
Abstract
Aims: We aimed to demonstrate whether coronary microvascular function is improved after ticagrelor administration compared to clopidogrel administration in STEMI subjects undergoing thrombolysis.
Methods and results: MIRTOS is a multicentre study of ticagrelor versus clopidogrel in STEMI subjects treated with fibrinolysis. We enrolled 335 patients <75 years old with STEMI eligible for thrombolysis, of whom 167 were randomised to receive clopidogrel and 168 to receive ticagrelor together with thrombolysis. Primary outcome was the difference in post-PCI corrected TIMI frame count (CTFC). All clinical events were recorded in a three-month follow-up period. From the 335 patients who were randomised, 259 underwent PCI (129 clopidogrel and 130 ticagrelor) and 154 angiographies were analysable for the study primary endpoint. No significant difference was found between the clopidogrel (n=85) and ticagrelor (n=69) groups for CTFC (24.33±17.35 vs 28.33±17.59, p=0.10). No significant differences were observed in MACE and major bleeding events between randomisation groups (OR 2.0, 95% CI: 0.18-22.2, p=0.99).
Conclusions: Thrombolysis with ticagrelor in patients <75 years old was not able to demonstrate superiority compared to clopidogrel in terms of microvascular injury, while there was no difference between the two groups in MACE and major bleeding events. Trial Registration. ClinicalTrials.gov Identifier: NCT02429271. EudraCT Number 2014-004082-25.
Introduction
Antiplatelet therapy is an essential part of the therapeutic regimen of patients with coronary artery disease. Clopidogrel, a P2Y12 receptor antagonist, and aspirin are the most commonly used antiplatelet agents for the treatment of cardiovascular disease. Newer P2Y12 antagonists have shown more potent antiplatelet action and prevent more ischaemic events than clopidogrel, tending to replace the latter in the treatment of acute coronary syndromes (ACS)1. Ticagrelor, an oral, direct acting and reversibly binding P2Y12 antagonist, yields greater inhibition of platelet aggregation than clopidogrel, which is a prodrug that requires metabolic activation and results in irreversible inhibition of the adenosine diphosphate-mediated pathway of platelet aggregation1,2,3. Ticagrelor’s onset of action is faster and its inhibitory effects are more pronounced and predictable than those of clopidogrel. Moreover, ticagrelor – beyond its robust antiplatelet action – increases extracellular adenosine concentrations by inhibiting red blood cell (RBC) re-uptake and induces adenosine triphosphate release from human RBCs4,5,6,7.
When performed early enough, primary percutaneous coronary intervention (PCI) has demonstrated better overall clinical results as reperfusion therapy for ST-segment elevation myocardial infarction (STEMI) compared to thrombolysis, thus constituting the reperfusion treatment of choice according to current guidelines8,9. Nevertheless, intravenous thrombolytic therapy remains a valuable option for many patients, especially in the first hour from symptom onset or whenever timely primary PCI is not feasible. Improvements in myocardial perfusion observed after thrombolytic therapy are a strong predictor of clinical outcomes10. The beneficial effects of co-administration of aspirin and clopidogrel with fibrinolytic therapy in STEMI patients are well established, and clopidogrel is recommended as the P2Y12 inhibitor of choice in this setting11.
Thrombolytic treatment induces a state of platelet hyperreactivity that peaks at 24 hours and affects P2Y12 receptor inhibition. This status may last up to three days after drug administration12. In addition, acute myocardial infarction is per se a clinical situation involving a platelet hyperreactivity status that could interfere with clopidogrel-mediated P2Y12 inhibition.
Despite recanalisation of the epicardial infarct-related artery, microvascular damage has been documented in 14% to 30% of patients with Thrombolysis In Myocardial Infarction (TIMI) 3 flow post PCI, meaning that visual restoration of flow does not always translate into myocardial recovery. In addition, stenting may increase serotonin release as a result of deep tissue injury and platelet activation, which may in turn result in microvascular vasospasm13. In line with this, a greater degree of platelet inhibition has been associated with improved myocardial perfusion before and after PCI. More specifically, glycoprotein IIb/IIIa receptor inhibition in the setting of STEMI has been associated with improved microvascular function post PCI14,15,16,17.
