Abstract
Background: The optimal antiplatelet strategy in the second year after percutaneous coronary intervention (PCI) remains unclear.
Aims: We aimed to compare ticagrelor monotherapy with aspirin monotherapy on clinical outcomes beyond 1 year post-PCI.
Methods: This post hoc subanalysis of the open-label, all-comers, randomised GLOBAL LEADERS trial, which compared 23-month ticagrelor monotherapy following 1-month dual antiplatelet therapy (DAPT) with 12-month aspirin monotherapy following 12-month DAPT, only included patients who, at 12 months, were free from ischaemic and bleeding events and were adherent to their assigned antiplatelet therapy. The incidences of ischaemic events (all-cause death, any myocardial infarction, or any stroke) and bleeding events (Bleeding Academic Research Consortium [BARC] type 3 or 5 bleeding) during the second year (12-24 months) were compared between patients receiving either ticagrelor or aspirin monotherapy.
Results: The present analysis included 11,121 (ticagrelor monotherapy n=5,308, and aspirin monotherapy n=5,813) of the 15,991 patients enrolled in GLOBAL LEADERS. During the second year, the ischaemic composite endpoint was lower with ticagrelor monotherapy compared to aspirin monotherapy (1.9% vs 2.6%: log-rank p=0.014, adjusted hazard ratio [HR] 0.74, 95% confidence interval [CI]: 0.58-0.96; p=0.022), which was primarily driven by a reduced risk of myocardial infarction. In contrast, BARC type 3 or 5 bleeding was numerically higher with ticagrelor monotherapy (0.5% vs 0.3%: log-rank p=0.051, adjusted HR 1.89, 95% CI: 1.03-3.45; p=0.005).
Conclusions: Patients free from events at the end of the first year post-PCI and who adhered to their prescribed regimen had a reduced risk of ischaemic events compared to aspirin monotherapy in the second year post-PCI. ClinicalTrials.gov: NCT01813435
Introduction
Antiplatelet therapy is an essential part of the standard of care in patients with coronary artery disease (CAD), especially after percutaneous coronary intervention (PCI)123. Recent trials indicate that P2Y12 inhibitor monotherapy reduces bleeding risks without increasing ischaemic risks, especially in the first year following PCI, and, therefore, could be an alternative to dual antiplatelet therapy (DAPT) post-PCI45.
Currently, beyond the first year after PCI, aspirin monotherapy is recommended for the secondary prevention of coronary ischaemic events, such as spontaneous myocardial infarction (MI)23. However, as demonstrated in the DAPT study, whilst continuing with DAPT for 12 to 30 months after PCI significantly reduces the risks of adverse ischaemic events, including stent thrombosis, compared to aspirin monotherapy, this comes at the expense of increased bleeding risks6. Recently, the HOST-EXAM trial demonstrated that in Asian patients clopidogrel monotherapy reduces adverse clinical events, compared to aspirin monotherapy, during the chronic maintenance period after PCI7. However, it remains unclear whether more potent but specific antiplatelet treatment improves clinical outcomes, compared to aspirin monotherapy, beyond 1 year in Western populations8.
The GLOBAL LEADERS trial, which was an open-label, all-comers, randomised controlled trial, aimed to investigate the safety and efficacy of a novel antiplatelet regimen, consisting of 23-month ticagrelor monotherapy following 1-month DAPT, compared to 12-month aspirin monotherapy following 12-month DAPT. In the second year of the trial, the experimental arm of ticagrelor monotherapy was compared to the reference arm of aspirin monotherapy. The objective of the current analysis of GLOBAL LEADERS is to compare the efficacy and safety of monotherapy with ticagrelor and aspirin amongst those patients who were free from ischaemic and bleeding events during the first year following PCI and continued to adhere to their allocated treatment regimen.
Methods
The GLOBAL LEADERS trial
The GLOBAL LEADERS trial4 was an investigator-initiated, prospective, randomised, multicentre, multicontinental, open-label trial designed to evaluate two antiplatelet therapy strategies after PCI, consistently using bivalirudin and biolimus A9-eluting stents (BioMatrix) in an all-comers population, with no restriction regarding clinical presentation (chronic coronary syndrome [CCS] or acute coronary syndrome [ACS]), lesion complexity or number of stents used. Patients who needed oral anticoagulation therapy after PCI, had a history of major bleeding, had surgery planned within 12 months of PCI or had severe hepatic impairment were not eligible for the study. In the experimental strategy, patients received aspirin 75-100 mg once daily, in combination with ticagrelor 90 mg twice daily for one month, followed by ticagrelor 90 mg monotherapy twice daily for 23 months (irrespective of the clinical presentation). In the reference strategy, patients received aspirin 75-100 mg daily, in combination with either clopidogrel 75 mg once daily in CCS patients or ticagrelor 90 mg twice daily in ACS patients for 1 year, followed by aspirin 75-100 mg monotherapy once daily for the following 12 months (from 12 to 24 months after PCI). The study was approved by the institutional review board at each participating institution. All patients provided informed consent. The study complied with the Declaration of Helsinki and Good Clinical Practice guidelines.
Substudy population
In the present substudy, patients who died or had experienced ischaemic events (stroke, myocardial infarction [MI], repeat revascularisation, or definite/probable stent thrombosis) or bleeding (the Bleeding Academic Research Consortium [BARC] criteria type 2, 3 or 5 bleeding) during the first year (up to 365 days after randomisation) were excluded, mainly because those events could lead to changes in antiplatelet regimen in clinical practice69. In addition, patients who were not adherent to their assigned treatment6, or for whom we did not have information on adherence up to 12 months, were excluded. To summarise, the current analysis included all those patients who were known to have adhered to their assigned treatment and had not had any ischaemic or bleeding events in the first year after their PCI.
