Advances in medical therapeutics usually follow a typical pattern, with safety and efficacy initially established among high-risk patients prior to broader application in lower-risk individuals. In this regard, the withdrawal of aspirin (ASA) after percutaneous coronary intervention (PCI), or “aspirin-free” strategies, were first explored among patients with atrial fibrillation (AF) undergoing PCI in whom bleeding risk rendered the obligatory need for triple therapy prohibitive1. Numerous clinical trials have since shown that early withdrawal of ASA among such patients significantly lowers bleeding without compromising ischaemic risk as long as an oral anticoagulant and P2Y12 inhibition are maintained. Another cohort of patients at elevated risk for both ischaemic and haemorrhagic events includes those presenting with acute coronary syndromes (ACS), even in the absence of AF. Traditionally, concerns for recurrent thrombosis have dominated clinical decision making in the setting of ACS. Current guidelines recommend at least one year of dual antiplatelet therapy, preferably with a potent P2Y12 inhibitor, following an index ACS event2. However, exposure to dual antiplatelet therapy (DAPT) results in a time-dependent accrual of bleeding risk, which also increases morbidity and mortality3. Importantly, the GLOBAL LEADERS investigators were the first to extend the aspirin-free paradigm to a broad population of non-AF patients with and without ACS undergoing PCI (n=15,968)4. In this seminal trial, ticagrelor monotherapy for 23 months after one month of DAPT was compared to a conventional antiplatelet strategy. Although negative with respect to the primary endpoint of death or Q-wave myocardial infarction (MI), important secondary analyses and ancillary studies have since been reported from this data set with important clinical implications.
One such investigation is the GLOBAL LEADERS Adjudication Sub-StudY (GLASSY), a dedicated analysis focusing on the top 20 enrolling sites coupled with formal independent adjudication of all endpoint events. This latter point is particularly relevant as reliance on site-reported events or claims introduces both misclassification and ascertainment bias for certain endpoints, particularly bleeding.
In this issue of EuroIntervention, Franzone et al have examined the effect of the ticagrelor monotherapy in the GLASSY cohort in relation to presentation with ACS (n=3,840) or non-ACS (n=3,745)5.
Over two years, allocation to the experimental arm led to a comparable reduction in the primary efficacy endpoint of death, MI, stroke or urgent target vessel revascularisation among those with (RR 0.82, 95% CI: 0.66-1.01) or without ACS (RR 0.89, 95% CI: 0.69-1.13) (pint=0.63). Analogous results were obtained for the individual components of this endpoint and other ischaemic events, such as stent thrombosis. With respect to the primary safety endpoint of Bleeding Academic Research Consortium (BARC) type 3 or 5 bleeding, the experimental strategy resulted in a numerical reduction (RR 0.76, 95% CI: 0.51-1.12) among those with ACS, while an increase was observed among those without ACS (RR 1.39, 95% CI: 0.91-2.12; pint=0.039). Similar patterns were observed for the more frequent outcome of BARC 2, 3 or 5 bleeding with stronger evidence of effect modification (pint<0.001). These results must be interpreted within the nuanced design of the GLOBAL LEADERS trial as stable patients randomised to the conventional arm received one year of DAPT with clopidogrel plus ASA whereas ACS patients were treated with ticagrelor plus ASA. After one year, all patients allocated to the conventional strategy were transitioned to ASA monotherapy. Thus, the ACS comparison presented by Franzone et al represents ticagrelor monotherapy versus ticagrelor plus ASA followed by ASA alone. Analogously, ticagrelor monotherapy is compared to clopidogrel plus ASA followed by ASA among non-ACS patients.