Since ticagrelor co-administration with thrombolysis in STEMI patients lacks any evidence, we sought to investigate whether ticagrelor – administered together with aspirin – could improve microvascular perfusion and potentially reduce post-infarction myocardial injury compared to clopidogrel, in patients with STEMI treated with thrombolysis.
Methods
We conducted a multicentre, prospective, randomised, open-label, blinded-endpoint (PROBE design), two-arm, parallel-group study. In the first study arm patients received clopidogrel and in the second arm they received ticagrelor. In all patients enrolled, thrombolysis – with a fibrin-specific agent – constituted the reperfusion modality of choice, since primary PCI was not feasible within two hours from first medical contact. Randomisation and administration of the P2Y12 inhibitor took place immediately before thrombolysis. All patients were loaded with aspirin 150-300 mg per os, according to guidelines for thrombolytic therapy. Clopidogrel group patients received a 300 mg loading dose and a 75 mg maintenance dose, and ticagrelor group patients received a 180 mg loading dose and a 90 mg bid maintenance dose. Concomitant therapy was left to the treating physician’s discretion. Laboratory tests were performed as in routine clinical practice. After thrombolysis, patients were transferred for catheterisation and PCI, according to guidelines and routine clinical practice, to a PCI centre. For the performance of coronary angiography, both radial access and femoral access were allowed. PCI – when indicated – was performed at the time of coronary angiography. Anticoagulation was either unfractionated heparin, bivalirudin or low molecular weight heparin, according to the treating physician’s judgement. Both drug-eluting stents and bare metal stents were allowed for use. In case of failed thrombolysis, patients were transferred immediately to the PCI centre for rescue PCI.
The study complies with the Declaration of Helsinki. The locally appointed ethics committee had approved the research protocol before study initiation. Written informed consent was obtained from all subjects.
The trial was conducted in 27 Greek hospitals – 14 community hospitals, where randomisation and thrombolysis were performed, and 13 PCI-capable hospitals, to which subjects were transferred for coronary angiography 0-72 hours post thrombolysis. It should be noted that in Greece thrombolysis remains the treatment of choice for approximately 30% of patients presenting with STEMI, mainly because of special geographic features14.
Angiograms were blindly evaluated in an independent core lab (Cardialysis, Rotterdam, the Netherlands) and pre- and post-PCI TIMI myocardial perfusion grade (MPG), corrected TIMI frame count (CTFC), TIMI flow grade, angiographic perfusion score, TIMI thrombus grade, minimal lumen diameter (MLD) and percentage of diameter stenosis were measured in patients who underwent PCI after diagnostic coronary angiography.
All patients were followed with regular visits at study sites after one and three months. Clinical evaluation, standard 12-lead electrocardiogram and echocardiography were performed at all visits. For bleeding events, the Bleeding Academic Research Consortium (BARC) definition was used15. Patients who did not undergo PCI after coronary angiography were excluded from the study and those who had been randomised to ticagrelor were switched to clopidogrel per os 75 mg/day (without any loading dose), on the next day after coronary angiography. Those patients were followed during their hospital stay for safety reasons (bleeding events).
The study primary endpoint was CTFC. Secondary endpoints were all other angiographic parameters studied, bleeding events and major adverse cardiovascular events (MACE) – death, myocardial infarction, revascularisation, stroke – during the three-month follow-up period.
STATISTICAL ANALYSIS
All enrolled patients were included in the analysis of primary, secondary and safety outcomes on an intention-to-treat basis. Categorical variables were compared between randomisation groups by chi-square tests; continuous variables were compared with t-tests or non-parametric tests, as appropriate. All analyses were performed with IBM SPSS Statistics for Windows, Version 21 (IBM Corp., Armonk, NY, USA).
