
Abstract
Aims: Antiplatelet treatment in the elderly post percutaneous coronary interventions (PCI) remains a complex issue. Here we report the results of the pre-specified subgroup analysis of the GLOBAL LEADERS trial evaluating the long-term safety and cardiovascular efficacy of ticagrelor monotherapy among patients categorised according to the pre-specified cut-off value of 75 years of age.
Methods and results: This was a pre-specified analysis of the randomised GLOBAL LEADERS trial (n=15,991), comparing 23-month ticagrelor monotherapy (after one month of DAPT) with the reference treatment (12-month DAPT followed by 12 months of aspirin). Among elderly patients (>75 years; n=2,565), the primary endpoint (two-year all-cause mortality or new Q-wave core lab-adjudicated myocardial infarction [MI]) occurred in 7.2% and 9.4% of patients in the ticagrelor monotherapy and the reference group, respectively (hazard ratio [HR] 0.75, 95% confidence interval [CI]: 0.58-0.99, p=0.041; pint=0.23); BARC-defined bleeding type 3/5 occurred in 5.2% and 4.1%, respectively (HR 1.29, 95% CI: 0.89-1.86; p=0.180; pint=0.06). The elderly with stable CAD had a higher rate of BARC 3/5 type bleeding (HR 2.05, 95% CI: 1.18-3.55) with ticagrelor monotherapy versus the reference treatment (pint=0.02). Elderly patients had a lower rate of definite or probable stent thrombosis (ST) with ticagrelor monotherapy (0.4% vs 1.4%, p=0.015, pint=0.01), compared with the reference group.
Conclusions: In this pre-specified, exploratory analysis of the overall neutral trial, there was no differential treatment effect of ticagrelor monotherapy (after one-month dual therapy with aspirin) found in elderly patients undergoing PCI with respect to the rate of the primary endpoint of all-cause death or new Q-wave MI. The lower rate of ST in the elderly with ticagrelor monotherapy is hypothesis-generating. ClinicalTrials.gov identifier: NCT01813435
Introduction
Age is associated with a high comorbidity burden and an increased risk of both ischaemic and bleeding complications1. The benefit–risk of antiplatelet therapies in elderly patients is complex and the current data remain inconclusive with regard to optimal potency and duration of antiplatelet regimen, compared with younger individuals1,2,3,4,5.
Elderly patients above 75 years of age represent more than one third of patients undergoing percutaneous coronary intervention (PCI) for acute coronary syndromes (ACS) and with an ageing society this percentage is expected to grow. Nevertheless, they still tend to be under-represented in clinical trials6. The remaining gap in evidence-based treatment of elderly patients, including a need to identify the most suitable antiplatelet strategies in this vulnerable patient subgroup, has been underlined by leading authorities and scientific associations2.
Recently, aspirin-free antiplatelet protocols after PCI have been advocated to preserve their anti-ischaemic effects without the bleeding risk associated with dual antiplatelet therapy (DAPT)7. In GLOBAL LEADERS, ticagrelor in combination with aspirin for one month followed by ticagrelor monotherapy for 23 months was not superior to 12 months of DAPT followed by 12 months of aspirin alone in the prevention of all-cause mortality or new Q-wave myocardial infarction (MI) in a broad patient population undergoing PCI8. Nevertheless, the risks and effects of different intensity antiplatelet therapies may differ substantially between younger and elderly adults, as the latter often carry the burden of multiple risk factors and comorbidities, and have been demonstrated to present altered platelet function, compared to younger individuals1,3.
Given this background, we report the results of the pre-specified subgroup analysis of the GLOBAL LEADERS trial evaluating the long-term safety and cardiovascular efficacy of ticagrelor monotherapy among patients categorised according to the pre-specified cut-off value for age of 75 years.
Methods
STUDY DESIGN AND PATIENT POPULATION
GLOBAL LEADERS (ClinicalTrials.gov NCT01813435) was an investigator-initiated, prospective, randomised, multicentre, multinational, open-label trial that compared two strategies of antiplatelet treatment in 15,991 patients scheduled for PCI8. Patients with stable coronary artery disease (CAD) or ACS were randomly allocated to either an experimental strategy of one-month aspirin and ticagrelor, followed by 23 months of ticagrelor alone, or to the reference strategy with 12-month DAPT consisting of aspirin in combination with either clopidogrel (for patients with stable CAD) or ticagrelor (for patients with ACS)7. All types of lesions were permitted, including left main, bifurcations, chronic total occlusions, interventions on grafted vessels, etc. Detailed inclusion criteria, exclusion criteria, and study procedures have been described previously (Supplementary Appendix 1, Supplementary Appendix 2),7,8. The trial was performed in compliance with the ethical principles of the Declaration of Helsinki. All participants provided written informed consent at enrolment. An independent data and safety monitoring board (DSMB) oversaw the safety of all patients.
CLINICAL ENDPOINTS
The primary endpoint comprised a composite of all-cause death or centrally adjudicated (by ECG core lab) new Q-wave MI up to two years after the index procedure. The key secondary safety endpoint was site-reported bleeding type 3 or 5 defined according to the Bleeding Academic Research Consortium (BARC) up to two-year follow-up. Further site-reported secondary endpoints included any stroke, site-reported MI, any revascularisation, target vessel revascularisation (TVR), definite stent thrombosis (ST) and the composite of definite or probable ST, according to the Academic Research Consortium (ARC) criteria (Supplementary Appendix 3). In addition, upon the request of the DSMB, data on the rates of possible ST were collected, and the composite endpoint of any ST (definite/probable/possible) was reported.
