
Abstract
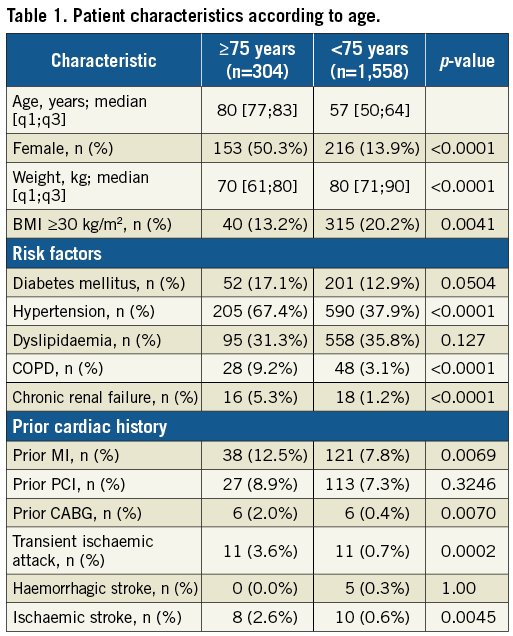
Aims: The aim of the study was to examine the main results of the ATLANTIC trial in patients with ST-elevation myocardial infarction (STEMI), randomised to pre- versus in-hospital ticagrelor, according to age.
Methods and results: Patients were evaluated by age class (<75 vs. ≥75 years) for demographics, prior cardiovascular history, risk factors, management, and outcomes. Elderly patients (≥75 years; 304/1,862) were more likely to be women, diabetic, lean, with a prior history of myocardial infarction and CABG, and with comorbidities (p<0.01 for all). Elderly patients presented more frequently with acute heart failure and less frequently had thromboaspiration, a stent implanted (p<0.01) and an aggressive antithrombotic regimen. Elderly patients had lower rates of pre- and post-PCI ≥70% ST-segment elevation resolution (43.9% vs. 51.6%; p=0.035), of pre- and post-PCI TIMI 3 flow (17.1% vs. 27.5%, p=0.0002), and a higher rate of the composite of death/MI/stroke/urgent revascularisation (9.9% vs. 2.9%; OR 3.67, 95% CI [2.27; 5.93], p<0.0001) and mortality (8.5% vs. 1.5%; OR 6.45, 95% CI [2.75; 15.11], p<0.0001). There was a non-significant trend towards more frequent major bleedings among elderly patients (TIMI major 2.3% vs. 1.1%; OR 2.13, 95% CI [0.88; 5.18], p=0.095). There was no significant interaction between time of ticagrelor administration (pre-hospital versus in-lab) and class of age for all outcomes.
Conclusions: Elderly patients, who represented one sixth of the patients randomised in the ATLANTIC trial, had less successful mechanical reperfusion and a sixfold increase in mortality at 30 days, probably due to comorbidities and possible undertreatment. The effect of early ticagrelor was consistent irrespective of age. ClinicalTrials.gov identifier: NCT01347580
Abbreviations
ACS: acute coronary syndrome
CABG: coronary artery bypass graft
ECG: electrocardiogram
MI: myocardial infarction
PCI: percutaneous coronary intervention
STEMI: ST-segment elevation myocardial infarction
TIMI: Thrombolysis In Myocardial Infarction
Introduction
Despite representing one third of the acute coronary syndrome (ACS) population, elderly patients (defined as aged 75 years or more) represent less than one sixth of the patient population in the pivotal trials that have established the superiority of ticagrelor or prasugrel over clopidogrel1,2. Dedicated trials for elderly patients have been shown to be feasible3-5 and these patients should receive evidence-based therapies6,7. In particular, special consideration with respect to dosage of oral antiplatelet therapy and contraindications has been implemented in order to limit bleeding complications8. Pre-specified analysis in patients ≥75 years has demonstrated no net benefit of prasugrel 60 mg load/10 mg o.d. over clopidogrel; the 5 mg daily dose was approved based on pharmacokinetic modelling9, further confirmed in a dedicated elderly patient trial4. On the other hand, the absolute benefit of ticagrelor over clopidogrel was amplified in the elderly (≥75) as compared to younger patients, although there were more fatal intracranial bleeding events and no interaction according to age for bleeding8.