The sample size required for the assessment of the primary endpoint was derived from the corrected CTFC improvement hypothesis. This is based on data from the ERAMI trial, in which post-PCI CTFC was significantly improved in STEMI patients treated early rather than in those treated late with abciximab (19±8 vs 27±20)16. We assumed that a difference of five units in CTFC represents the minimum difference necessary to detect clinical significance. Sample size calculations were based on a two-sided significance level of 5%, 80% power and a difference in CTFC of five units.
Sample size calculations for 80% power required at least 284 patients to be randomised, in order to detect as significant – at the 5% level – a minimum clinically important difference in CTFC of five units with an assumed SD=15.
Eventually, 335 patients were randomised (allowing for 10% dropout); 259 underwent PCI, but for technical reasons the number of patients who had CTFC assessed was 154 (85 clopidogrel, 69 ticagrelor). Based on that sample size, power to detect the difference drops to 54%.
Results
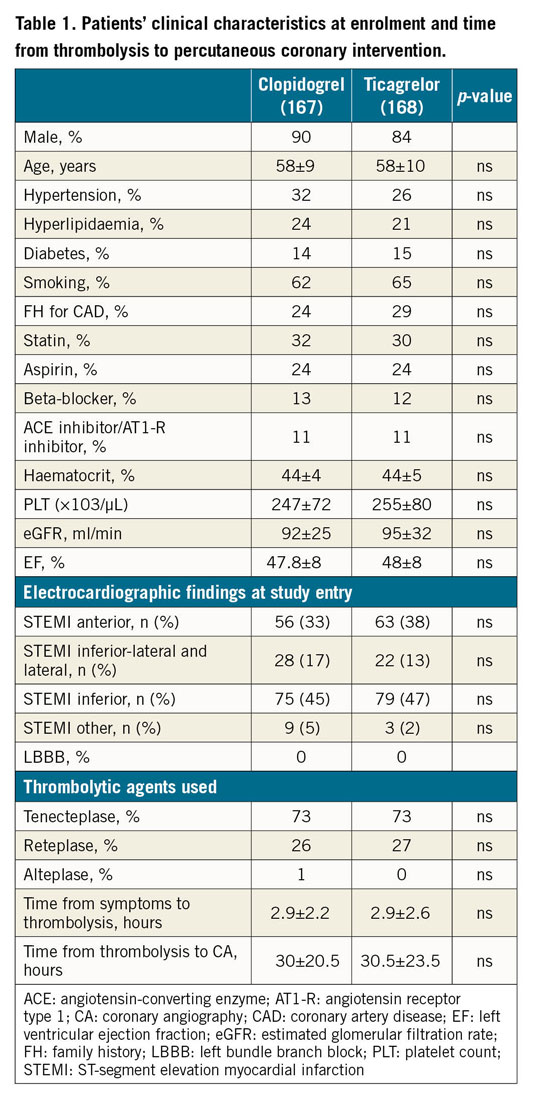
Between October 2015 and July 2018, 335 patients were recruited, 259 of whom underwent PCI and were included in the primary endpoint analysis. All the rest were followed for any clinical events during their hospitalisation. The three-month follow-up period for the last enrolled patient ended in October 2018. In Figure 1, we present the study flow. Table 1 summarises subjects’ baseline characteristics, whereas the numbers of subjects having completed each study phase are presented in Supplementary Table 1.