Finally, the rates of the ARC 2-defined patient-oriented composite endpoint (POCE: all-cause death, any stroke, site-reported MI and any revascularisation) and net adverse clinical events (NACE: POCE and BARC 3 or 5 type bleeding) were reported up to two years.
Landmark analyses were performed within the elderly subgroup using the pre-specified time cut-offs: at 30 days (corresponding to the planned dates of discontinuation of aspirin in the experimental group) and one year (corresponding to the planned dates of discontinuation of a P2Y12 receptor antagonist in the reference group) after the index procedure.
The trial was monitored for event under-reporting and event definition consistency; no central adjudication of clinical events was planned7,8.
STATISTICAL ANALYSIS
Sample size consideration and statistical analysis for the primary and secondary endpoints in GLOBAL LEADERS have been described previously8.
Clinical endpoints were evaluated using the Mantel-Cox log-rank method up to the time point when the first of this type of event occurred, reporting hazard ratios (HR) with 95% confidence intervals (CI). Pre-specified analyses of the primary endpoint, secondary efficacy and safety endpoints were performed with tests for treatment-by-age interaction using the predefined cut-off value of 75 years of age. All analyses were performed following the intention-to-treat definition using SPSS software, Version 25 (IBM Corp., Armonk, NY, USA). A two-sided p-value of <0.05 was considered statistically significant.
Results
STUDY POPULATION
The GLOBAL LEADERS trial recruited and randomly assigned 15,991 participants. As 23 patients subsequently withdrew consent and requested deletion of their data from the database, a total of 15,968 patients remained in the study8. There were 2,565 (16.1%) patients aged >75 years, further referred to as elderly patients and 13,403 aged ≤75 years, further referred to as younger patients. The baseline characteristics were balanced between the experimental and the reference arm for both age subgroups, except for a higher proportion of patients with a history of prior PCI or CABG in the experimental versus the reference arm among elderly patients (Table 1, Supplementary Table 1).
CLINICAL ENDPOINTS IN THE PRE-SPECIFIED AGE SUBGROUPS
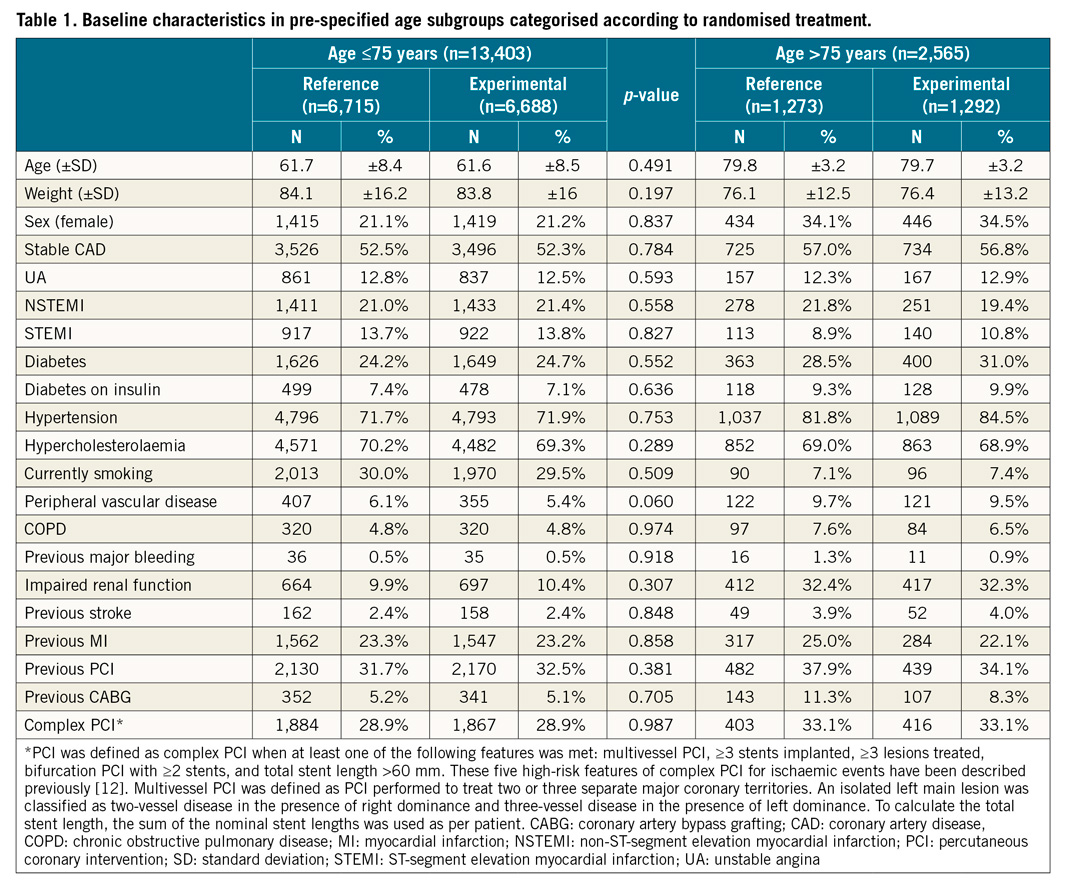
Amongst elderly patients, the primary endpoint occurred in 93 (7.2%) patients in the experimental treatment strategy group and in 120 (9.4%) patients in the reference treatment strategy group (HR 0.75, 95% CI: 0.58-0.99; p=0.041) at two years (pint=0.23) (Table 2).