The present findings with respect to ACS are consonant with those from other trials comparing ticagrelor monotherapy after a short duration of DAPT versus guideline-endorsed ticagrelor plus ASA (Figure 1),6,7. In aggregate, these results demonstrate a net clinical benefit characterised by a reduction in both minor and major bleeding without an apparent penalty with regard to ischaemic risk upon withdrawal of ASA and continuation of ticagrelor alone after a short duration of DAPT (1-3 months). These clinical observations are also supported by pharmacodynamic studies demonstrating that ASA withdrawal does not modulate in vitro or ex vivo markers of blood thrombogenicity in the presence of potent P2Y12 blockade or other antithrombotic regimens8,9. Thus, it appears that the need for long-term ASA may be obviated among ACS patients treated with ticagrelor, challenging the existing construct and recommendation for DAPT in all ACS patients undergoing PCI. However, it is likely that clinical adoption will be met with some degree of reluctance given the long-standing imperative for DAPT after PCI and ACS. As this clinical and investigative space evolves, other bleeding-avoidance strategies will also need to be compared to one another. These include guided de-escalation of DAPT based upon results of platelet function or genetic testing and use of prasugrel monotherapy2. Moreover, questions persist surrounding the extension of aspirin-free strategies vis-à-vis long-term secondary prevention. Clinical trials have shown that prolonged DAPT, or the combination of a low-dose direct oral anticoagulant with ASA, significantly lowers thrombotic events as compared with ASA alone among patients with a history of MI or enriched with atherosclerotic cardiovascular disease. The extent to which ASA will remain a necessary component of such long-term therapies remains unknown.
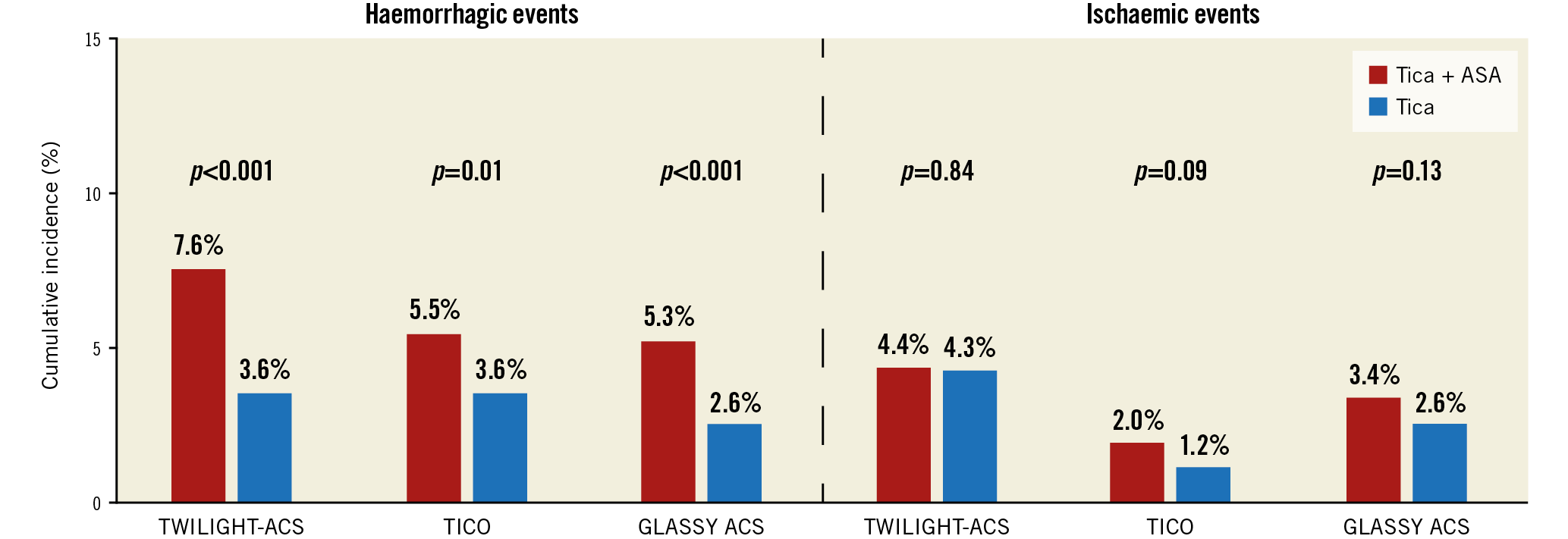
Figure 1. Effect of ticagrelor monotherapy versus ticagrelor plus aspirin on haemorrhagic and ischaemic events among patients with acute coronary syndromes (ACS). TWILIGHT: Ticagrelor With Aspirin or Alone in High-Risk Patients After Coronary Intervention; TICO: Ticagrelor Monotherapy After three Months in the Patients Treated With New Generation Sirolimus-eluting Stent for Acute Coronary Syndrome; GLASSY: GLOBAL LEADERS Adjudication Sub-StudY. Haemorrhagic events were defined as Bleeding Academic Research Consortium (BARC) type 2, 3 or 5 in TWILIGHT and GLASSY-ACS; Thrombolysis In Myocardial Infarction (TIMI) minor or major in TICO. Ischaemic events included death, myocardial infarction or stroke in TWILIGHT; cardiac death, acute MI, stent thrombosis, target vessel revascularisation (TVR) in TICO; death, MI, stroke or urgent TVR in GLASSY. Events in GLASSY-ACS occurring between 30 days and one year are depicted to provide a comparison between ticagrelor monotherapy and ticagrelor plus ASA. ASA: aspirin; Tica: ticagrelor