Endpoints
The primary endpoint of the present study was a composite of all-cause mortality, any site-reported MI (periprocedural or spontaneous), in accordance with the third universal definition10, and any stroke (ischaemic, haemorrhagic or uncertain) during the second year (from 12 to 24 months) following randomisation. The secondary safety endpoint was site-reported major bleeding events, according to the BARC criteria type 3 or 511. Other endpoints included new Q-wave MI, defined as MI with development of new pathological Q-waves, any revascularisation (target-vessel or non-target vessel), definite or probable stent thrombosis, according to the Academic Research Consortium (ARC) definition12, and BARC type 2, 3, or 5 bleeding. Moreover, patient-oriented composite endpoints (POCE), defined as a composite of all-cause mortality, any stroke, any MI and any revascularisation, and net adverse clinical events (NACE), defined as a composite of POCE and BARC type 3 or 5 bleeding, were also reported to clarify the net benefit and risk13.
All events, other than new Q-wave MI, were site reported without independent adjudication.
Statistical analysis
All the analyses were performed on the intention-to-treat population. Continuous variables are expressed as mean±standard deviation and were compared using the independent t-test. Categorical variables are presented as counts and percentages and compared using the chi-square test or Fisher’s exact test, as appropriate.
The Kaplan-Meier method was used to estimate the cumulative event rates, and the log-rank test was performed to examine the differences between the experimental strategy (ticagrelor monotherapy) versus the reference strategy (aspirin monotherapy), with the calculation of absolute risk reduction (ARR) and number needed to treat (NNT) based on those event rates14. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated, in comparison with the two randomised arms in the unadjusted and adjusted Cox proportional hazards models, to adjust for potential bias from patients excluded due to clinical events or non-adherence to the assigned regimen during the first year. The covariables in the adjusted model included age, sex, body mass index (BMI), clinical presentation (ACS or CCS), diabetes, peripheral vascular disease (PVD), chronic obstructive pulmonary disease (COPD), current smoker, renal failure, previous stroke, previous MI, previous bleeding, left main PCI, and multivessel PCI, with these variables selected based on prior knowledge of their association with outcomes15. The composite endpoints were analysed according to time-to-first event analysis.
In addition, risk-differences between the two randomised groups for the primary ischaemic endpoint (death, MI, or stroke) and secondary bleeding endpoint (BARC type 3 or 5 bleeding) were assessed, stratifying patients by prespecified subgroups of clinical presentation (CCS or ACS), age (≥75 or ≤75 years of age), sex (men or women), diabetes or non-diabetes, and with or without chronic kidney disease (CKD)4, with evaluation of the treatment-by-subgroup interactions, using adjusted Cox proportional hazards models.