ST-segment elevation myocardial infarction (STEMI) is a high thrombotic burden situation where a strategy of early administration of oral P2Y12 inhibitors is recommended10. Evidence is weak for clopidogrel and limited data are available for both ticagrelor and prasugrel, the recommended first-line oral P2Y12 inhibitor therapies11. The randomised ATLANTIC study12 demonstrated that early administration of ticagrelor was safe but did not improve pre-PCI coronary reperfusion in STEMI patients. Of note, there were differences in platelet inhibition and immediate reperfusion after (but not before) PCI, associated with reductions in ischaemic endpoints over the first 24 hours with early versus delayed ticagrelor administration13.
Data on pre-treatment in the elderly with STEMI are scarce. In addition, high on-treatment platelet reactivity is common among these patients14 who receive multiple medications with a potential delayed action of pre-treatment. In the ATLANTIC-Elderly pre-specified subgroup analysis, we examine whether the main results of the ATLANTIC trial in STEMI patients, randomised to pre- versus in-hospital ticagrelor, differ according to age class.
Methods
STUDY DESIGN AND PROCEDURES
ATLANTIC was an international study that randomised patients presenting with ongoing STEMI to receive double-blind treatment with a 180 mg loading dose of ticagrelor either pre-hospital (in-ambulance) or in-hospital (in-catheterisation laboratory), in addition to aspirin and standard care. The coordinating centre was the ACTION Study Group (www.action-coeur.org) and the study was funded by AstraZeneca. Detailed methods and results have been published previously. In summary, patients diagnosed with STEMI (<6 hrs from onset) and scheduled for primary PCI were randomised in the pre-hospital setting to receive a pre- versus in-hospital ticagrelor 180 mg loading dose. All patients then received maintenance treatment with ticagrelor 90 mg twice daily for at least 30 days, up to a maximum of 12 months. The co-primary endpoint was the absence of pre-PCI ≥70% ST-segment elevation resolution and/or TIMI flow grade 3 in the infarct-related artery at initial angiography. Other treatments were left to the physician’s discretion.
The aim of the present analysis was to examine whether the main results of the ATLANTIC trial in STEMI patients differ according to age class using a cut-off of 75 years old. Interaction with prior cardiovascular history, comorbidities, initial clinical features, procedural characteristics and timing were all taken into consideration.
DATA MANAGEMENT AND STATISTICAL METHODS
Subjects were classified according to age class for the index event or PCI. Continuous variables are presented as mean and standard deviation (SD) or median, as appropriate, and compared using the Student’s t-test in case of Gaussian distribution or the Mann-Whitney U test in case of non-Gaussian distribution. Categorical variables are presented as counts and percentages and compared using the chi-square test or Fisher’s exact test. The association between age class and clinical endpoints was assessed by fitting logistic regression models with age as the only covariate, for those subjects with data available for the relevant endpoint. Odds ratios and p-values for pre- versus in-hospital ticagrelor were calculated using a univariate logistic regression model. Adjustments for major baseline characteristics including gender, body mass index, diabetes, hypertension, prior coronary intervention or myocardial infarction and mechanical complications/cardiogenic shock were performed using multiple logistic regression with sets of variables forced into the model. Kaplan-Meier probability curves of time-to-event clinical endpoints were produced for the period of 0 up to 30 days after PCI and compared using a Cox proportional hazards model. Bleeding outcomes were evaluated in patients who underwent PCI for the index event. All analyses were performed using SAS, version 9.3 (SAS Institute Inc., Cary, NC, USA).