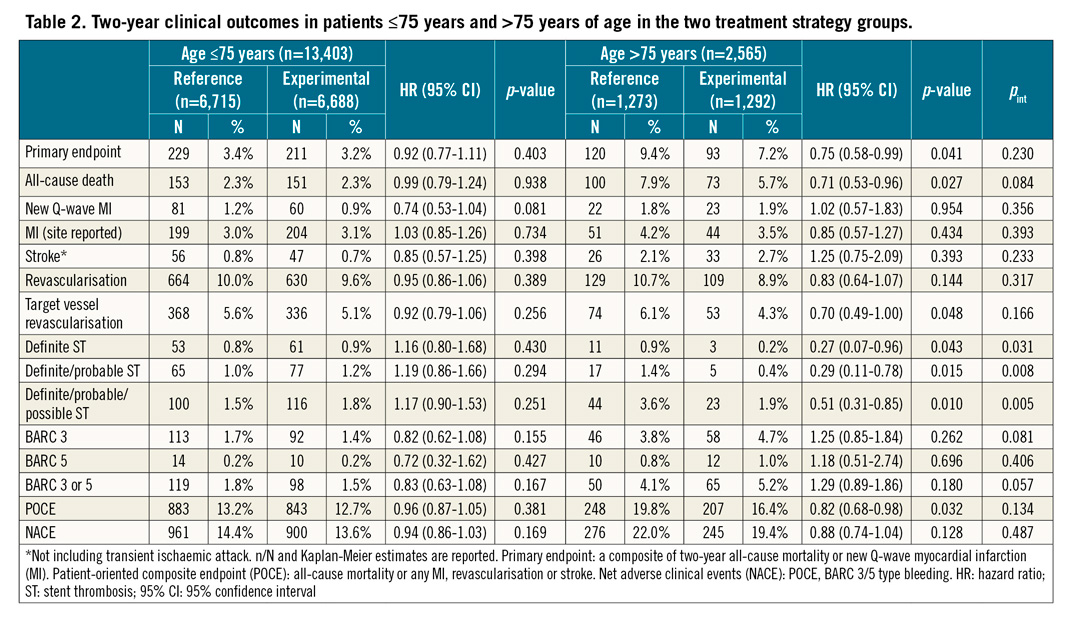
The elderly patients in the experimental group had a lower rate of all-cause death (5.7% vs 7.9%; p=0.027), POCE (16.4% vs 19.8%, p=0.032), TVR (4.3% vs 6.1%, p=0.048), definite (0.2% vs 0.9%, p=0.043) and definite or probable ST (0.4% vs 1.4%; p=0.015) at two years, as compared with the reference arm; BARC 3 or 5 type bleedings were numerically more frequent in the experimental as compared to the reference group (5.2% vs 4.1%, p=0.180), though not statistically different between the two treatment groups (pint=0.06) (Figure 1, Figure 2, Supplementary Table 2).
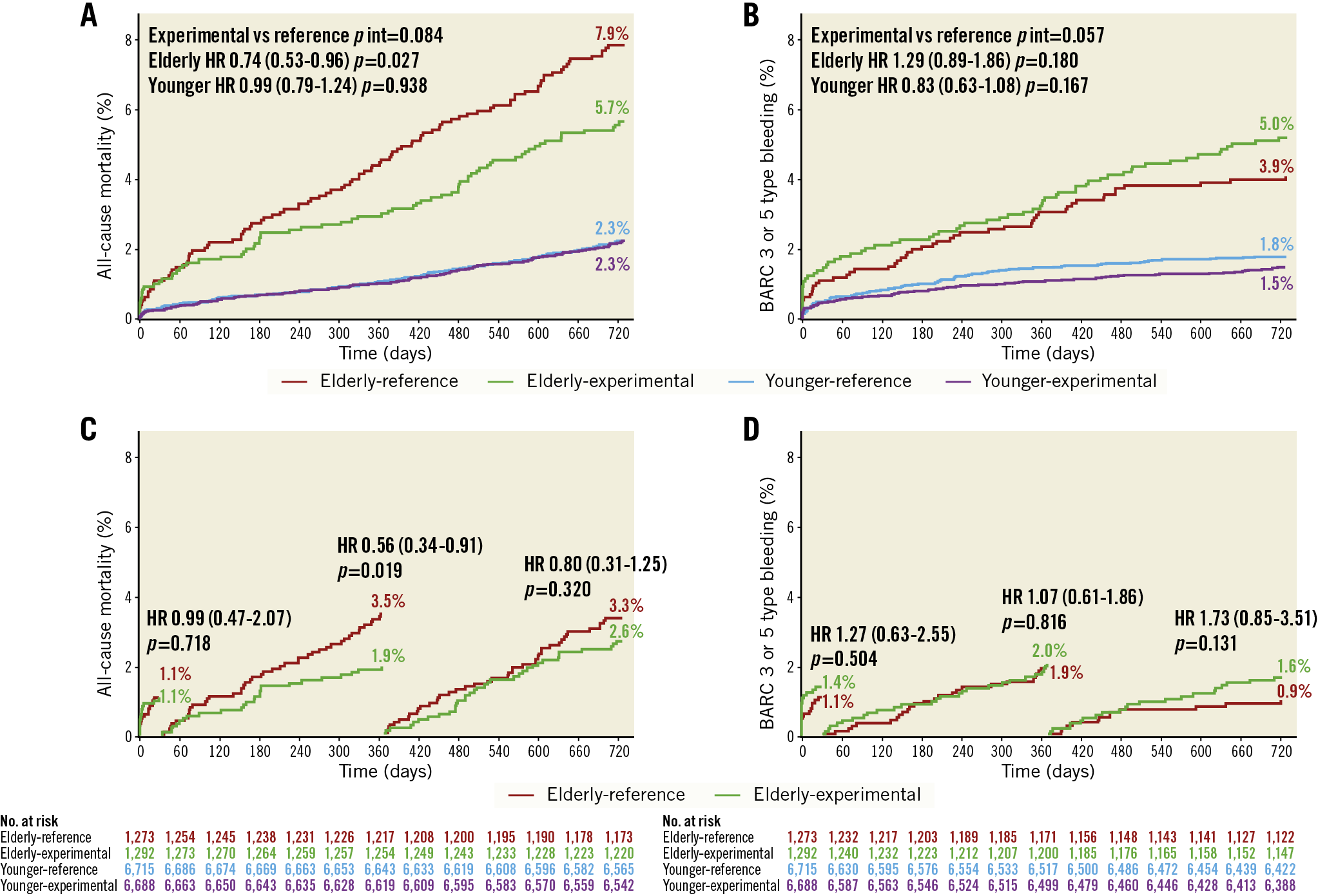
Figure 1. Kaplan-Meier curves for all-cause mortality and BARC 3 or 5 type bleeding. All-cause mortality (A) and BARC 3 or 5 type bleeding (B) at two years categorised according to age and randomised treatment (n=15,968). Landmark analyses at 30 days, 31-365 days and 366-730 days in elderly patients (n=2,565) for all-cause mortality (C) and BARC 3 or 5 type bleeding (D).
