10,000 patients had been enrolled. The end of enrolment is projected for September 2015.
Conclusion
The GLOBAL LEADERS trial includes minimally selected, consecutive patients undergoing PCI with bivalirudin and unrestricted use of BES. The protocol also allows for patients exposed to clopidogrel/prasugrel, both as maintenance treatment and loading dose. Therefore, the trial will reflect the full spectrum of patients managed in routine PCI practice with different clinical presentations (with the exception of thrombolytic and anticoagulant therapy at the time of randomisation), and will provide a pivotal comparison of the efficacy and safety of a short course of ticagrelor plus aspirin followed by 23-month ticagrelor monotherapy versus the contemporary standard-of-care DAPT regimen.
The GLOBAL LEADERS trial challenges the concept that, against the background of ticagrelor, ongoing aspirin treatment, beyond the initial one month time period of high stent thrombosis risk, is well serving. More potent, more consistent antiplatelet inhibition with ticagrelor may be a better foundation for long-term antiplatelet therapy compared to aspirin in at-risk patients. GLOBAL LEADERS will also provide preliminary 30-day efficacy and safety data on a randomised comparison of ticagrelor plus low-dose aspirin versus clopidogrel and aspirin following DES use in a cohort of stable CAD patients. The GLOBAL LEADERS trial may pave the way for future studies of ticagrelor as antiplatelet monotherapy across a wide spectrum of patients with CAD and is the first large-scale study on ticagrelor in elective stenting.
| Impact on daily practice The GLOBAL LEADERS trial challenges the concept that, against the background of ticagrelor, ongoing aspirin treatment beyond the initial one-month time period of high stent thrombosis risk, is well serving. The GLOBAL LEADERS trial may pave the way for future studies of ticagrelor as antiplatelet monotherapy across a wide spectrum of patients with coronary artery disease and is the first large-scale study on ticagrelor in elective coronary artery stenting. |
Guest Editor
This paper was guest edited by George Dangas, MD, PhD; Division of Cardiology, Mount Sinai Medical Center, New York, NY, USA; Cardiovascular Research Foundation, New York, NY, USA.
Conflict of interest statement
C. Hamm discloses the following relationships: advisory and speakers honoraria from AstraZeneca. P. Jüni received research grants to the institution from AstraZeneca, Biotronik, Biosensors International, Eli Lilly and The Medicines Company, and unpaid membership of the steering group of trials funded by AstraZeneca, Biotronik, Biosensors, St. Jude Medical and The Medicines Company. P.W. Serruys declares a research grant (to INSERM U1148) from Sanofi, and Servier; speaking or consulting honoraria from Abbott Laboratories, AstraZeneca, Biotronic, Medtronic, Sino Medical Sciences Technology, Stentys, Svelte Medical Systems, Volcano, unpaid membership of the advisory board of Abbott Vascular, unpaid membership of the steering group of trials funded by Medtronic. P. Steg received research grants (to INSERM U1148) from Sanofi, and Servier; speaking or consulting honoraria from Amarin, AstraZeneca, Bayer, Boehringer-Ingelheim, Bristol-Myers Squibb, CSL Behring, Daiichi-Sankyo, GlaxoSmithKline, Janssen, Lilly, Novartis, Pfizer, Regeneron, Roche, Sanofi, Servier, The Medicines Company; and owns stocks from Aterovax. M. Valgimigli received speaking or consulting honoraria from Biosensors, The Medicines Company and AstraZeneca and research grants from AstraZeneca and The Medicines Company. P. Vranckx received speaking or consulting honoraria from Bayer, Daiichi-Sankyo, The Medicines Company and AstraZeneca. S. Windecker received research grants to the institution from Abbott, Boston Scientific, Biotronik, Medtronic. The other authors have no conflicts of interest to declare. The Guest Editor receives speaker honoraria (modest level) from The Medicines Company. His spouse is on the Advisory Board (modest level) of AstraZeneca.
SUPPLEMENTARY DATA
Online Appendix 1
PRE-SPECIFIED SUBGROUP ANALYSES OF THE PRIMARY ENDPOINT ACCORDING TO SPECIFIC ANGIOGRAPHIC AND CLINICAL RISK ESTIMATES
We will check for interactions with treatment regimen towards cardiovascular efficacy and safety outcomes on the following items:
CLINICAL PRESENTATION
1. Treatment regimen in relation to clinical presentation (acute coronary syndrome vs. stable, elective) overall.
2. Treatment regimen in relation to clinical presentation (ST-segment elevation ACS, non-STE ACS) overall.
3. Treatment regimen in relation to clinical presentation and angiographic risk profile (ST ACS vs. SYNTAX score >8) overall.