For exploratory purposes, we also stratified subgroups according to DAPT score (high: ≥2, low: <2)16, PRECISE-DAPT score (high: ≥25, low: <25)17, CRUSADE score (high: >40, low-moderate: ≤40)18, ACUITY score (high: >20, low-moderate: ≤20)19, complex PCI criteria (multivessel PCI, >3 stents implanted, >3 lesions treated, bifurcation PCI with >2 stents, or total stent length >60 mm)120, TWILIGHT trial high-risk criteria (Supplementary Appendix 1)5, anatomical SYNTAX score (high: ≥22, low-intermediate: <22), logistic clinical SYNTAX score (≥median value or
Statistical significance was considered if the two-sided p-value ≤0.05. All analyses were performed in SPSS Statistics, version 26 (IBM Corp.) and R software, version 3.5.1 (R Foundation for Statistical Computing).
Results
The GLOBAL LEADERS trial enrolled 15,991 patients between July 2013 and November 2015. Twenty-three patients withdrew consent and requested that their data be deleted from the database, leaving a total of 15,968 patients of whom 7,980 (50.0%) and 7,988 (50.0%) were assigned to the experimental and reference strategies, respectively.
The patient flowchart of the present analysis is presented in Figure 1. At 12 months, 2,672 (33.5%) patients in the experimental arm and 2,175 (27.2%) patients in the reference arm were excluded due to ischaemic or bleeding events within 12 months, loss to follow-up, non-adherence to the study regimen or missing information on adherence. Hence, the cohort for this study comprised 11,121 patients, including 5,308 (66.5%) patients receiving ticagrelor monotherapy and 5,813 (72.8%) patients receiving aspirin monotherapy.
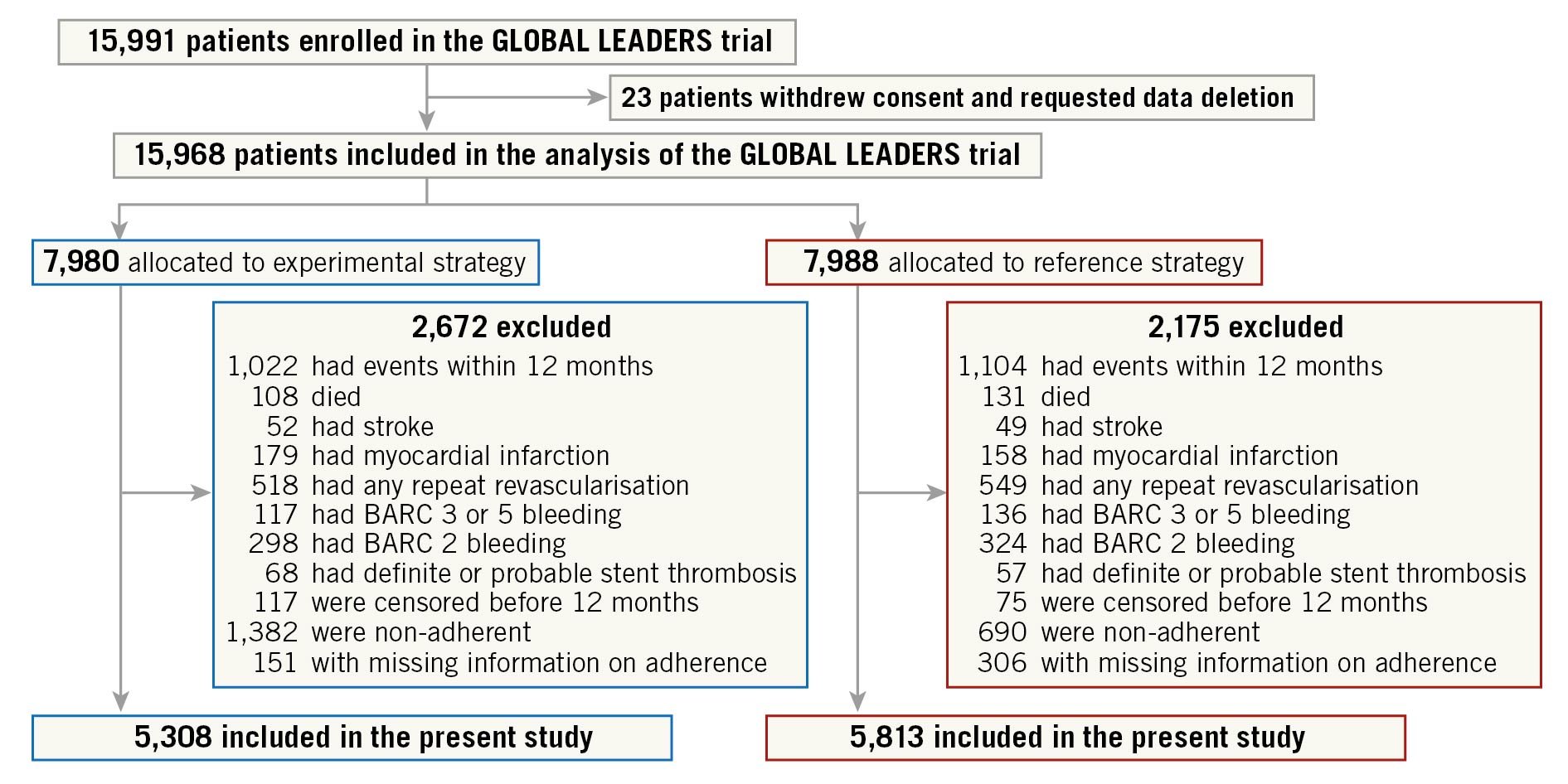
Figure 1. Study flowchart. Patients who had ischaemic or bleeding events during the first year after index PCI or were not adherent to the assigned antiplatelet therapy were excluded from the current study. BARC: Bleeding Academic Research Consortium
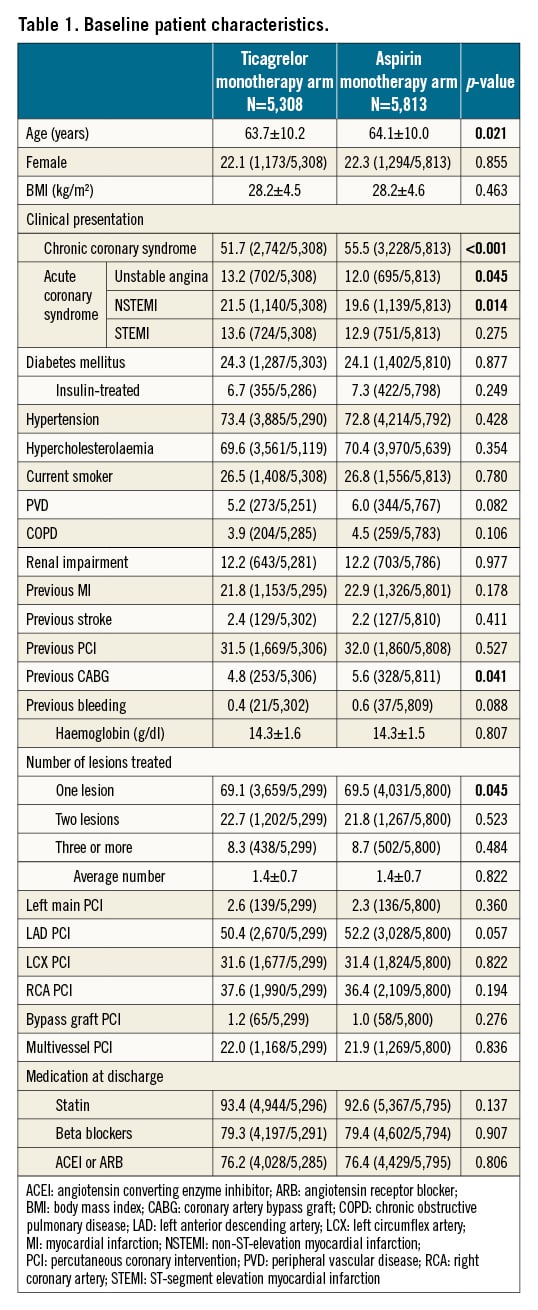
The baseline characteristics of the included patients were well balanced, except for age (63.7±10.2 vs 64.1±10.0 years; p=0.021) and more frequent CCS (51.7% vs 55.5%; p <0.001) (Table 1).
Clinical outcomes
The clinical outcomes for the landmark analysis beyond 1 year are presented in the Central illustration and Table 2. In this selected population, the composite of death, MI, or stroke was significantly lower with ticagrelor monotherapy compared to aspirin monotherapy (1.9% vs 2.6%: unadjusted HR 0.73, 95% CI: 0.57-0.94; p=0.014, adjusted HR 0.74, 95% CI: 0.58-0.96; p=0.022), which was mainly driven by the significantly reduced risk of spontaneous MI (0.7% vs 1.2%: unadjusted HR 0.57, 95% CI: 0.38-0.85; p=0.006, adjusted HR 0.54, 95% CI: 0.36-0.82; p=0.003). The risk of any repeat revascularisation was lower with the ticagrelor monotherapy (2.8% vs 3.5%: unadjusted HR 0.79, 95% CI: 0.64-0.98; p=0.029, adjusted HR 0.80, 95% CI: 0.64-0.99; p=0.037), whilst the risk of definite/probable stent thrombosis was comparable (ticagrelor 0.2% vs aspirin 0.3%: unadjusted HR 0.68, 95% CI: 0.31-1.51; p=0.347). The risk of new Q-wave MI did not differ in unadjusted (unadjusted HR 1.09, 95% CI: 0.62-1.93; p=0.755) or adjusted models (adjusted HR 1.03, 95% CI: 0.58-1.84; p=0.923), such that the composite of all-cause mortality and new Q-wave MI was also comparable (adjusted HR 0.95, 95% CI: 0.69-1.30; p=0.734) (Table 2).