Results
PATIENT CHARACTERISTICS AND MANAGEMENT
The ATLANTIC study included 1,862 patients of whom 304 (16.3%) were aged 75 years or more. Elderly patients were more likely to be women, diabetic, of low body weight, and with a prior history of myocardial infarction, CABG or stroke. Comorbidities were at least three times more frequent in elderly versus younger patients (Table 1). Overall, the main indicators of risk or severity were more frequent in elderly patients who had significantly higher TIMI risk score and more frequently presented with acute heart failure compared to younger patients despite similar rates of secondary transfer (Table 2). Diagnostic delays, management delays and total ischaemic time were significantly longer in the elderly (Table 2). There were no significant differences in terms of culprit coronary artery. Elderly patients were significantly less likely to be treated with GP IIb/IIIa inhibitors, thromboaspiration and drug-eluting stents (Table 2). They also less frequently received intra-hospital parenteral anticoagulation and a maintenance dose of P2Y12 inhibitors corresponding to PCI being performed less frequently and less frequent use of stents.
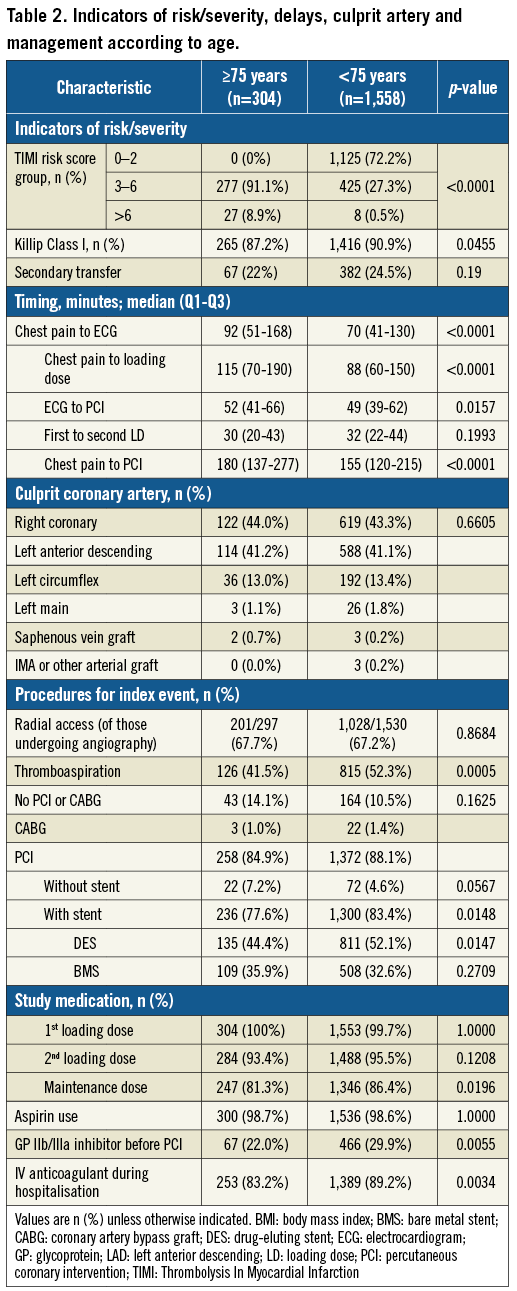
Elderly patients with mechanical complications (n=6) were more likely to have diabetes, more frequently presented with heart failure, and less frequently underwent coronary revascularisation, compared to elderly patients without mechanical complications. There were no differences in baseline characteristics and management between elderly patients receiving (n=135) and not receiving DES (n=101). Elderly patients who underwent a radial approach (n=201/304) for primary PCI were significantly more likely to undergo thrombus aspiration (96 [47.8%] vs. 30 [31.9%], p=0.0104) and PCI (183 [91.0%] vs. 74 [78.7%], p=0.0072) and were more frequently exposed to a maintenance dose of aspirin (194 [96.5%] vs. 84 [89.4%], p=0.0140) and P2Y12 inhibitors (175 [87.1%] vs. 71 [75.5%], p=0.0131) versus those undergoing a non-radial approach.
