
Abstract
Aims: Ticagrelor has shown greater, more rapid and more consistent platelet inhibition than clopidogrel. However, the superiority of ticagrelor for preventing ischaemic damage in STEMI patients has not been proven. The aim of this trial was to assess whether ticagrelor is superior to clopidogrel in preventing microvascular injury in ST-elevation myocardial infarction (STEMI).
Methods and results: Patients with STEMI underwent prospective random assignment to receive a loading dose (LD) of clopidogrel 600 mg or ticagrelor 180 mg (1:1 ratio) before primary percutaneous coronary intervention (PCI). As the primary endpoint, the index of microcirculatory resistance (IMR) was measured immediately after primary PCI. The secondary endpoint was the infarct size estimated from the wall motion score index (WMSI). A total of 76 patients were enrolled (clopidogrel group=38, ticagrelor group=38). The IMR in the ticagrelor group was significantly lower than that in the clopidogrel group (22.2±18.0 vs. 34.4±18.8 U, p=0.005). Cardiac enzymes were less elevated in the ticagrelor group than in the clopidogrel group (CK peak; 2,651±1,710 vs. 3,139±2,698 ng/ml, p=0.06). Infarct size, estimated by WMSI, was not different between the ticagrelor and clopidogrel groups at baseline (1.55±0.30 vs. 1.61±0.29, p=0.41) or after three months (1.42±0.33 vs. 1.47±0.33, p=0.57).
Conclusions: In patients with STEMI treated by primary PCI, a 180 mg LD of ticagrelor might be more effective in reducing microvascular injury than a 600 mg LD of clopidogrel, as demonstrated by IMR immediately after primary PCI.
Abbreviations
ACS: acute coronary syndrome
CFR: coronary flow reserve
CK: creatine kinase
FFR: fractional flow reserve
HPR: high platelet reactivity
IMR: index of microcirculatory resistance
LD: loading dose
LVEF: left ventricular ejection fraction
PCI: percutaneous coronary intervention
STEMI: ST-segment elevation myocardial infarction
TIMI: Thrombolysis In Myocardial Infarction
Tmn: mean transit time
TMPG: TIMI myocardial perfusion grade
TTE: transthoracic echocardiography
WMSI: wall motion score index
Introduction
Ticagrelor is a non-thienopyridine direct P2Y12 receptor blocker. It is more potent than clopidogrel, and is associated with less inter-individual variability1,2. The PLATO trial showed ticagrelor to be more clinically effective than clopidogrel in terms of reduction of ischaemic events in patients with acute coronary syndrome (ACS)3.
In addition to the potent inhibition of platelet function, ticagrelor has previously been shown to increase adenosine levels by inhibiting adenosine re-uptake at the tissue level and to induce adenosine triphosphate release from human red blood cells, stimulating vasodilation4. A recent study confirms that ticagrelor increases the plasma concentration of adenosine in patients with ACS when compared to clopidogrel5.
However, there have been no data on the role of the higher plasma adenosine and stronger inhibition of platelets from ticagrelor in the protection against microvascular injury in ST-segment elevation myocardial infarction (STEMI). Therefore, we designed a clinical study to compare the protective effects from microvascular injury of clopidogrel and ticagrelor in patients with STEMI.
Methods
STUDY RECRUITMENT
The CV-TIME trial was a single-centre, randomised, open-label parallel-arm trial designed to compare the effectiveness of a loading dose (LD) of ticagrelor with an LD of clopidogrel for preventing microvascular injury in STEMI patients. From September 2013 to November 2014, we consecutively enrolled STEMI patients at Inha University Hospital. Patients of at least 18 years of age, within 12 hours of onset of symptoms of STEMI with documented ischaemia due to a significant lesion in a native coronary artery were recruited. Reasons for exclusion were unprotected left main stem disease, culprit lesion located at a side branch, stent thrombosis, high-degree AV block, cardiogenic shock, contraindication to adenosine, history of previous cerebrovascular accident or myocardial infarction, or a final Thrombolysis In Myocardial Infarction (TIMI) grade <3. Figure 1 shows a brief flow chart summary of the study protocol.