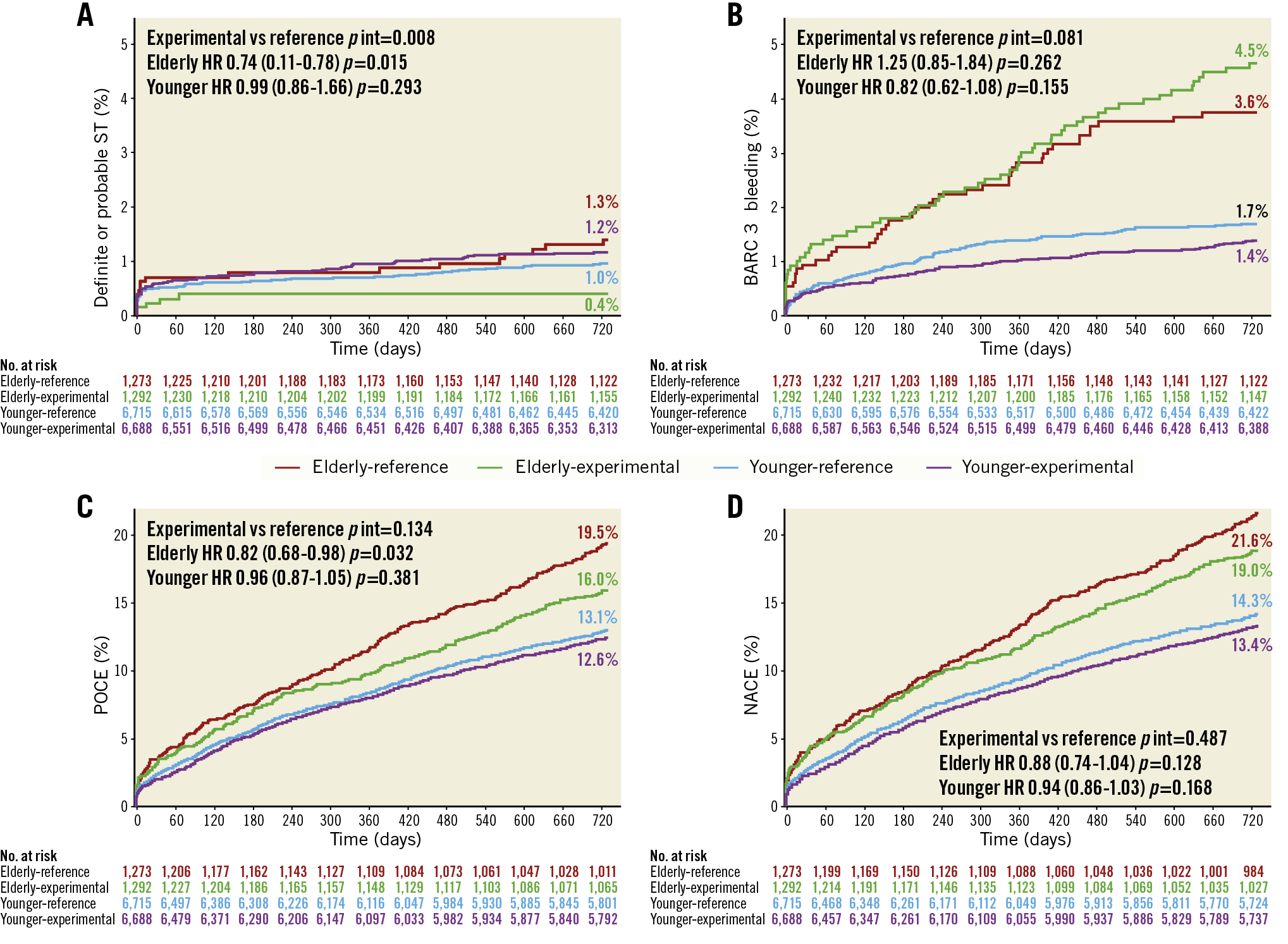
Figure 2. Kaplan-Meier curves. Definite or probable stent thrombosis (A), BARC 3 type bleeding (B) and the composite endpoints patient-oriented composite endpoint (POCE) (C) and net adverse clinical events (NACE) (D) at two years categorised according to age and randomised treatment (n=15,968).