OUTCOMES
There were more elderly patients with absence of pre-PCI TIMI 3 flow and only a trend for fewer elderly patients with pre-PCI ≥70% ST-segment elevation resolution (Table 3). More elderly patients had absence of post-PCI ≥70% ST-segment elevation resolution and significantly more elderly patients had absence of post-PCI TIMI 3 flow (Table 3). Bail-out use of GP IIb/IIIa inhibitors within 24 hours of the first loading dose was less frequently used in elderly than in younger patients (Table 3). The composite endpoint of death/MI/urgent revascularisation was increased by threefold in elderly versus younger patients; these differences were mainly driven by mortality (Figure 1). The absolute number of deaths was 26 (8.55%) and 23 (1.48%) in elderly versus non-elderly patients, respectively. Among the elderly, 15 (10.6%) and 11 (6.9%) patients died in the pre- versus in-hospital ticagrelor groups (p=ns), respectively. Major bleedings according to PLATO, TIMI and STEEPLE definitions were increased by at least twofold in elderly versus non-elderly patients (Table 4). Differences in clinical outcomes between elderly and non-elderly patients persisted after adjustments for mechanical complications or shock and for major comorbidities. Finally, there was a consistent effect of pre-hospital versus in-hospital ticagrelor administration to reduce acute stent thrombosis (OR 0.19, 95% CI [0.02; 0.88], p=0.0226) with no significant interaction by age class (p=0.694) (Figure 2). Age was an independent predictor of major adverse cardiac and cerebrovascular events (MACCE) and of bleeding events.
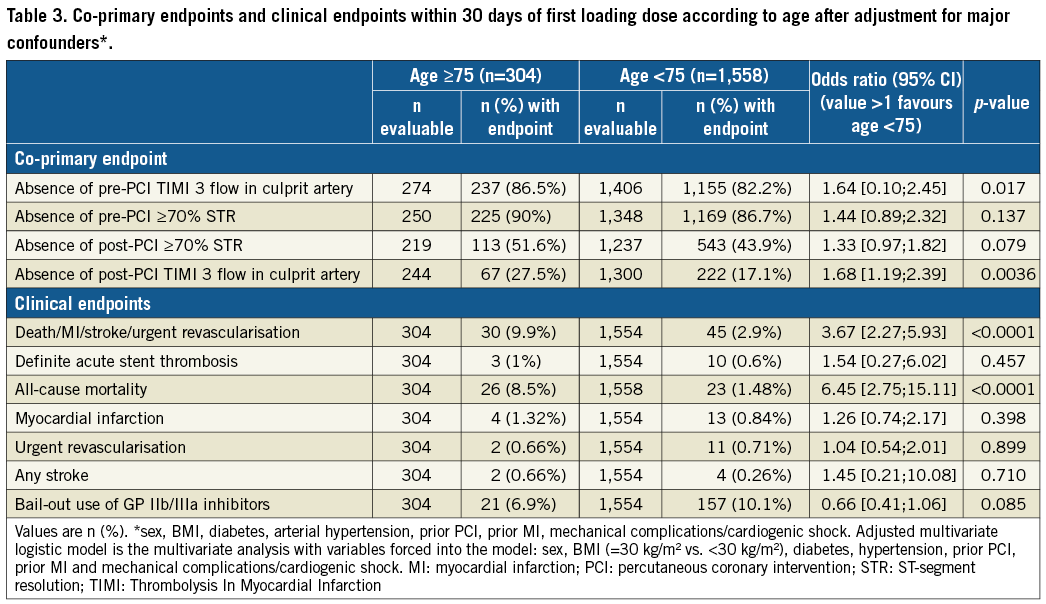
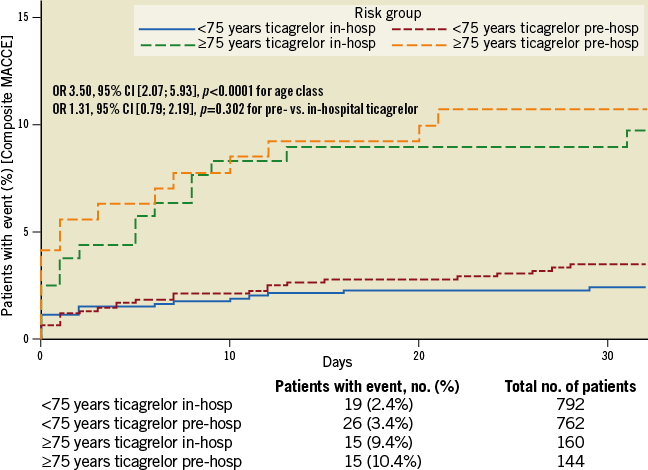
Figure 1. Composite of death/MI/urgent revascularisation/definite stent thrombosis/bail-out glycoprotein IIb/IIIa inhibitor use at 30 days according to age class.
