The benefits of an invasive approach and early revascularisation are well established, and clinical practice guidelines recommend that elderly patients should not be treated differently to younger patients. However, elderly patients are at higher risk of complications and subsequent mortality due to more frequent comorbidities, drug interactions, chronic renal failure, anaemia and frailty1,2. Especially in elderly patients, antiplatelet therapy can be a double-edged sword that reduces the risk of ischaemic events but exposes the patient to an increased risk of major bleeding and subsequent mortality3. As age is a leading factor associated with both ischaemic and bleeding events, the management of elderly patients is challenging. There is a lack of evidence in the elderly from randomised controlled trials which selected patients at low bleeding risk and excluded or limited the inclusion of elderly patients, resulting in a low representation of elderly patients in the population of clinical trials (10%) and a slightly higher proportion in registries (35%)4.
The challenge of managing bleeding risk has become more serious as technical progress and stent development have drastically reduced the risk of stent thrombosis, leaving major bleeding the most feared complication of percutaneous coronary intervention (PCI), particularly in the elderly. Many therapeutic strategies have therefore been suggested to reduce this risk. One of these is antithrombotic treatment de-escalation, as suggested by Tomaniak et al5 in this issue of EuroIntervention where they report the results of a sub-analysis of the GLOBAL LEADERS trial focusing on elderly patients.
GLOBAL LEADERS was a randomised clinical trial that aimed to compare two antiplatelet therapy strategies after stent implantation. The experimental arm consisted of one-month dual antiplatelet therapy (DAPT) with ticagrelor plus aspirin followed by a single antiplatelet inhibition with ticagrelor for 23 months, while the reference arm received 12-month DAPT with aspirin in combination with either ticagrelor (in patients with acute coronary syndrome [ACS]) or with clopidogrel (in patients with stable coronary artery disease [CAD]), followed by 12-month aspirin monotherapy6. The hypothesis of superior efficacy and safety of early and long-term ticagrelor monotherapy was rejected in the overall population as there were no significant differences in ischaemic and bleeding events between the two groups at 24 months6. However, despite these neutral results, ticagrelor monotherapy is not without clinical interest. In the TWILIGHT trial, patients who had not had clinical events during the first three months of DAPT continued to take ticagrelor with aspirin or placebo for one year. In this trial, the incidence of clinically relevant bleeding events was lower among patients treated with ticagrelor alone than in those treated with ticagrelor plus aspirin without a significant price to pay in terms of ischaemic events (non-inferiority)7.
To provide further data on this highly relevant topic, and to attempt to select patient profiles which could benefit from this therapeutic strategy, Tomaniak et al assessed the effects of single antiplatelet inhibition with ticagrelor in the GLOBAL LEADERS subgroup of elderly patients (≥75 years; n=2,565, 16.1%). The authors observed a meaningful reduction in all-cause mortality, target vessel revascularisation and a patient-oriented composite endpoint (all-cause death, any stroke, myocardial infarction [MI] and any revascularisation) in elderly patients treated with ticagrelor monotherapy compared with the reference strategy. However, we should keep in mind that the GLOBAL LEADERS trial was neutral, and the investigators did not find an impact of age on the primary endpoint of the main study (a composite of either all-cause mortality or new Q-wave MI). In consequence, the reported results are hypothesis-generating only. Even though the number of patients was large, findings derived from subgroups may be only the play of chance, particularly since the number of events was low and the authors did not control for multiple comparisons. In an appropriately cautious interpretation due to these limitations, a safety signal should be considered. Despite the withdrawal of aspirin, the rate of major bleeding events (BARC 3/5 type bleeding) was numerically higher in the elderly patients with ticagrelor monotherapy for 23 months than with DAPT for 12 months and the difference reached statistical significance among stable CAD patients. In addition, treatment discontinuation was more frequent in the elderly than in the overall study population, and significantly higher in the experimental strategy than in the reference arm. This poor treatment compliance is a tricky issue as it may increase the risk of secondary ischaemic events. Unfortunately, the authors did not specifically evaluate this in the present sub-analysis. Considering the higher rates of bleeding events and treatment discontinuation, partially related to the necessity of twice daily dosing, ticagrelor monotherapy has no clear benefit for secondary prevention in elderly patients. Given that no trial is currently planned to evaluate P2Y12 monotherapy in this specific population, further data from the elderly population of the TWILIGHT trial7 should be helpful in order to provide clarity.