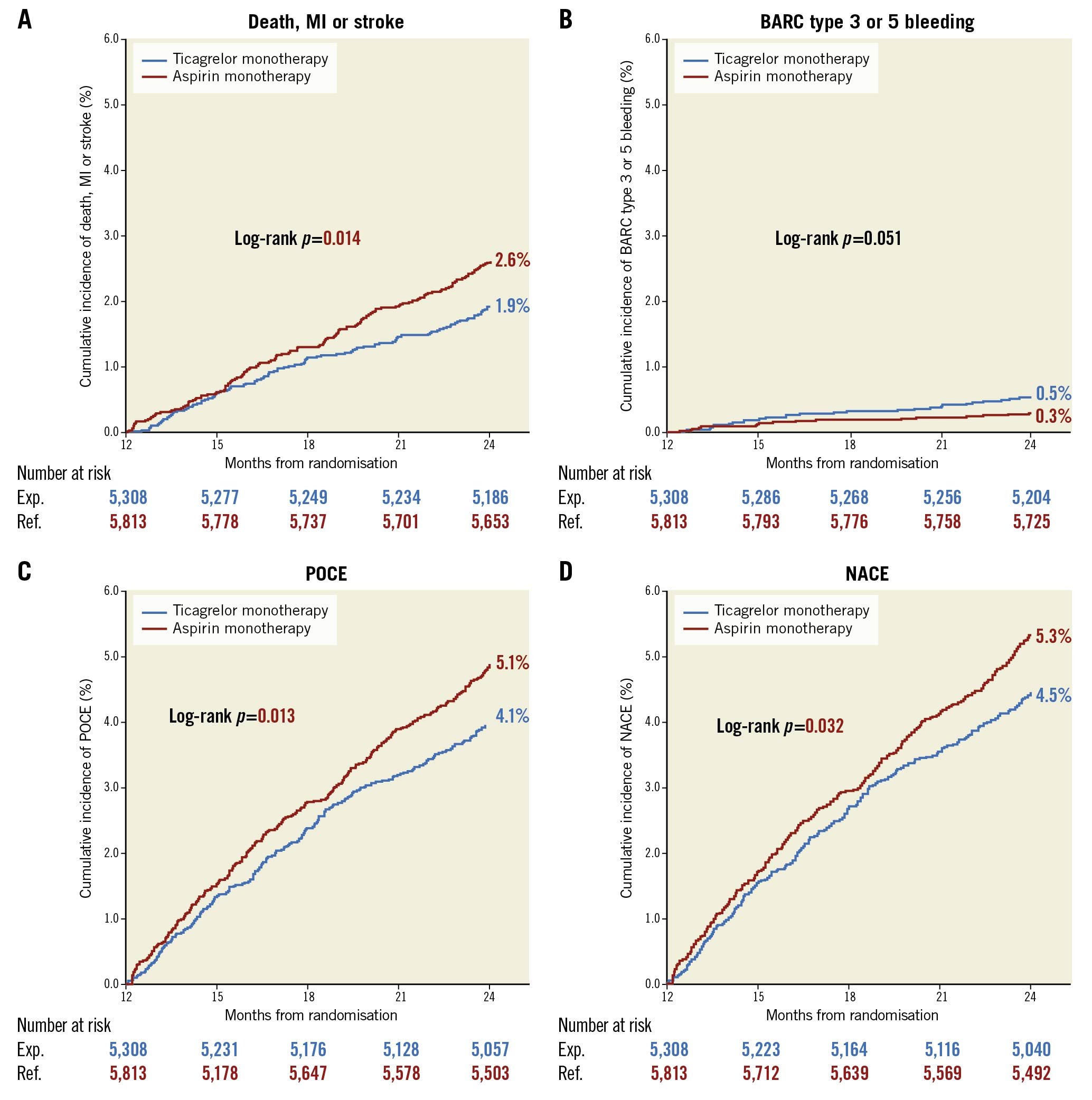
Central illustration. Cumulative Kaplan-Meier estimates of clinical events from 12 to 24 months in patients with ticagrelor monotherapy or aspirin monotherapy. From 12 months to 24 months, ticagrelor monotherapy reduced incidences of ischaemic events (death, MI, or stroke) but numerically increased bleeding events (BARC type 3 or 5 bleeding). BARC: Bleeding Academic Research Consortium; MI: myocardial infarction; NACE: net adverse clinical events; POCE: patient-oriented composite endpoints
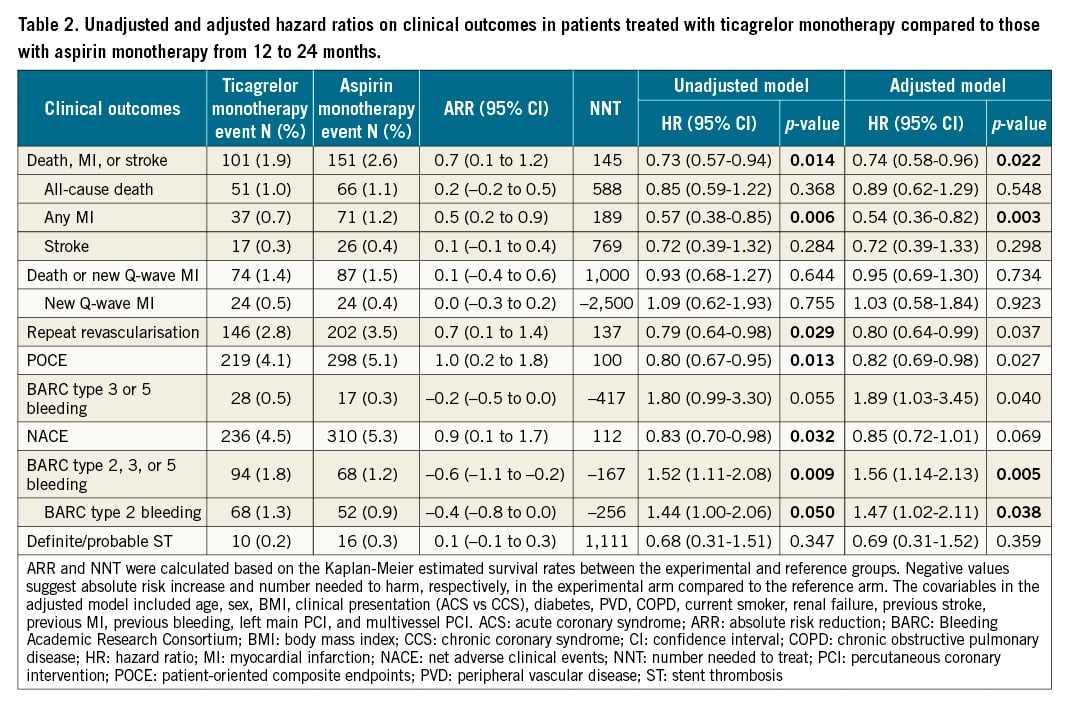
Ticagrelor monotherapy led to a numerically higher rate of BARC type 3 or 5 bleeding (0.5% vs 0.3%: unadjusted HR 1.80, 95% CI: 0.99-3.30; p=0.055), which was only significant after adjusting for confounders (adjusted HR 1.89, 95% CI: 1.03-3.45; p=0.040). BARC type 2 bleeding was significantly higher with ticagrelor monotherapy than with aspirin monotherapy (1.3% vs 0.9%: unadjusted HR 1.44, 95% CI: 1.00-2.06; p=0.050, adjusted HR 1.47, 95% CI: 1.02-2.11; p=0.027), resulting in a significantly higher risk of BARC type 2, 3, or 5 bleeding with ticagrelor monotherapy (1.8% vs 1.2%: unadjusted HR 1.52, 95% CI: 1.11-2.08; p=0.009, adjusted HR 1.56, 95% CI: 1.14-2.13; p=0.005).
Consequently, compared to the aspirin monotherapy, ticagrelor monotherapy was associated with a significant reduction in the risk of POCE (4.1% vs 5.1%: unadjusted HR 0.80, 95% CI: 0.67-0.95; p=0.013, adjusted HR 0.82, 95% CI: 0.69-0.98; p=0.027) but not in the adjusted risk of NACE (4.4% vs 5.3%: unadjusted HR 0.83, 95% CI: 0.70-0.98; p=0.032, adjusted HR 0.85, 95% CI: 0.72-1.01; p=0.069).
Prespecified subgroup analysis
The adjusted risk differences between ticagrelor and aspirin monotherapy, in terms of the primary (death, MI, or stroke) and secondary endpoints (BARC type 3 or 5 bleeding), were assessed among the prespecified subgroups: clinical presentation, age, sex, and diabetic status (Figure 2). Overall the treatment-by-subgroup interactions were not significant across strata, except for the presence or absence of CKD in terms of serious bleeding: only patients in the subgroup without CKD (p for interaction=0.026) showed higher BARC type 3 or 5 bleeding in the ticagrelor monotherapy arm than in the aspirin monotherapy arm.
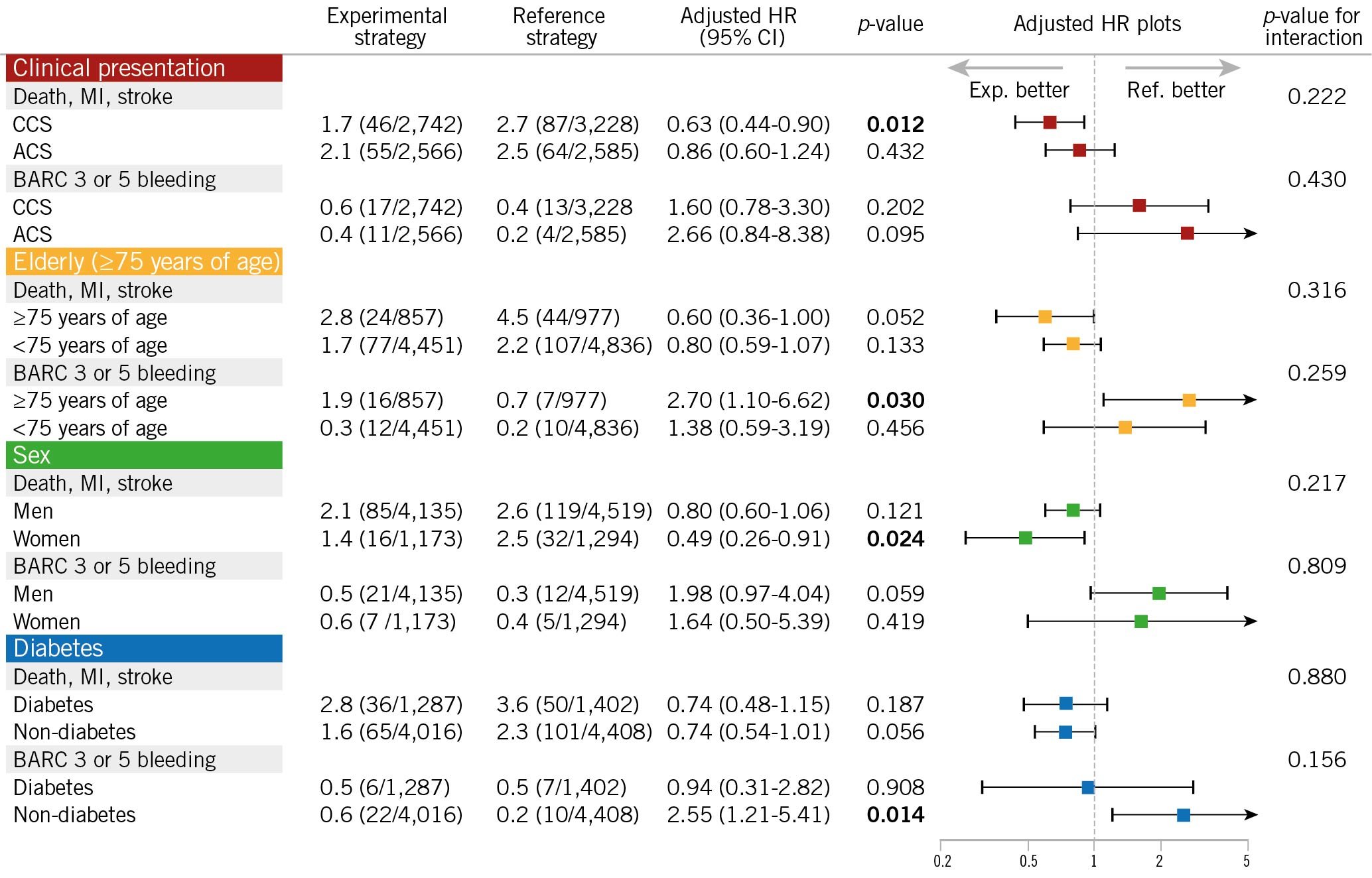
Figure 2. Hazard ratio of ticagrelor monotherapy over aspirin monotherapy in patients stratified by prespecified subgroups in the GLOBAL LEADERS trial.There was a significant treatment-by-subgroup interaction observed between antiplatelet strategy and the presence of chronic kidney disease (CKD), in terms of BARC type 3 or 5 bleeding, where patients without CKD showed treatment benefit from aspirin monotherapy, compared to ticagrelor monotherapy, while it was not observed among patients with CKD. In all other subgroups, no significant treatment-by-subgroup interactions were observed among patients treated with ticagrelor monotherapy or aspirin monotherapy during the second year. Adjusted covariates are listed in Table 2. ACS: acute coronary syndrome; BARC: Bleeding Academic Research Consortium; CCS: chronic coronary syndrome; CI: confidence interval; HR: hazard ratio; MI: myocardial infarction
Subgroups stratified according to available risk scores or criteria