No significant differences in clinical outcome rates were found between the two treatment strategy groups in younger patients (Figure 2, Figure 3, Supplementary Figure 1).
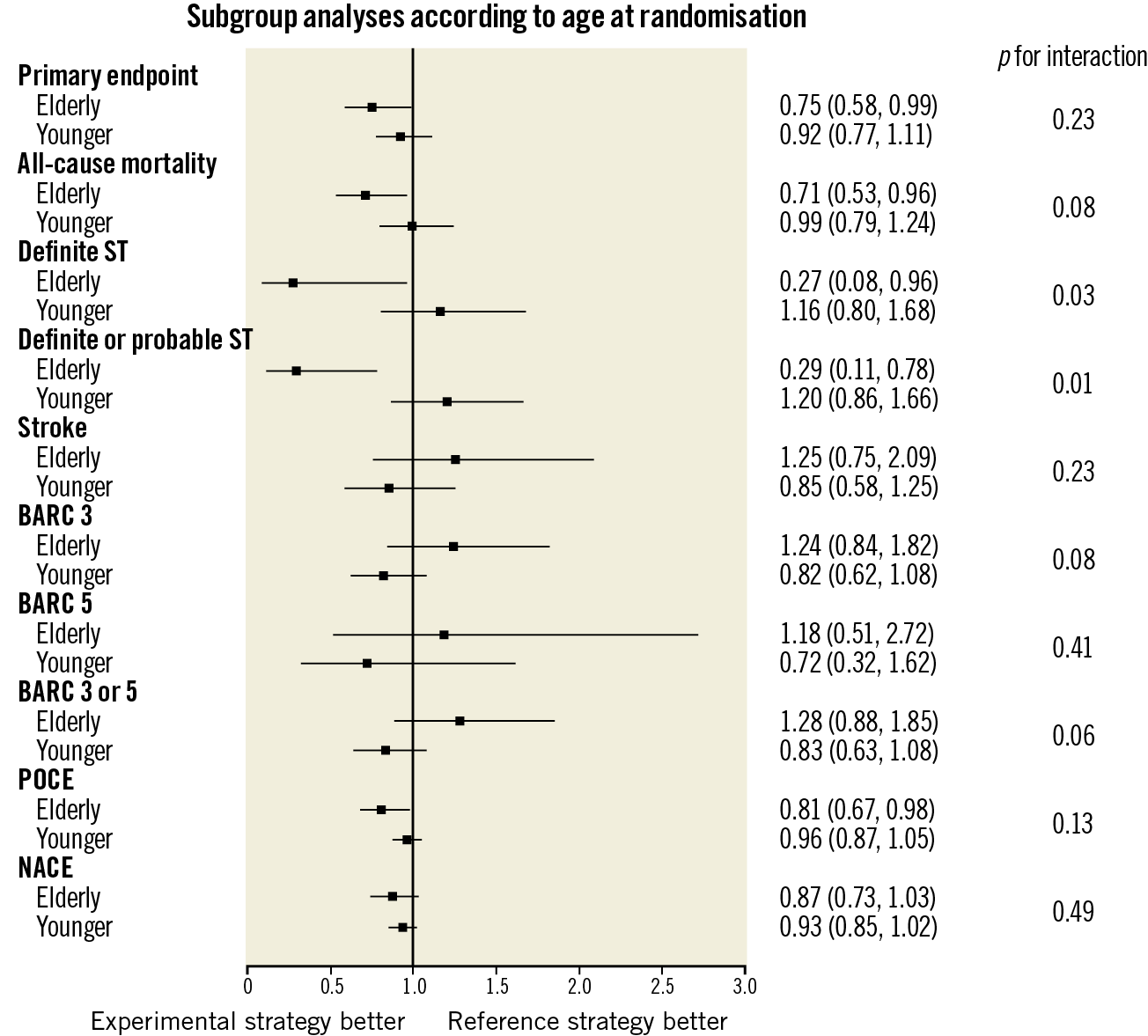
Figure 3. Subgroup analyses of clinical outcomes categorised according to the pre-specified age cut-off of 75 years and the randomised treatment.
A significant interaction was found between age and the treatment effects favouring the experimental strategy for definite ST (pint=0.03), definite/probable ST (pint=0.01) and any ST (definite/probable/possible ST) (pint=0.01) risk reduction in elderly patients (Table 2, Supplementary Table 3, Figure 2, Figure 4, Supplementary Figure 1). The clinical events in which ST was identified as an underlying mechanism are shown in Figure 4 and Supplementary Table 3.