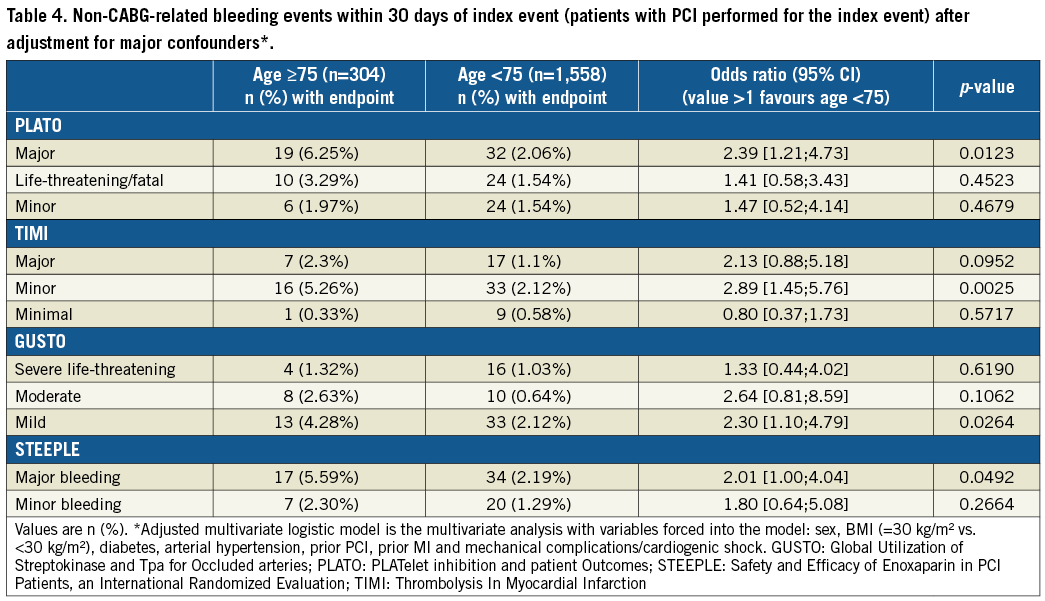
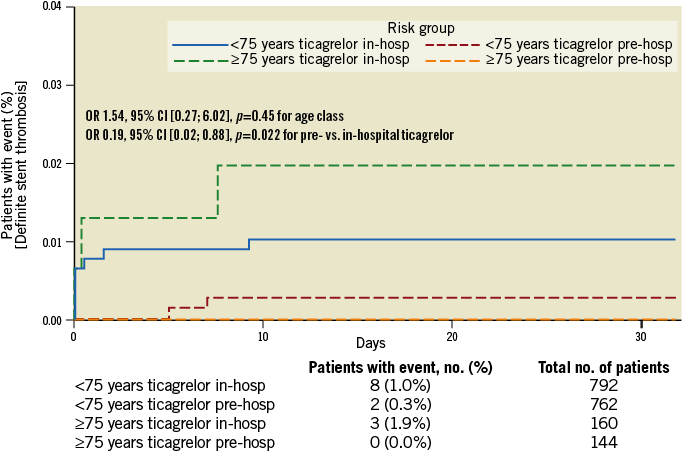
Figure 2. Definite stent thrombosis within 30 days of index PCI according to age class and ticagrelor administration (p interaction=NS).