However, the early dropping of aspirin following PCI remains a relevant and promising therapeutic option to reduce bleeding risk. Recently, the SMART-CHOICE trial demonstrated that three months of DAPT followed by nine months of clopidogrel monotherapy was non-inferior to one year of standard DAPT in terms of major adverse cardiac and cerebrovascular events8. In the STOPDAPT-2 trial, one-month DAPT followed by 11 months of clopidogrel monotherapy was superior to a standard 12 months of DAPT in terms of net clinical benefit9.
A number of other therapeutic strategies have been proposed in the overall population of patients with CAD. Several of these have been associated with significant reduction of bleeding events (Figure 1) such as antiplatelet de-escalation by switching from a more potent P2Y12 inhibitor to clopidogrel after the first month (TOPIC trial10). Although few elderly patients were included in these trials, the strategies may be particularly beneficial to elderly patients considering the inherent higher bleeding risk. The potential benefit from shorter DAPT duration in the elderly population was also supported by the SENIOR trial which confirmed the safety of drug-eluting stents in elderly patients, reporting fewer ischaemic events than in patients treated with bare metal stents despite a short bare metal stent-like DAPT regimen (one month in stable patients and six months in ACS patients)2. Finally, platelet function testing (PFT) offers the possibility of adjusting antithrombotic treatment to a pre-specified target. However, the randomised ANTARCTIC trial11 failed to demonstrate a benefit of PFT on a clinical endpoint among elderly ACS patients (≥75 years). Consistently, the pre-specified analysis of the randomised TROPICAL ACS trial12 failed to demonstrate the benefit of PFT-guided de-escalation of antiplatelet treatment in ACS elderly patients. Patients over the age of 70 years had a higher rate of clinically relevant bleeding events overall, but there was no significant difference between standard and PFT-guided strategies. Therefore, routine use of PFT to adjust antithrombotic treatment in elderly patients is not recommended.
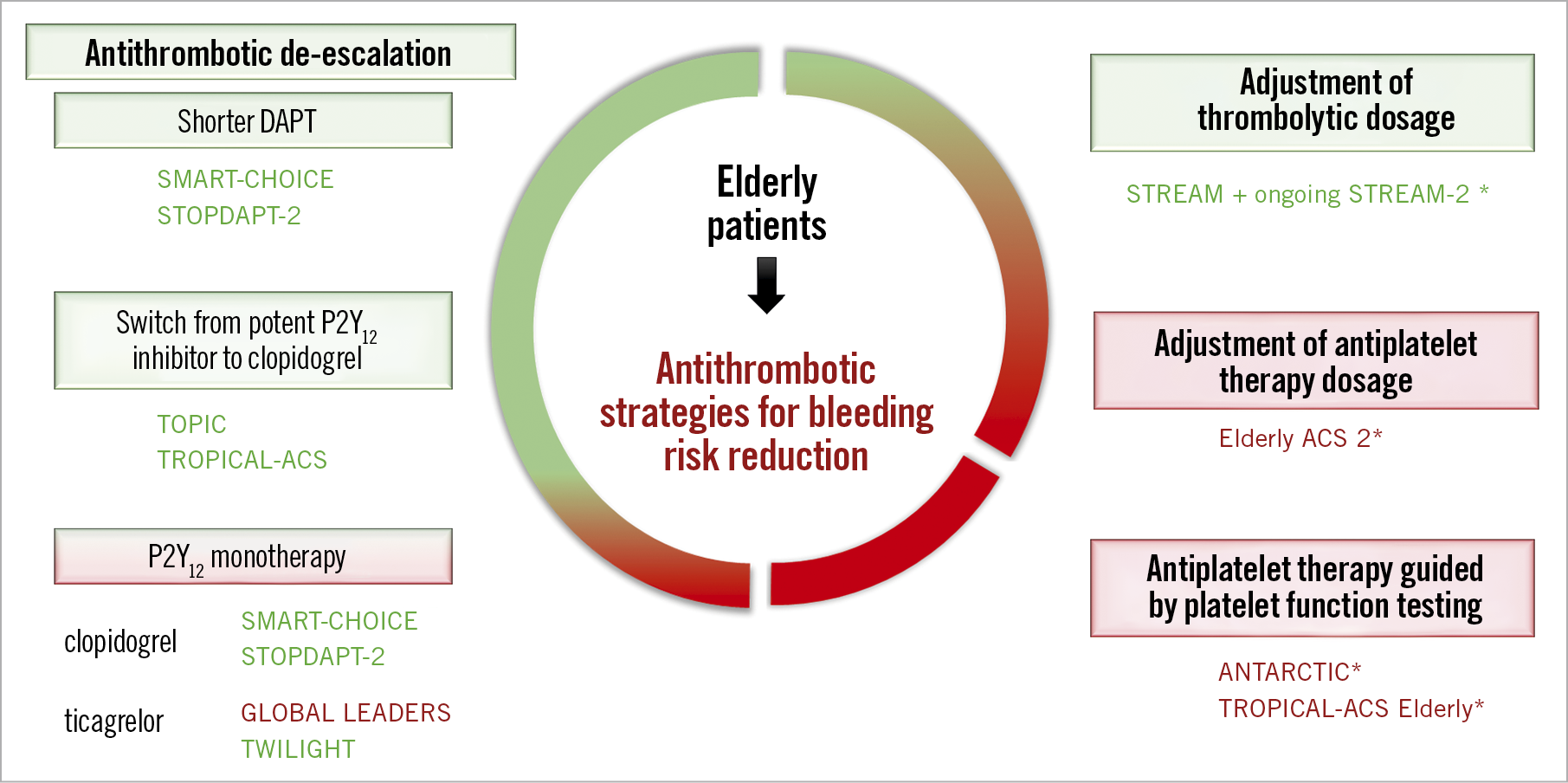