Early withdrawal of ASA among stable patients undergoing PCI represents an intuitive “next step” in the iterative application of the aspirin-free strategy. However, as these patients are at relatively low risk for bleeding, the clinical basis for such an approach appears less robust. Indeed, the present analysis demonstrates that ticagrelor monotherapy increases bleeding events without a reduction in ischaemic complications when compared to a conventional antiplatelet strategy in stable patients. Nonetheless, it is plausible that stable patients enriched with clinical and/or angiographic markers of excess thrombotic or haemorrhagic risk may be suitable for early withdrawal of ASA. A post hoc analysis from the GLOBAL LEADERS trial found that the experimental treatment yielded an accentuated benefit among those undergoing complex versus non-complex PCI10. Similar findings were reported from the Ticagrelor With Aspirin or Alone in High-Risk Patients After Coronary Intervention (TWILIGHT) trial, although direct comparisons across studies is confounded by differences in referent strategies11.
The role of ASA as a foundation for DAPT in all patients undergoing PCI should no longer be considered a default and mandatory assumption. Initially challenged in the setting of AF and PCI, contemporary clinical and pharmacodynamic data have now extended this paradigm to the ACS setting. Ongoing studies will further clarify the need, or lack thereof, for ASA in lower-risk patients after PCI.
Conflict of interest statement
U. Baber has received personal fees from Amgen, Boston Scientific and AstraZeneca. R. Mehran reports institutional research grants from Abbott Laboratories, Abiomed, Applied Therapeutics, AstraZeneca, Bayer, Beth Israel Deaconess, Bristol-Myers Squibb, CERC, Chiesi, Concept Medical, CSL Behring, DSI, Medtronic, Novartis Pharmaceuticals, OrbusNeich; consultant fees from Abbott Laboratories, Boston Scientific, CardiaWave, Chiesi, Janssen Scientific Affairs, Medscape/WebMD, Medtelligence (Janssen Scientific Affairs), Roivant Sciences, Sanofi, Siemens Medical Solutions; consultant fees paid to the institution from Abbott Laboratories, Bristol-Myers Squibb; advisory board, funding paid to the institution from Spectranetics/Philips/Volcano Corp; consultant (spouse) from Abiomed, The Medicines Company, Merck; Equity <1% from Claret Medical, Elixir Medical; DSMB Membership fees paid to the institution from Watermark Research Partners; consulting (no fee) from Idorsia Pharmaceuticals Ltd., Regeneron Pharmaceuticals; and is an Associate Editor for ACC, AMA.
Supplementary data
To read the full content of this article, please download the PDF.