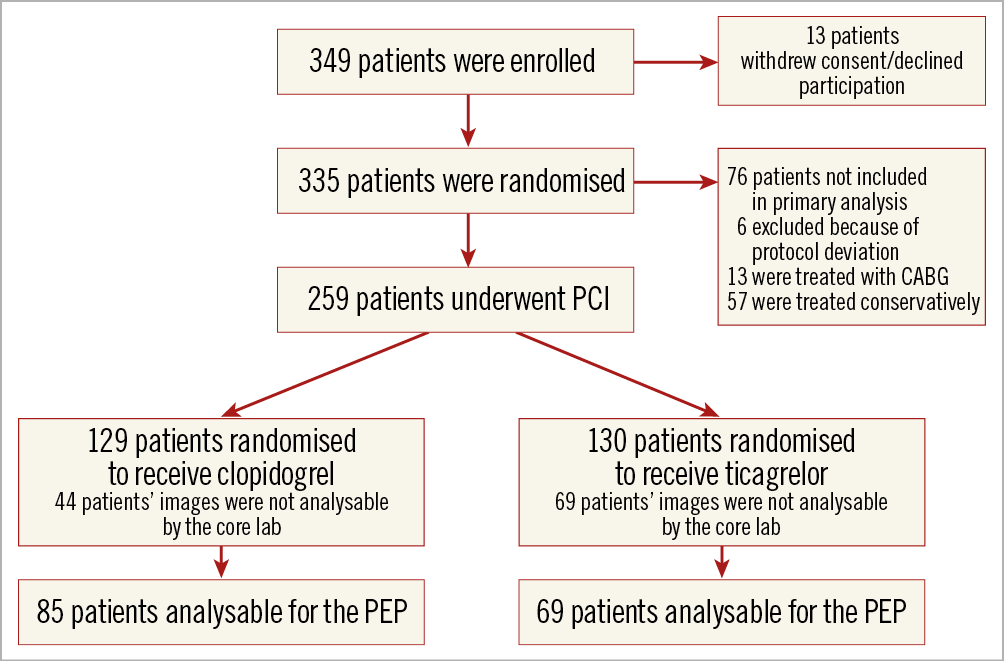
Figure 1. Study flow diagram.
STUDY PRIMARY ENDPOINT
From the 259 patients who underwent PCI, 154 angiograms were analysable for the primary endpoint (60.5%). The rest could not be analysed by the core lab, because either image quality or projections performed were not appropriate for a valid analysis. No statistically significant difference was found in CTFC values between clopidogrel (n=85) and ticagrelor (n=69) groups (24.33±17.35 vs 28.33±17.59, p=0.10) (Figure 2).
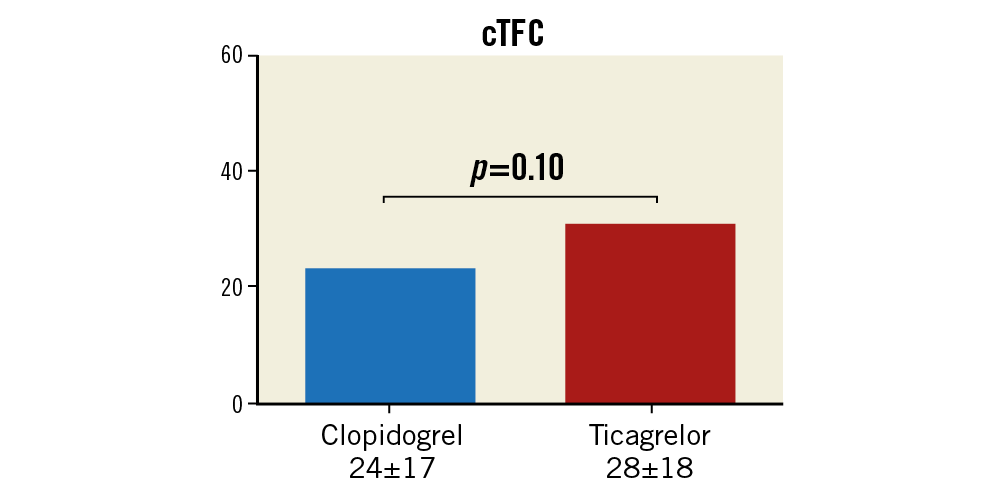
Figure 2. Mean corrected Thrombolysis In Myocardial Infarction (TIMI) frame count values (cTFC) in each randomisation group.
Subjects’ primary angiographic characteristics are summarised in Supplementary Table 2.
An additional analysis for the 154 patients with analysable angiograms was performed. Clinical characteristics are presented in Supplementary Table 3 and angiographic characteristics in Supplementary Table 4. The results of this additional analysis are in line with the initial intention-to-treat analysis.