4. Treatment regimen in stable patients up to 30 days, 30 days to end of trial (30-day landmark).
5. Treatment regimen in relation to ECG changes at presentation.
CONCOMITANT DISEASE/BASELINE RISK FACTORS
6. Treatment regimen in relation to renal function (and dialysis) at time of randomisation.
7. Treatment regimen in relation to diabetes (type 1 & 2) status at randomisation.
8. Treatment regimen in relation to weight (body mass index ≥27) at the time of randomisation.
9. Treatment regimen in relation to age at the time of randomisation.
10. Treatment regimen in relation to gender.
11. Treatment regimen in relation to history of cardiovascular (cardiac, peripheral, or combined) disease (including coronary artery bypass grafting).
12. Treatment regimen in relation to history of prior stroke or transient ischaemic attack.
13. Treatment regimen in relation to the GRACE risk score at admission.
14. Treatment regimen in relation to the SYNTAX score (I & II) at admission. Including: value of incorporating baseline ECG changes into the SYNTAX I and II scores. Including: impact of residual (after PCI) SYNTAX score with respect to treatment effect and outcomes.
15. Impact of CRUSADE and or ACUITY bleeding scores in risk stratifying bleeding and ischaemic events and its interaction with treatment strategies.
GEOGRAPHIC REGION
16. Treatment regimen in relation to geographic region (region-by-treatment interaction) prospectively defined as South America, Canada, Europe, Asia.
TREATMENT ELEMENTS
DRUG
17. Treatment regimen in relation to the access site used during the index procedure.
18. Treatment regimen in relation to periprocedural anticoagulation. Including: safety and efficacy of switching strategies on anticoagulation before and during the procedure.
19. Treatment regimen and outcome in patients in need of CABG.
20. Treatment regimen and outcome in patients with non-cardiac surgery.
21. Treatment regimen in relation to previous exposure to aspirin and/or P2Y12 receptor blocker (vs. naïve patients). The ONSET study. Including: safety and efficacy of switching strategies with respect to P2Y12 inhibition before PCI.
22. Treatment regimen in relation to proton pump inhibitor use.
23. Treatment regimen in relation to post-procedure anticoagulation.
STENT PROCEDURE
24. Treatment regimen in relation to staged procedures.
25. Treatment regimen in relation to specific lesion subsets including:
a. Left main
b. Bifurcations
ANALYSIS
26. Treatment regimen with a landmark analysis at 12 months.
27. BARC bleeding analysis including case fatality hazard.
28. OFFSET trial. Treatment regimen in relation to prolonged follow-up.
29. Treatment regimen in relation to repeat events (including bleeding).
Online Appendix 2
STEERING COMMITTEE
VOTING:
P.W. Serruys (chair)
S. Windecker (co-principal investigator)
M. Valgimigli (co-principal investigator)
P. Vranckx (co-principal investigator)
P.G. Steg
C. Hamm
G.A. van Es (ECRI)
P. Jüni (statistician)
CORE LABORATORY
ANGIOGRAPHY:
An independent angiographic core laboratory, located at Cardialysis, Rotterdam, The Netherlands, will analyse all diagnostic angiograms and define the SYNTAX score blinded to the therapy. Members of the angiographic core laboratory are not involved as investigators or co-investigators in this study.
ECG:
ECGs will be collected according to the schedule of investigations and will be assessed by an independent core laboratory, located at Cardialysis, Rotterdam, The Netherlands.
DATA SAFETY AND MONITORING BOARD:
F.W.A. Verheugt, Onze Lieve Vrouw Gasthuis, Amsterdam, The Netherlands (Chairman)
J.G.P.Tijssen, Academic Medical Center, Amsterdam, The Netherlands (Biostatistician)
L. Mauri, Brigham and Women’s Hospital, Boston, USA.
BLINDED INDEPENDENT MEDICAL REVISOR:
E. McFadden, Cork University Hospital, Cork, Ireland.
DATA MONITORING:
CARDIALYSIS
92 Westblaak, 3012 KM Rotterdam, The Netherlands.
THEOREM
1016 West Ninth Avenue, King of Prussia, PA, 19406, USA.
SPONSOR:
The European Cardiovascular Research Institute
(ECRI; www.ECRI-trials.com)