Figure 3 presents several subgroups classified in accordance with risk stratification by the DAPT score, PRECISE-DAPT score, CRUSADE score, ACUITY score, complex PCI criteria, TWILIGHT trial criteria, anatomical SYNTAX score, logistic clinical SYNTAX score, or the ARC-high bleeding risk trade-off model. The results were reasonably consistent, with reduced ischaemic risk with ticagrelor monotherapy, compared to aspirin monotherapy overall, and no treatment-by-subgroup interactions evident in terms of ischaemic events (death, MI, or stroke) or bleeding events (BARC type 3 or 5 bleeding).
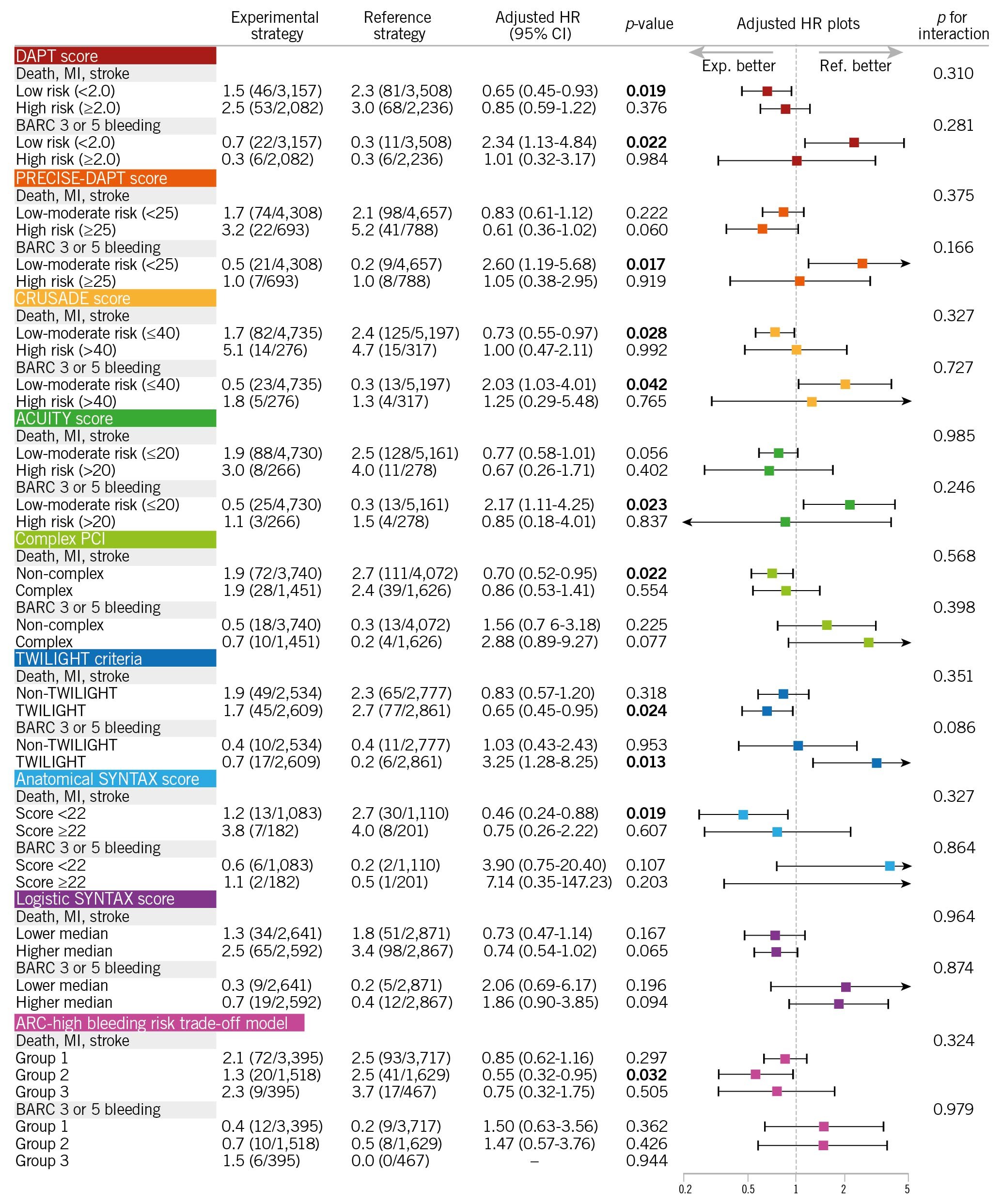
Figure 3. Hazard ratio of ticagrelor monotherapy over aspirin monotherapy in patients stratified by specific subgroups in the GLOBAL LEADERS trial. Patients were stratified by: A) DAPT score, PRECISE-DAPT score, CRUSADE score, and ACUITY score; B) complex PCI or non-complex PCI, eligible or ineligible for the TWILIGHT criteria, anatomical SYNTAX score of ≥22 or <22, and ≥median (2.16% 2-year mortality risk) or The main finding from our study is that in a selected population that was adherent to its assigned antiplatelet regimen and was free from clinical events in the first year of follow-up, ticagrelor monotherapy was associated with lower ischaemic events (death, MI, or stroke) and with numerically increased serious bleeding events (BARC type 3 or 5 bleeding). The majority of events with contemporary DES occur during the first year of follow-up after PCI. Beyond 1 year, whilst there is an annual 0.2-0.6% incremental rate of stent thrombosis2526, most events are related to progressive atherosclerosis within (neoatherosclerosis) or unrelated to the stented segment, and typically aspirin is prescribed to prevent associated thrombotic events. Our findings corroborate the results of the DAPT study where, compared to aspirin monotherapy, more potent antiplatelet strategies (clopidogrel or prasugrel on top of aspirin [in the DAPT study] and on top of ticagrelor monotherapy [in the current study]) reduced ischaemic cardiovascular events, at the expense of an increased risk in bleeding events6. In the present study, the rate of definite/probable stent thrombosis between 12 and 24 months was 0.2% and 0.3% with ticagrelor and aspirin monotherapy, respectively. In comparison, in the DAPT study, rates of stent thrombosis between 12 and 30 months were, respectively, 0.4% and 1.4% with DAPT and aspirin monotherapy. Although the follow-up duration of the DAPT trial was 6 months longer than the present study, the risk of stent thrombosis among patients treated with aspirin monotherapy was nearly 5 times higher in the DAPT study6. This is highly likely to be due to the substantial use of first-generation DES in the DAPT trial (approximately 38% of all stents)6, whilst in the GLOBAL LEADERS trial patients uniformly received a BioMatrix second-generation biodegradable polymer DES. The remaining unanswered question is whether secondary prevention using ticagrelor monotherapy is superior to DAPT, or other P2Y12 inhibitor monotherapy, especially in the 12 months after PCI using a contemporary DES. In the HOST-EXAM study, the favourable effects of clopidogrel monotherapy over aspirin monotherapy were observed not only for ischaemic endpoints, mainly driven by a significantly lower risk of ACS readmission, but also bleeding endpoints (BARC type ≥3 or haemorrhagic stroke), whereas there were no significant