STUDY SECONDARY ANGIOGRAPHIC ENDPOINTS
THROMBUS GRADE PRE-PCI
Angiograms of 184 subjects were analysable for thrombus grade (TG) pre-PCI. No significant difference was found between the clopidogrel (n=110) and ticagrelor (n=74) groups (1.1±1.47 vs 1.2±1.63, p=0.82).
THROMBUS GRADE POST PCI
Angiograms of 166 patients were analysable for TG post PCI. No significant difference was found between the clopidogrel (n=100) and ticagrelor (n=66) groups (0.081±0.4 vs 0.12±0.54, p=0.63).
TIMI FLOW PRE-PCI
Angiograms of 259 patients were analysable for TIMI flow (TF) pre-PCI. No significant difference was found between the clopidogrel and ticagrelor groups (2.49±0.99 vs 2.51±0.98, p=0.93).
TIMI FLOW POST PCI
Angiograms of 245 patients were analysable for TF post PCI. No significant difference was found between the clopidogrel (n=128) and ticagrelor (n=117) groups (2.88±0.48 vs 2.91±0.36, p=0.92).
MYOCARDIAL BLUSH GRADE (MBG)
Angiograms of 119 patients were analysable for MBG. No significant difference was found between the clopidogrel (n=54) and ticagrelor (n=65) groups (1.8±0.8 vs 1.75±0.85, p=0.78).
BLEEDING EVENTS
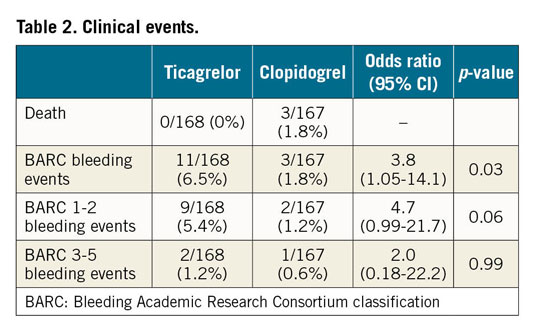
All bleeding events were recorded and classified according to BARC during the three-month study period. For major bleeding events (BARC ≥3), no statistically significant difference was observed between randomisation groups (one patient with BARC 3A bleeding in each group). Minor bleeding events (BARC 1 & 2) were numerically higher in the ticagrelor group, but this finding did not reach statistical significance (odds ratio [OR] 4.7 [0.99-21.7], p=0.06) (Table 2). From the 9 minor bleeding events in the ticagrelor group, 7 were BARC 1 and 2 BARC 2 and for clopidogrel 1 and 1, respectively.
OTHER CLINICAL EVENTS
All 335 patients were followed during the study period for any clinical events. Deaths, myocardial infarctions, strokes and any unplanned revascularisation were recorded. No statistically significant difference was observed for any of the above clinical events (Table 2).
Discussion
MIRTOS is the first randomised trial comparing ticagrelor co-administration with aspirin, versus the conventional clopidogrel-based therapy, in STEMI patients <75 years receiving thrombolysis. The primary findings were as follows:
1. Ticagrelor was not superior to clopidogrel in the population studied for post-PCI CTFC, which was the study’s primary endpoint.
2. Major bleeding events were similar between the study groups.
Post-PCI CTFC was similar in both study groups; thus, the primary study endpoint hypothesis was neutral. Moreover, there were no differences in any secondary angiographic endpoints studied.
Ticagrelor is a potent antiplatelet agent that has proved better efficacy and has been associated with reduced mortality compared to clopidogrel in ACS, albeit at the cost of increased bleeding risk1,17. In addition, several animal and human studies have shown that ticagrelor demonstrates several pleiotropic effects through adenosine-mediated or other unknown pathways4,5.
In the PLATO study, thrombolysis-treated STEMI patients were excluded, while clinical experience with ticagrelor in this patient population is limited1. In the TREAT trial, ticagrelor was administered approximately 12 hours post thrombolysis in 1,800 patients and proved to be a safe alternative to clopidogrel in terms of bleeding events, while no difference in ischaemic events was observed; however, the study was not powered to demonstrate such differences18. There are a few small studies showing that ticagrelor administration some hours after thrombolytic therapy with clopidogrel and aspirin significantly improves platelet inhibition19,20,21, while only anecdotal experience on administration of ticagrelor at the same time as thrombolytics has been published22.