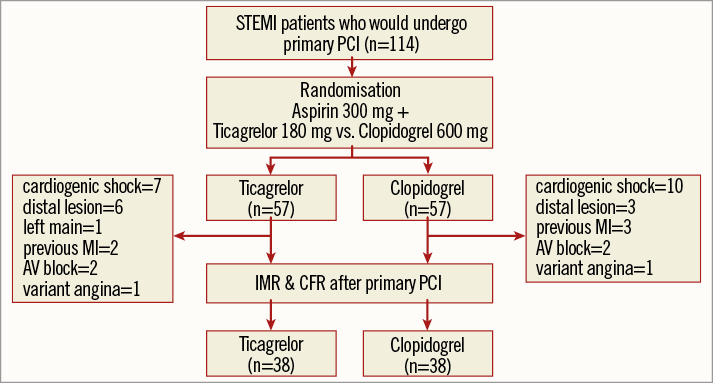
Figure 1. Flow chart of the entire study. AV block: atrioventricular block; CFR: coronary flow reserve; IMR: index of microcirculatory resistance; PCI: percutaneous coronary intervention; STEMI: ST-segment elevation myocardial infarction
STUDY TREATMENT
Patients were randomly assigned to receive either clopidogrel or ticagrelor using a random number table that was independently managed at the Inha University Hospital Cardiovascular Research Center. The patients received 300 mg aspirin plus an LD of either 600 mg clopidogrel or 180 mg ticagrelor before primary PCI. Administration of glycoprotein IIb/IIIa inhibitors was at the physician’s discretion. All patients included in this trial were treated according to the current ACC/AHA guidelines regarding post-stenting management, which specify treatment with at least 100 mg of aspirin daily, and 75 mg clopidogrel or 180 mg ticagrelor daily for at least 12 months after PCI.
CORONARY PHYSIOLOGIC ASSESSMENT
The index of microcirculatory resistance (IMR) measured at the infarct-related artery after primary PCI is a strong predictor of myocardial damage6 and can be used as a quantitative assessment of microvascular injury in ACS patients7. We assessed the microvascular injury using IMR measured at the infarct-related artery immediately after reperfusion therapy8,9. An intracoronary combined pressure-temperature sensor-tipped guidewire (Radi PressureWire 5; Radi Medical Systems, Uppsala, Sweden) was used to measure the thermodilution-derived IMR. The pressure sensor was placed at the distal two thirds of the infarct-related artery. Intracoronary nitroglycerine was administered (200 μg), and hyperaemia was induced using an adenosine infusion (140 μg/kg/min) administered via the femoral or antecubital vein. Aortic and distal coronary pressures were measured during hyperaemia, and the fractional flow reserve (FFR) was calculated by using the formula: FFR=distal coronary pressure/aortic pressure. The IMR was calculated from the ratio of the mean distal coronary pressure at maximal hyperaemia to the inverse of the hyperaemic mean transit time (Tmn) as follows: IMR=distal pressure×Tmn during hyperaemia8,10. Since it can be unsafe to measure wedge pressure in the setting of acute MI, Yong’s method was used for calculating an IMR without a wedge pressure11. Figure 2 shows the thermodilution coronary flow reserve (CFR), calculated by dividing the resting Tmn by the hyperaemic Tmn12.
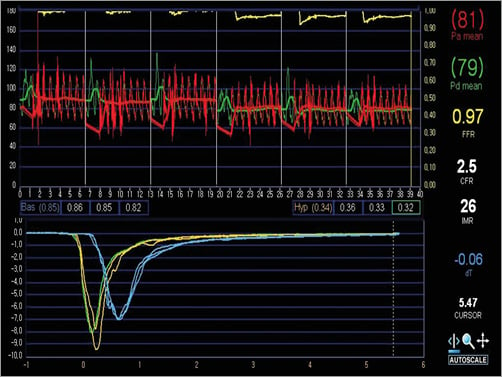
Figure 2. Thermodilution curves. The index of microcirculatory resistance (IMR) was calculated from the ratio of the mean distal coronary pressure (Pd) at maximal hyperaemia to the inverse of the hyperaemic mean transit time (blue lines). Thermodilution coronary flow reserve (CFR) was calculated by dividing the resting mean transit time (yellow lines) by the hyperaemic mean transit time.
INFARCT SIZE MEASUREMENTS
Transthoracic echocardiograms (TTEs) were obtained less than 24 hours after primary PCI, and again three months later. The LV ejection fraction (LVEF) was measured from apical four- and two-chamber views, using the modified Simpson’s rule. As recommended by the American Society of Echocardiography, wall motion score index (WMSI) was assessed in a 16-segment model13. An experienced cardiologist who was blinded to the IMR rated the segmental wall motion as follows: normal or hyperkinetic=1, hypokinetic=2, akinetic=3, and dyskinetic or aneurysmatic=4. WMSI was calculated as the sum of all scores divided by the number of segments visualised.