risk differences between the two antiplatelet regimens in terms of non-fatal MI or repeat revascularisation7. In Western populations, who generally have higher ischaemic risk but lower bleeding risk than Asian populations, ticagrelor monotherapy, with its more potent antiplatelet effect, might yield further reductions in ischaemic events, compared to clopidogrel monotherapy. However, this needs to be investigated in dedicated randomised studies. In the current study, the superior efficacy of ticagrelor monotherapy over aspirin monotherapy was demonstrated with a reduced ischaemic risk seen in the overall population, albeit at the expense of a numerically increased risk of bleeding. For exploratory purposes, we tried to identify a specific population who had a net clinical benefit with reduced ischaemic events and no increased bleeding among the study’s prespecified subgroups, as well as in subgroups stratified by available risk scores or criteria. Some subgroups, for example, females, had favourable anti-ischaemic effects with ticagrelor monotherapy, compared to aspirin monotherapy, without any increased bleeding events. However, due to the limited sample size for these stratified analyses, they were underpowered, and the 95% CIs were too wide to reliably estimate the risk difference between the two antiplatelet strategies in any subgroup. Theoretically, potent antiplatelet therapy would be more effective in patients with high-ischaemic risk, such as ACS patients27 or those undergoing complex PCI20. However, no amplification of the anti-ischaemic benefits of monotherapy with ticagrelor, compared to aspirin, was seen amongst those high-ischaemic risk subgroups. Only among patients with or without CKD was the treatment-by-subgroup interaction statistically significant in terms of BARC type 3 or 5 bleeding (Figure 2), suggesting that for patients without CKD the reference strategy (aspirin monotherapy) might be better than the experimental strategy (ticagrelor monotherapy) to avoid an unnecessary increased risk of serious bleeding. Our findings strengthen the call for further studies to evaluate the efficacy and risk of novel antiplatelet strategies for secondary prevention. The current European Society of Cardiology (ESC) guidelines on antiplatelet therapy recommend aspirin monotherapy after 6 and 12 months of DAPT, following PCI for CCS and ACS, respectively, with the use of P2Y12 inhibitor monotherapy yet to be debated in that clinical context12. Although recent trials tend to shorten the duration of DAPT, followed by a switch to monotherapy with aspirin or a P2Y12 inhibitor, to date no randomised trial has compared aspirin monotherapy with a potent P2Y12 inhibitor monotherapy after PCI, with respect to clinical endpoints. We acknowledge that the current study did not compare these two antiplatelet strategies in the first year, and that beyond the first year after PCI there may be lower requirements for potent antiplatelet therapy. In fact, the NNT was substantially high to yield a treatment benefit of ticagrelor monotherapy, compared to aspirin monotherapy, during the second year; it was more than 100 in every clinical endpoint in the current study (Table 2). Taking into account the increased bleeding risk, as well as the higher cost of ticagrelor than aspirin, the current results are a weak incentive to use routine ticagrelor