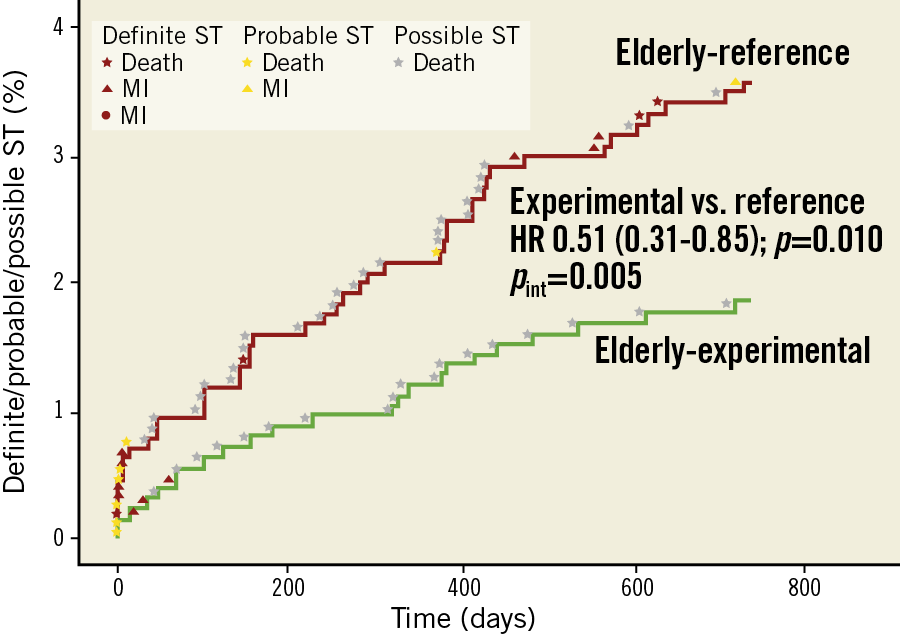
Figure 4. Cumulative incidence of definite/probable/possible stent thrombosis in elderly patients (according to ARC definition). Shown are definite ST (red), probable ST (yellow) and possible ST (grey) among the elderly in the experimental and the reference group, along with the worst hierarchical clinical outcome, over 730 days of follow-up.
ONE-YEAR CLINICAL OUTCOMES
At one year, amongst elderly patients there were no differences found in the rates of the primary endpoint or the key safety endpoint of BARC 3 or 5 type bleeding; however, there was a differential treatment effect observed with regard to the rate of the composite endpoint of definite/probable/possible ST which was found to be lower in the experimental arm (pint=0.03) (Supplementary Table 4).
TREATMENT ADHERENCE IN THE PRE-SPECIFIED AGE SUBGROUPS
At each follow-up visit from discharge up to 24-month follow-up, the elderly presented lower treatment adherence, compared to younger patients (Supplementary Table 5). From the three-month follow-up visit onwards, the adherence rates were lower in the experimental than in the reference arm, both among the elderly and among younger patients (Supplementary Table 6).
LANDMARK ANALYSES
By landmark analyses at 30 days and one year, in elderly patients, the difference in all-cause mortality rates between treatment groups was found to be driven by lower mortality in the experimental arm, occurring mainly between 30 days and one year (Figure 1). The rates of BARC 3 or 5 type bleedings at each time point did not differ significantly in either treatment group, though they were numerically higher between one and two years in the experimental group (Figure 1).
EXPLORATORY ANALYSES IN THE ELDERLY CATEGORISED ACCORDING TO CLINICAL PRESENTATION: ACS VERSUS STABLE CAD
Exploratory analyses in elderly patients categorised according to clinical presentation did not demonstrate any differences in the rates of the primary endpoint in either treatment group (Supplementary Table 7, Supplementary Table 8). However, there was a differential effect of the experimental treatment strategy found with regard to risk of bleeding BARC 3 type and BARC 3 or 5 type (Supplementary Table 7, Supplementary Figure 2, Supplementary Figure 3). Amongst the elderly presenting with stable CAD who had been allocated to the experimental treatment group, there was a higher risk of BARC 3 (HR 2.06, 95% CI: 1.15-3.67, p=0.015, pint=0.016) and BARC 3 or 5 type bleeding (HR 2.05, 95% CI: 1.18-3.55, p=0.012, pint=0.018).
Discussion
The elderly subgroup analysis from the GLOBAL LEADERS trial represents the first study dedicated to evaluating the long-term safety and efficacy of ticagrelor monotherapy following PCI in relation to age. The main findings of the present study can be summarised as follows:
i) In the GLOBAL LEADERS trial, there was no experimental treatment effect modification by age of either all-cause mortality or new Q-wave MI.
ii) In elderly patients, ticagrelor monotherapy, compared with the reference treatment, was associated with lower rates of the primary endpoint, all-cause mortality, POCE and TVR, although there was no significant differential treatment effect observed in the elderly versus younger patients.
iii) Landmark analyses suggest that these differences are driven by differences in all-cause mortality during the period from 30 days to one year (i.e., ticagrelor monotherapy vs DAPT), while there is no clear excess bleeding risk during the same period.
Contrary to some studies that excluded very old patients4, GLOBAL LEADERS included patients at a more varied and advanced age on admission – 1,169 (7.3%) were octogenarians, of whom 237 were at least 85 years old (1.5%).
The finding of no differential treatment effect of the experimental strategy with regard to the primary endpoint among elderly patients appears reassuring. There was a borderline significant increase in major bleeding risk (pint=0.06) found in the elderly treated with experimental treatment, that was mainly related to the higher bleeding rates observed in elderly patients presenting with stable CAD. Interestingly, higher bleeding rates in the experimental arm amongst the elderly with stable CAD were observed, despite lower PRECISE DAPT and PARIS score-defined bleeding risk in the stable CAD subgroup, as compared with ACS individuals. It has to be noted that previous studies documented a higher rate of bleeding events after ACS in the elderly, in particular within the first months after it9,10. Nevertheless, it should be underlined that GLOBAL LEADERS did not compare two drugs, but assessed two treatment strategies, and the reference treatment strategy was different in patients presenting with ACS (ticagrelor and aspirin for 12 months), compared to patients presenting with stable CAD (clopidogrel and aspirin for 12 months)11. As a consequence, in the experimental group stable CAD patients – representing 53% of the overall study – received ticagrelor monotherapy and were therefore (as stable patients) unduly exposed to potent platelet inhibitors, guideline-recommended primarily for ACS.