ANGIOGRAPHIC AND LABORATORY ANALYSIS
The TIMI flow grade and the TIMI myocardial perfusion grade (TMPG) were rated from grade 0 to grade 3 based on the images obtained after reperfusion therapy14. Cardiac enzymes including creatine kinase (CK), CK-MB and troponin I were measured every eight hours after until the cardiac enzyme began to decline.
ENDPOINTS
The primary endpoint of the study was the IMR, measured immediately after primary PCI. The secondary endpoint was the left ventricular WMSI on echocardiography at baseline and three months after the index PCI.
STATISTICAL ANALYSES
The objective was to determine whether ticagrelor has any benefit over clopidogrel in preventing microvascular injury in STEMI patients. Based on previous reports for IMR values in STEMI, we assumed that the mean IMR in the clopidogrel group would be approximately 29 U with a standard deviation of 1415,16. To prove ticagrelor superior to clopidogrel, the IMR in the ticagrelor group should be less than 30% of the IMR in the clopidogrel group. We enrolled 38 patients in the ticagrelor and the clopidogrel groups, respectively, with 80% power to detect a group difference of 9 U in the change of the IMR value at a two-sided alpha level of 0.05.
All of the primary and secondary endpoints were analysed on an intention-to-treat basis, with all patients analysed as part of their assigned treatment group. The baseline characteristics of the study patients were summarised in terms of frequencies and percentages for categorical variables and by using means with standard deviations for continuous variables. Categorical variables were compared using Fisher’s exact test. Continuous variables were compared using the two-sample t-test. A p-value of 0.05 was established as the level of statistical significance for all tests.
Results
BASELINE CHARACTERISTICS
Among the 114 patients who were randomised and then underwent primary PCI, 38 patients were excluded for various reasons (Figure 1). Physiological studies were available in 76 patients. Therefore, the analysis population consisted of 76 patients: 38 assigned to clopidogrel and 38 to ticagrelor.
The mean age of the study population was 59±13 years. The mean door-to-balloon and symptom-to-balloon times were 91±83 minutes and 369±350 minutes, respectively. The infarct was located in the left anterior descending artery (LAD) in 46 patients (60.5%), left circumflex artery (LCX) in nine patients (11.8%), and right coronary artery (RCA) in 21 patients (27.6%).
COMPARISON OF ANGIOGRAPHIC AND LABORATORY FINDINGS
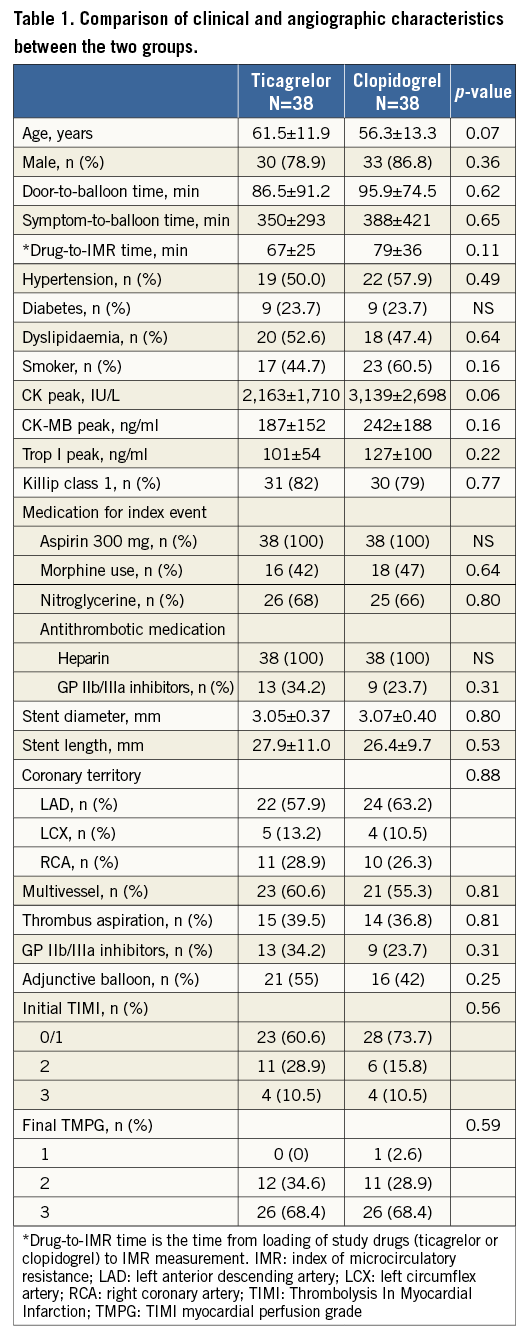
Baseline angiographic and laboratory findings are displayed in Table 1. On pre-PCI angiography, the prevalence of TIMI 2~3 did not differ between the ticagrelor group and the clopidogrel group (39 vs. 26%, p=0.22). There was no difference in the use of thrombus aspiration (p=0.81), glycoprotein IIb/IIIa inhibitors (p=0.31) and adjunctive balloon (p=0.25) between the groups. On final angiography, the TMPG was similar in both groups (p=0.59). The peak cardiac enzyme level in the ticagrelor group was less than that in the clopidogrel group (CK peak; 2,651±1,710 vs. 3,139±2,698 ng/ml, p=0.06).