monotherapy beyond 1 year after PCI. However, our findings provide further insights into the clinical question of whether monotherapy with aspirin or a P2Y12 inhibitor would be the optimal antiplatelet strategy in individual patients after PCI. First, this study is a post hoc, non-prespecified subanalysis of a randomised controlled trial. Therefore, all the findings should be considered as hypothesis-generating and non-confirmatory. Second, we previously reported the second-year results of ticagrelor monotherapy versus aspirin monotherapy in the GLOBAL LEADERS subpopulation that was eligible in accordance with the DAPT study criteria16. However, a number of patients were excluded, due to events in the first year or non-adherence to treatment. Particularly in the experimental arm, the number of patients who were not adherent to the assigned antiplatelet therapy (ticagrelor monotherapy) was substantially higher than those in the reference arm (1,382 vs 690) (Figure 1), which might introduce a selection bias. In fact, some variables, such as age or clinical presentation, were imbalanced between the two groups suggesting selection biases derived from the excluded population (Table 1). Therefore, in the current study to minimise such bias, we also performed multivariable adjustments for confounding factors. In addition, we also evaluated the clinical effects of ticagrelor monotherapy over aspirin monotherapy in specific subgroups. However, these subgroup analyses may be underpowered to evaluate clinical risk differences in each subgroup. Third, in the current guidelines, updated in 20182, the recommended maintenance dose of ticagrelor during the chronic phase (beyond 1 year) is 60 mg bid on top of aspirin, instead of 90 mg bid as implemented in the current study. The GLOBAL LEADERS trial was initially designed in 2013; at that time the clinical value of a lower dose of ticagrelor (60 mg bid) was not established as treatment during the chronic maintenance period. Hence, the use of ticagrelor 60 mg bid might lead to results at variance with the current ones. Finally, in the GLOBAL LEADERS trial, there was no central independent adjudication of clinical events, and all events were site-reported without adjudication. However, the GLASSY study28, which is a prespecified ancillary study of the GLOBAL LEADERS trial with central independent event adjudication, reported results consistent with site reporting, with the incidence of MI significantly lower with ticagrelor monotherapy, compared to aspirin monotherapy, in the second year of follow-up (rate ratio 0.54, 95% CI: 0.33-0.88), even when nonadherent patients were excluded (rate ratio 0.54, 95% CI: 0.31-0.93, Supplementary Table 1)28. In patients free from events at the end of the first year post-PCI and who adhered to their prescribed regimen, ticagrelor monotherapy was associated with a reduced risk of ischaemic events with a numerically increased risk of bleeding events compared to aspirin monotherapy in the second year post-PCI. Therefore, ticagrelor monotherapy may be a good alternative to aspirin monotherapy for secondary prevention 12 months after PCI in patients who are event-free and adherent to the regimen at 12 months. Beyond 1-year post-PCI, in patients free from events at the end of the first year post-PCI and who adhered to their prescribed regimen up to 1 year, ticagrelor