An intriguing observation from this study is the low rate of definite or probable ST (0.4%) in the experimental strategy arm among elderly patients, which contrasts with a higher proportion of ST (2.5%) within elderly patients (>75 years) in the LEADERS FREE trial5. These patients were treated with the polymer-free drug-coated stent and one-month DAPT post PCI, although followed by aspirin rather than ticagrelor monotherapy5.
As our study was not powered for evaluation of an ST clinical endpoint, which occurred in a low number of patients in this cohort, we cannot exclude that this finding could have arisen from chance alone. With the caveat of the inherent limitations related to subgroup analyses, the observed difference in ST rates between two treatment groups among the elderly may also point towards some potentially age-dependent effects of the experimental strategy1,2,4. Interestingly, ADP-mediated platelet aggregation has been demonstrated to increase with age, whereas no difference was observed for aspirin response3. This could explain some differences in the efficacy of the experimental treatment strategy, including ticagrelor instead of aspirin monotherapy, in elderly versus younger patients. ST represents a mechanistic explanation for clinically relevant adverse events (Supplementary Table 3). All-cause death, POCE and TVR were all consistently lower among the elderly in the experimental arm versus the reference arm, though no significant interaction terms were found for age effects, with a borderline effect for all-cause mortality (pint=0.08) (Figure 4).
WHO COULD ULTIMATELY BENEFIT FROM POTENT P2Y12 ANTAGONIST MONOTHERAPY AMONG THE ELDERLY?
Although any simplified definition of complex PCI cannot fully account for the vast spectrum of PCI complexity encountered in daily clinical practice12,13, complex PCIs were more frequent among the elderly compared with younger patients in this cohort (Supplementary Table 1).
Notably, patients who underwent complex PCI treated with the experimental treatment strategy had a significant reduction in the risk of the primary endpoint as well as POCE, while maintaining a similar risk of bleeding, thereby resulting in a net clinical benefit at two years in the overall GLOBAL LEADERS trial population12,13. A clinically oriented interpretation may suggest that patients at increased risk of both ischaemic and bleeding events, as expressed by high-risk clinical characteristics (such as advanced age) and high-risk procedural features, might represent the target group for potent P2Y12 monotherapy; in GLOBAL LEADERS the elderly presenting with ACS benefitted from the anti-ischaemic effect of the drug without an excess in bleeding associated with DAPT up to one year after PCI, having a potential reduction in definite and probable stent thrombosis. Indeed, age and complex procedural features also represented enrichment criteria in the protocol of TWILIGHT – a trial that recently showed reduction of the BARC 2, 3 or 5 type bleeding with ticagrelor monotherapy following a three-month event-free period of DAPT after PCI and non-inferiority of such a strategy with regard to the composite of all-cause death, non-fatal MI, or stroke, compared with a standard DAPT regimen14. However, the electronic case report form in GLOBAL LEADERS did not include information on rotational atherectomy or other tools used to modify calcified stenoses. As severely calcified lesions are relatively frequent among the elderly, the safety of novel antiplatelet regimens still remains to be evaluated more specifically among patients requiring advanced lesion preparation. This clinically oriented interpretation would need to be formally confirmed in a dedicated trial.
Limitations
Since the superiority criteria for the primary endpoint were not met in the overall trial, no formal procedure was planned to account for multiple testing and the present study did not have sufficient power to reach statistical significance in comparisons between treatment groups, all reported findings should be considered strictly as hypothesis-generating and exploratory. Secondly, some imbalance may exist between the treatment groups within elderly patients, as the randomisation was not stratified by age. No central adjudication was planned to ascertain secondary outcomes. However, GLOBAL LEADERS was monitored for event under-reporting and consistency of event definitions8. Finally, adherence to randomised treatment was lower in the experimental arm, in particular amongst the elderly – a finding in line with previous reports15. Nevertheless, discontinuation rates were comparable to previous trials investigating ticagrelor16. According to protocol, ticagrelor monotherapy was used at the dose of 90 mg twice daily. A lower dose, 60 mg twice daily, may be better tolerated while retaining a high level of platelet inhibition16,17 and could be preferred in future studies. Given that patients who take any medication twice a day are more likely to have reduced compliance, it could also appear justified to consider testing prasugrel – a P2Y12 inhibitor administered once daily – in monotherapy, as a means to improve adherence amongst the elderly. Nevertheless, among patients at a more advanced age, the use of prasugrel remains controversial: TRITON TIMI-38, TRILOGY ACS and Elderly ACS 2 demonstrated no net clinical benefit of prasugrel over clopidogrel amongst the elderly, although prasugrel was evaluated in these trials as part of dual, not single, antiplatelet therapy. Recently, in an ACS population, ISAR-REACT 5 showed a significantly lower incidence of death, MI, or stroke with prasugrel, as compared with ticagrelor, with no significantly different incidence of major bleeding (bleeding BARC 3, 4 or 5 type) between groups; however, the outcomes specifically among elderly patients have not been reported18.