Figure 1. Different tested therapeutic strategies to reduce bleeding risk following percutaneous coronary intervention. The green boxes represent strategies associated with significant reduction of bleeding events and the red ones neutral/negative trials without significant reduction of bleeding events. *Trials that specifically included elderly patients. ANTARCTIC11: Assessment of a Normal Versus Tailored Dose of Prasugrel After Stenting in Patients Aged >75 Years to Reduce the Composite of Bleeding, Stent Thrombosis and Ischemic Complications; GLOBAL LEADERS6: A Clinical Study Comparing Two Forms of Anti-platelet Therapy After Stent Implantation; Elderly ACS 213: Comparison of Reduced-dose Prasugrel and Clopidogrel in Elderly Patients With Acute Coronary Syndrome Undergoing Early Percutaneous Coronary Intervention; SMART-CHOICE8: Smart Angioplasty Research Team: Comparison Between P2Y12 Antagonist Monotherapy vs Dual Antiplatelet Therapy in Patients Undergoing Implantation of Coronary Drug-Eluting Stents; STOPDAPT-29: Short and Optimal Duration of Dual Antiplatelet Therapy After Everolimus-Eluting Cobalt-Chromium Stent trial 2; STREAM 2 (NCT02777580): STrategic Reperfusion in Elderly Patients Early After Myocardial Infarction; TOPIC10: Timing of Optimal Platelet Inhibition After Acute Coronary Syndrome; TROPICAL-ACS12: Testing Responsiveness to Platelet Inhibition on Chronic Antiplatelet Treatment For Acute Coronary Syndromes Trial; TWILIGHT: Ticagrelor With Aspirin or Alone in High-Risk Patients After Coronary Intervention7.
Antithrombotic management of elderly patients remains challenging but, despite relatively few data from randomised trials in this population, specific strategies to reduce the risk of bleeding should be at the forefront of patient management. With the options of shorter DAPT duration, de-escalation of P2Y12 treatment and possibly potent P2Y12 monotherapy, cardiologists are now armed to provide an individualised approach considering the patient’s comorbidities as well as the severity and complexity of coronary artery disease.
Conflict of interest statement
B. Lattuca has received research grants from Biotronik, Daiichi Sankyo, Fédération Française de Cardiologie and Institute of Cardiometabolism and Nutrition, consultant fees from Daiichi Sankyo, Eli Lilly and Medtronic, and lecture fees from AstraZeneca and Novartis. G. Cayla has received research grants from Abbott, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Bristol Myers Squibb, Medtronic, Pfizer, and Sanofi.
Supplementary data
To read the full content of this article, please download the PDF.