monotherapy was associated with a reduced risk of ischaemic composite endpoints and a numerically increased risk of major bleeding, compared to aspirin monotherapy. Further studies are warranted to evaluate the efficacy and risk of the novel antiplatelet strategy of a potent P2Y12 inhibitor monotherapy for secondary prevention. GLOBAL LEADERS was sponsored by the European Clinical Research Institute, which received funding from AstraZeneca, Biosensors International, and the Medicines Company. The study funders had no role in the design, data collection, management, analysis, interpretation, or writing of the report. H. Hara reports a grant for studying overseas from the Japanese Circulation Society and a grant from the Fukuda Foundation for Medical Technology, outside the submitted work. J. Piek reports personal fees and non-financial support from Philips/Volcano, outside the submitted work. C. Hamm reports speaker fees from AstraZeneca, not directly related to this study. G. Steg reports grants and personal fees from Amarin, AstraZeneca, Bayer/Janssen, Merck, Sanofi, and Servier; personal fees from Amgen, Boehringer-Ingelheim, Bristol Myers Squibb, Idorsia, Lilly, Mylan, Novartis, Novo Nordisk, Pfizer, and Regeneron, outside the submitted work. M. Valgimigli reports personal fees from Abbott Vascular, AstraZeneca, Alvimedica/CID, Bayer, Bristol Myers Squib SA, CoreFLOW, Daiichi Sankyo, Idorsia Pharmaceuticals Ltd, iVascular, Medscape, Opsens, Universität Basel, Dept. Klinische Forschung, Vifor; and grants and personal fees from Terumo, outside the submitted work. S. Windecker serves as unpaid advisory board member and/or unpaid member of the steering/executive group of trials funded by Abbott, Abiomed, Amgen, AstraZeneca, Biotronik, BMS, Boston Scientific, Cardiovalve, Edwards Lifesciences, MedAlliance, Medtronic, Novartis, Polares, Sinomed, V-Wave, and Xeltis, but has not received personal payments by pharmaceutical companies or device manufacturers. He is also member of the steering/executive committee group of several investigator-initiated trials that receive funding by industry, without impact on his personal remuneration. Dr. Windecker is an unpaid member of the Pfizer Research Award selection committee in Switzerland and of the Women as One Awards Committee. He is a member of the Clinical Study Group of the Deutsches Zentrum für Herz Kreislauf-Forschung and of the Advisory Board of the Australian Victorian Heart Institute. He is chairperson of the ESC Congress Program Committee, former chairperson of the ESC Clinical Practice Guidelines Committee and Deputy Editor of JACC CV Interventions. P. Vranckx reports personal fees from Bayer Health Care, CLS Bhering, Novartis, and Daiichi Sankyo, outside the submitted work. P.W. Serruys reports personal fees from Biosensors, HeartFlow, Micel Technologies, Philips/Volcano, Sinomedical Sciences Technology, and Xeltis, outside the submitted work. The other authors have no conflicts of interest to declare. To read the full content of this article, please download the PDF.Discussion
Long-term secondary prevention beyond 1 year after PCI
The efficacy of ticagrelor monotherapy over aspirin monotherapy for secondary prevention in subgroups
Clinical perspective
Limitations
Conclusions
Impact on daily practice
Funding
Role of the funding source
Conflict of interest statement
Supplementary data