Discussion
Elderly patients represented one sixth of the randomised patients in the ATLANTIC study. They displayed more comorbidities, more severe presentation and worse outcomes compared with younger patients. Early administration of ticagrelor led to better post-PCI TIMI 3 flow and less acute stent thrombosis irrespective of class of age, whereas it had no effect on pre-PCI TIMI 3 flow or ST resolution. The ATLANTIC-Elderly study suggests that, when the diagnosis of STEMI is made, oral P2Y12 inhibitor therapy should be administered as soon as possible irrespective of age when primary PCI is the chosen reperfusion strategy (Figure 3).
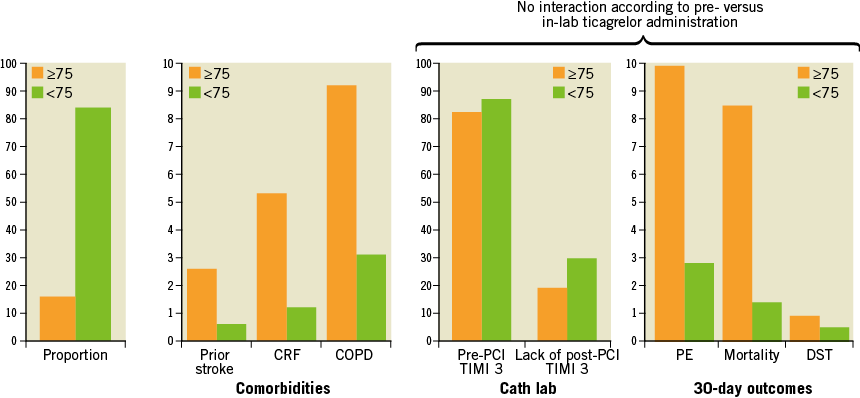
Figure 3. Key findings of the ATLANTIC-Elderly substudy. CRF: chronic renal failure; PE: primary endpoint; DST: definite stent thrombosis
The concept of pre-treatment, a treatment given when the diagnosis is suspected and before the coronary artery status is known, was first introduced for patients with non-ST-elevation ACS loaded with clopidogrel. It has been revisited in the ACCOAST study in the context of early catheterisation in higher-risk patients with prasugrel; no benefit was demonstrated15. There was a significant increased bleeding risk particularly among patients with type 2 MI who do not require antiplatelet therapy but also in PCI-treated patients16. One sixth were elderly patients, of whom a substantial proportion had no significant obstructive CAD, suggesting caution with treatment preceding the coronary angiogram.
STEMI differs from NSTEMI given a stronger thrombotic burden, an easier diagnosis and subsequently a much higher probability of coronary intervention. For example, primary stenting was performed in almost 90% of patients in the ATLANTIC study with a significantly lower rate among elderly versus younger patients. The clinical benefit of pre-treatment with clopidogrel in primary PCI is established although randomised trials have been underpowered17-19. Data with prasugrel are scarce whereas early administration of ticagrelor in STEMI reduced early stent thrombosis compared with in-hospital administration with no effect on ST-segment resolution and TIMI 3 flow before primary PCI, the co-primary study endpoints of the ATLANTIC study.