Conclusions
In this pre-specified, exploratory analysis of the overall neutral trial, there was no differential treatment effect of ticagrelor monotherapy (after one-month dual therapy with aspirin) in elderly patients with respect to the rate of the primary endpoint of all-cause death or new Q-wave MI after PCI, but the elderly had a borderline excess in bleeding risk. The elderly presented lower rates of ST with ticagrelor monotherapy; however, given the low rate of ST, this finding remains hypothesis-generating.
|
Impact on daily practice The data presented on the safety profile of ticagrelor from a large-scale contemporary PCI cohort may facilitate better informed clinical decisions on newer P2Y12 antagonist use in the elderly. Further research could establish whether the experimental strategy represents a good treatment alternative in selected elderly patients undergoing PCI for acute coronary syndromes, in whom the standard dual antiplatelet therapy is perceived by clinicians as not possible due to an expected excess in bleeding risk. The population of patients at increased risk of both ischaemic and bleeding complications, as expressed by clinical (including age) and procedural high-risk criteria, might represent the target group for ticagrelor monotherapy. In the GLOBAL LEADERS trial, the elderly presenting with ACS were able to benefit from the anti-ischaemic effect of the drug without an excess in bleeding associated with DAPT up to one year after PCI, having a potential reduction in definite and probable stent thrombosis. |
Appendix. Authors’ affiliations
1. Department of Cardiology, Erasmus Medical Center, Rotterdam, the Netherlands; 2. First Department of Cardiology, Medical University of Warsaw, Warsaw, Poland; 3. Amsterdam UMC, Amsterdam, the Netherlands; 4. Division of Cardiology, Department of Internal Medicine, Prince of Songkla University, Songkhla, Thailand; 5. Department of Internal Medicine, Cardiology Division, University of Campinas (UNICAMP), Campinas, Brazil; 6. Cardialysis Core Laboratories and Clinical Trial Management, Rotterdam, the Netherlands; 7. PAKS Dabrowa, Dabrowa Gornicza, Poland; 8. Department of Cardiology, Radboud UMC, Nijmegen, the Netherlands; 9. “St. Marina” University Hospital, Varna, Bulgaria; 10. Department of Cardiology, Medical Faculty, Johannes Kepler University, Linz, Austria; 11. Uniklinikum Tübingen, Tübingen, Germany; 12. PAKS Kozle, Kedzierzyn-Kozle, Poland; 13. Clinic Hospital Barcelona, Barcelona, Spain; 14. Krakowski Szpital Specjalistyczny im. Jana Pawła II, Krakow, Poland; 15. Applied Health Research Centre, Li Ka Shing Knowledge Institute, St Michael’s Hospital, University of Toronto, Toronto, Canada; 16. University of Giessen, Giessen, Germany; 17. FACT, Université Paris Diderot, Hôpital Bichat, Assistance Publique-Hôpitaux de Paris, Paris, France; 18. Department of Cardiology, National University of Ireland, Galway (NUIG), Galway, Ireland; 19. Department of Cardiology and Critical Care Medicine, Hartcentrum Hasselt, Jessa Ziekenhuis, Hasselt, Belgium; 20. Department of Cardiology, Bern University Hospital, Inselspital, University of Bern, Bern, Switzerland; 21. Mount Sinai Heart, Mount Sinai Medical Center, New York, NY, USA; 22. University Hospital of Wales, Cardiff, United Kingdom; 23. Azienda Ospedaliera S. Maria, Terni, Italy. The list of the GLOBAL LEADERS trial investigators is presented in Supplementary Appendix 4.
Guest Editor
This paper was guest edited by William Wijns, MD, PhD; National University of Ireland Galway, Lambe Institute, Galway, Ireland.
Funding
This work was supported by the European Clinical Research Institute, which received unrestricted grants from Biosensors International, AstraZeneca, and The Medicines Company.
Conflict of interest statement
M. Tomaniak acknowledges funding received as the Laureate of the European Society of Cardiology Research and Training Programme in the form of ESC 2018 Grant. P. Chichareon has received a research grant from Biosensors. R. Modolo has received research grants from Biosensors. E. Spitzer has received institutional grants from the European Cardiovascular Research Institute. T. Geisler has received personal fees from AstraZeneca, Bayer Healthcare, Boehringer Ingelheim, Bristol Myers Squibb, Pfizer, Daiichi Sankyo, Eli Lilly, The Medicines Company, MSD, Siemens Healthcare, and Spartan Bioscience. M. Sabaté has received personal fees from Abbott Vascular. P. Jüni reports being an unpaid member of the steering group of trials funded by AstraZeneca, Biotronik, Biosensors, St. Jude Medical and The Medicines Company. C. Hamm reports personal fees from AstraZeneca. P.G. Steg reports grants/personal fees from Bayer/Janssen, Merck, Sanofi, Amarin, Amgen, Bristol Myers Squibb, Boehringer-Ingelheim, Pfizer, Novartis, Regeneron, Lilly, AstraZeneca, and Servier. M. Valgimigli reports grants/personal fees from Abbott, Chiesi, Bayer, Daiichi Sankyo, Amgen, Terumo, Alvimedica, Medicure, AstraZeneca, Biosensors, and Idorsia. P. Vranckx reports personal fees from AstraZeneca, The Medicines Company, Bayer HealthCare, Terumo, and Daiichi Sankyo. J. Tijssen reports personal fees from Cardialysis, for DSMB membership in the GLOBAL LEADERS trial. S. Windecker reports grants from Abbott, Amgen, Bayer, Biotronik, Boston Scientific, Edwards Lifesciences, Medtronic, St. Jude Medical, Symetis SA, and Terumo. P.W. Serruys reports personal fees from Abbott Laboratories, AstraZeneca, Biosensors, Biotronik, Cardialysis, GLG Research, Medtronic, Micel Technologies, Qualimed, Sino Medical Sciences Technology, Société Europa Digital & Publishing, St. Jude Medical, Stentys, Svelte Medical Systems, Philips/Volcano, Xeltis, StentIt, and HeartFlow. The other authors have no conflicts of interest to declare. The Guest Editor has no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