Our study was the first to test the administration of ticagrelor with fibrinolytic agents and aspirin and compare it to the standard of practice, which is clopidogrel administration. In the 2017 ESC guidelines for STEMI management, a 2- to 24-hour time window from thrombolysis to coronary angiography is recommended. All centres participating in MIRTOS were strongly encouraged to comply with the ESC guidelines. Nevertheless, because of specific geographic features in the Greek landscape, this was not always possible. Thus, a time window up to 72 hours was permitted.
Our primary endpoint was angiographic, and our hypothesis was based on the theoretical advantages of ticagrelor over clopidogrel in terms of antiplatelet action and potential pleiotropic effects. The study results failed to prove any difference in primary and angiographic secondary endpoints between ticagrelor and clopidogrel. In line with our results, in the PLATO angiographic substudy no difference was observed between ticagrelor and clopidogrel for the same angiographic parameters23. According to our hypothesis, in the present study the time from treatment to angiography and PCI was much longer than in PLATO. This could have provided ticagrelor the time to exhibit its potent antiplatelet and pleiotropic effects that theoretically could have decreased microvascular injury and myocardial damage. Our results do not support this hypothesis. One of the potential explanations might lie in the large number of angiographies that could not be analysed for the primary endpoint in the core lab (almost 40%), thus decreasing the study’s statistical power. Moreover, ticagrelor’s pleiotropic effects are still under investigation; results from different studies are contradictory. It is known that ticagrelor inhibits adenosine transporter 1, which in turn leads to increased extracellular adenosine concentration and increased adenosine receptor activation locally; however, the clinical relevance of this is still unclear24.
On the other hand, it is important that in the MIRTOS study – in line with TREAT results, where ticagrelor was administered about 11 hours later and not together with thrombolysis – ticagrelor was not associated with increased major bleeding events (≥BARC 3), which were identical in the two study groups. The total number of bleeding events was greater in the ticagrelor group, due to increased minor bleedings (Table 2), especially BARC 1 (7 of the 8 minor bleeding events in the ticagrelor group). This finding is in concordance with what is already known from previous studies1,17,18. In the PLATO study1, fibrinolysis was an exclusion criterion and, due to lack of relevant data, current guidelines do not recommend ticagrelor for patients with STEMI undergoing fibrinolysis8.
Administration of a potent antiplatelet agent such as ticagrelor together with fibrinolysis could involve an increased bleeding risk. Although our study was not powered for testing differences in bleeding events, these results are encouraging because evidence with ticagrelor and thrombolytics is missing and those exploratory findings may drive larger adequately powered studies to focus on bleeding events. Ticagrelor has been associated with a significant decrease in MACE compared to clopidogrel throughout the spectrum of ACS patients; thus, we could extrapolate that thrombolysis with ticagrelor might be a safe choice with a potential to decrease MACE compared to clopidogrel. This hypothesis needs confirmation in larger studies. Our results concerning ticagrelor safety can be considered only as exploratory and hypothesis-generating.
Limitations
The final number of patients with analysable angiograms for the study primary endpoint was 154 (60.5% of the number initially planned). The study was open-label and patients were informed about their antiplatelet medication. Nevertheless, it should be emphasised that the primary endpoint was evaluated in an independent core lab which was blinded to the patients’ treatment.