Overall, these data support an early administration of oral P2Y12 inhibitors when the diagnosis of STEMI is made, as suggested in the guidelines with a class I recommendation and a level of evidence B. The current evidence from this substudy suggests the same recommendation for the elderly. Elderly patients represent over one third of patients admitted with MI, and two thirds dying from MI are over 75 years. The elderly are more vulnerable, have more drug side effects, are more likely to experience morphine-related side effects, more frequently experience poor response to oral P2Y12 inhibitors, and have longer chest pain to first medical contact and less pre-PCI TIMI 3 flow than younger patients, factors that may be supportive of an early administration of oral P2Y12 inhibitors. On the other hand, elderly patients have less post-PCI TIMI 3 flow and an increased risk of stent thrombosis. Of importance, all these common features were observed in ATLANTIC-Elderly, a pre-specified subgroup analysis. Age was associated with increased mortality and increased thrombotic risk and bleedings. These poor outcomes were confirmed after adjustments for major confounders including diabetes, gender, hypertension, prior PCI, prior MI and cardiogenic shock. Finally, the most important and novel finding of ATLANTIC-Elderly is the lack of interaction between early versus delayed administration of ticagrelor and class of age.
Age-related organ changes may affect drug pharmacokinetics such as decreased absorption, less effective first pass metabolism, increased free action in plasma of highly protein-bound drugs, and impaired elimination. This may discourage the one-size-fits-all approach including pre-treatment with oral P2Y12 inhibitors in elderly STEMI patients. In particular, the use of ticagrelor has been cautioned in patients with advanced sinoatrial disease20 asthma and/or chronic obstructive pulmonary disease, which are more common among the elderly. However, there was no age-treatment interaction in the elderly subgroup, in particular with respect to mortality21. No particular side effects were reported in the elderly assigned to pre-hospital administration of ticagrelor in the ATLANTIC study. Personalised treatment remains a matter of debate when the treatment decisions, such as antiplatelet therapy in the elderly, are difficult to make22. Individualised treatment according to the level of platelet reactivity has been tested in elderly patients without any significant benefit3.
Limitations
Absolute numbers of elderly patients and outcomes preclude any robust conclusions. Elderly STEMI patients presented late with more severe clinical status and had less successful mechanical reperfusion and less favourable clinical outcomes probably due to comorbidities. However, the effect of early administration of ticagrelor was consistent irrespective of age.
Conclusions
Elderly patients represented one sixth of the patients randomised in the ATLANTIC trial, had less successful mechanical reperfusion and a sixfold increase in mortality at 30 days, probably due to comorbidities and possible undertreatment. The effect of early ticagrelor was consistent irrespective of age.
| Impact on daily practice Administration of oral P2Y12 inhibitors is recommended at first medical contact in STEMI patients in whom the decision to proceed for primary PCI has been made. The effect of early administration of a ticagrelor loading dose on ST resolution and pre-PCI TIMI 3 flow is consistent irrespective of age in patients undergoing primary PCI. Whether this may lead to a better clinical benefit warrants further investigation. |
Appendix. Collaborators
Jens Flensted Lassen, MD, PhD; Department of Cardiology B, Aarhus University Hospital, Skejby, Aarhus N, Denmark. Leonardo Bolognese, MD; Cardiovascular and Neurological Department, Azienda Ospedaliera Arezzo, Arezzo, Italy. Warren J. Cantor, MD; Southlake Regional Health Centre, University of Toronto, Newmarket, Ontario, Canada. Angel Cequier, MD; Heart Disease Institute, Hospital Universitario de Bellvitge, University of Barcelona, L’Hospitalet de Llobregat, Barcelona, Spain. Mohamed Chettibi, MD, PhD; Centre Hospito-universitaire Frantz Fanon, Blida, Algeria. Shaun G. Goodman, MD; Canadian Heart Research Centre, Division of Cardiology, St Michael’s Hospital, University of Toronto, Toronto, Canada. Christopher J. Hammett, MB, ChB; Department of Cardiology, Royal Brisbane and Women’s Hospital, Brisbane, Queensland, Australia. Kurt Huber, MD; 3rd Department of Medicine, Cardiology and Intensive Care Medicine, Wilhelminenhospital, and Sigmund Freud Private University, Medical School, Vienna, Austria. Magnus Janzon, MD, PhD; Department of Cardiology and Department of Medical and Health Sciences, Linköping University, Linköping, Sweden. Béla Merkely, MD, PhD; Heart and Vascular Center, Semmelweis University, Budapest, Hungary. Robert F. Storey, MD, DM; Department of Infection, Immunity and Cardiovascular Disease, University of Sheffield, Sheffield, United Kingdom. Jurrien M. ten Berg, MD, PhD; Department of Cardiology, St Antonius Hospital Nieuwegein, Nieuwegein, the Netherlands. Uwe Zeymer, MD; Klinikum Ludwigshafen and Institut für Herzinfarktforschung Ludwigshafen, Ludwigshafen, Germany. Muriel Licour, MsC; AstraZeneca, La Défense, France. Anne Tsatsaris, MD; AstraZeneca, La Défense, France.
Acknowledgements
Editorial support was provided by Yves Champollion.
Funding
The ATLANTIC study was executed by the ACTION Study Group (www.action-coeur.org) and was sponsored by AstraZeneca.
Conflict of interest statement
J-P. Collet has received research grants from Bristol-Myers Squibb, Medtronic, Fédération Française de Cardiologie, and Société Française de Cardiologie; consulting fees from Sanofi, Eli Lilly, and Bristol-Myers Squibb; and lecture fees from Bristol-Myers Squibb, Sanofi, Eli Lilly, and AstraZeneca. J. Silvain has received research grants to the institution from AstraZeneca, Brahms, Daiichi Sankyo, Eli Lilly, Institute of Cardiometabolism (ICAN), INSERM, Fédération Française de Cardiologie, Fondation de France, Société Française de Cardiologie, and Sanofi; consulting fees from Actelion, AstraZeneca, Daiichi Sankyo, Eli Lilly, and Sanofi; lecture fees from Algorythm, AstraZeneca, and Bristol-Myers Squibb; and travel fees from AstraZeneca, Braun, Bristol-Myers Squibb, and Pfizer. F. Lapostolle has received research grants or honoraria from AstraZeneca, Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, Daiichi Sankyo, Eli Lilly, Pfizer, and Teleflex. E. Vicaut has received consulting or lecture fees from Abbott, Bristol-Myers Squibb, Celgene, Daiichi Sankyo, Eli Lilly, Fresenius, European Cardiovascular Research Center, LFB, Hexacath, Medtronic, Novartis, Pfizer, Sanofi, and Sorin; and grants to his institution (APHP) for clinical trials from AstraZeneca, Boehringer Ingelheim, and Sanofi. C. Hamm has received advisory board and speaker honoraria from AstraZeneca. A. van ‘t Hof has received research and educational grants from Abbott, AstraZeneca, Eli Lilly/Daiichi Sankyo, Merck/Correvio, Medtronic, and The Medicines Company; and speaker fees from Boehringer Ingelheim, Abbott, AstraZeneca, Eli Lilly/Daiichi Sankyo, Merck/Correvio, Medtronic, The Medicines Company, Pfizer, and Sanofi. G. Montalescot has received research grants to the institution or consulting/lecture fees from Acuitude, ADIR, Amgen, AstraZeneca, Bayer, Berlin Chimie AG, Boehringer Ingelheim, Bristol-Myers Squibb, Brigham Women’s Hospital, Cardiovascular Research Foundation, Celladon, CME Resources, Daiichi Sankyo, Eli Lilly, Europa, Fédération Française de Cardiologie, Gilead, Hopitaux Universitaires Genève, ICAN, Janssen-Cilag, Lead-Up, Medcon International, Menarini, Medtronic, MSD, Pfizer, Recor, Sanofi, Stentys, The Medicines Company, TIMI Study Group, Universitat Basel, WebMD, and Zoll Medical. The other authors have no conflicts of interest to declare.