Conclusions
Thrombolysis with ticagrelor in patients younger than 75 years was not superior to clopidogrel in terms of microvascular injury and microvascular integrity, as assessed by different angiographic endpoints. In terms of clinical events, there was no difference between the two groups in MACE and major bleeding events.
|
Impact on daily practice The MIRTOS study tested, for the first time, ticagrelor together with thrombolysis in patients with STEMI and did not prove superiority compared to clopidogrel in terms of microvascular integrity. Significant bleeding events were similar between randomisation drugs, indicating that thrombolysis with ticagrelor might be a safe choice for high-risk STEMI patients, a hypothesis that requires confirmation in a larger clinical trial. |
Appendix. Study collaborators
Ioannis Mantas, MD; Cardiology Department, Halkida General Hospital, Halkida, Greece. Emmanouil Foukarakis, MD, PhD; Cardiology Department, Venizeleio General Hospital, Heraklion, Greece. Ioannis Kanonidis, MD, PhD; 2nd Cardiology Department, Hippokration Hospital, Thessaloniki, Greece. Antonios Ziakas, MD, PhD; 3rd Cardiology Department, Hippokration Hospital, Thessaloniki, Greece. Stergios Tzikas, MD, PhD; 1st Cardiology Department, AHEPA University Hospital, Thessaloniki, Greece. Georgios Papaioannou, MD; Cardiology Department, Livadia Hospital, Livadia, Greece. Antonios Draganigos, MD; Cardiology Department, Corfu Hospital, Corfu, Greece. Grigorios Ikonomou, MD; Cardiology Department, Preveza Hospital, Preveza, Greece. Anastasia Damelou, MD, PhD; Cardiology Department, Korinthos Hospital, Korinthos, Greece. Ilias Samiotis, MD; Cardiology Department, Rethymnon Hospital, Rethymnon, Greece. Nikolaos Kampouridis, MD; Cardiology Department, Kavala Hospital, Kavala, Greece. Ioannis Tsounos, MD; Cardiology Department, Agios Pavlos Hospital, Thessaloniki, Greece. Gregory Chlouverakis, PhD; Department of Biostatistics, Medical School, University of Crete, Crete, Greece. George Kochiadakis, MD, PhD; Stylianos Petousis, MD; Emmanouil Skalidis, MD, PhD; Evangelos Zacharis, MD, PhD; Fragkiskos Parthenakis, MD, PhD; Cardiology Department, University Hospital of Heraklion, Heraklion, Greece. Anastasia Nikolaou, MD, PhD; Hellenic Cardiovascular Research Society (HCRS), Athens, Greece.
Funding
This work was supported by AstraZeneca and was sponsored by the Hellenic Cardiovascular Research Society.
Conflict of interest statement
M. Hamilos reports grants and personal fees from HCRS, and personal fees from AstraZeneca and Sanofi. J. Kanakakis reports grants from HCRS, and personal fees from AstraZeneca and Sanofi. I. Anastasiou reports non-financial support from HCRS. C. Karvounis reports non-financial support from HCRS, and personal fees from AstraZeneca. J. Goudevenos reports non-financial support from HCRS. L. Michalis reports personal fees from Sanofi, AstraZeneca, Elpen and Bayer Hellas. M. Koutouzis reports grants from HCRS. I. Tsiafoutis reports grants from HCRS and AstraZeneca. K. Raisakis reports grants from HCRS. D. Stakos reports grants from HCRS. P. Vardas reports personal fees from Menarini International, Dean Medicus, Servier, the European Society of Cardiology and Hygeia Hospitals Group. I. Mantas reports non-financial support from HCRS. E. Foukarakis reports grants from HCRS. G. Papaioannou reports non-financial support from HCRS. G. Ikonomou reports non-financial support from HCRS. A. Damelou reports grants from HCRS. I. Samiotis reports non-financial support from HCRS. N. Kampouridis reports non-financial support from HCRS. I. Tsounos reports non-financial support from HCRS. G. Chlouverakis reports grants from HCRS. G. Kochiadakis reports non-financial support from HCRS, personal fees from AstraZeneca and Sanofi, and grants from Innovis, Servier and Abbott. S. Petousis reports grants from HCRS. E. Skalidis reports grants from HCRS, and personal fees from AstraZeneca, Medtronic, Boehringer-Ingelheim and Pfizer. E. Zacharis reports non-financial support from HCRS, and personal fees from Sanofi and Amgen. F. Parthenakis reports grants from HCRS. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