MICROVASCULAR INJURY ESTIMATED BY IMR IMMEDIATELY AFTER PRIMARY PCI
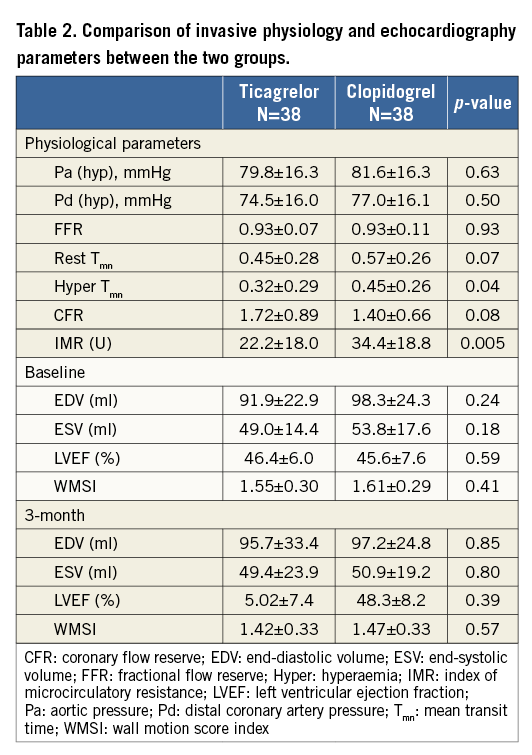
Intracoronary physiology parameters were measured in 76 infarct-related arteries after successful reperfusion therapy. The mean time from loading of study drugs (ticagrelor or clopidogrel) to IMR measurement was 73 minutes. The mean FFR immediately after PCI was 0.93±0.19. The mean IMR was 28.3±19.3 U and CFR was 1.66±1.25. Between the ticagrelor and the clopidogrel groups, there were no significant differences in hyperaemic aortic (80±16 vs. 82±16 mmHg, p=0.63) or distal coronary artery pressures (75±16 vs. 77±16, p=0.5), FFR (0.93±0.07 vs. 0.93±0.11, p=0.93). As a primary endpoint, the IMR in the ticagrelor group was significantly lower than that in the clopidogrel group (22.2±18.0 vs. 34.4±18.8, p=0.005) (Figure 3). The CFR in the ticagrelor group was more preserved than in the clopidogrel group (1.72±0.89 vs. 1.40±0.66, p=0.08) (Table 2).
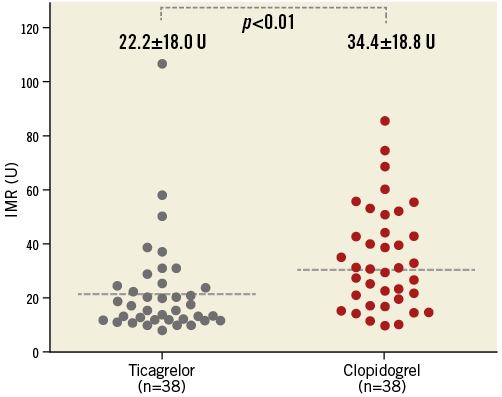
Figure 3. Comparison of index of microcirculatory resistance in ticagrelor and clopidogrel groups. A comparison of microvascular injury estimated by index of microcirculatory resistance in STEMI patients with a 180 mg loading dose of ticagrelor or a 600 mg loading dose of clopidogrel.
COMPARISON OF INFARCT SIZE BY WMSI
Baseline echocardiography was performed within 24 hrs after primary PCI in all enrolled patients. There was no difference in LVEF (46.4±6.0 vs. 45.6±7.6%, p=0.59) or WMSI (1.55±0.30 vs. 1.61±0.29, p=0.41) between the ticagrelor and clopidogrel groups (Table 2). On the TTE three months post primary PCI, the WMSI was similar between the ticagrelor group and the clopidogrel group (1.42±0.33 vs. 1.47±0.33, p=0.57). On paired comparison between the WMSI at baseline and at three months, a significant improvement was shown both in the ticagrelor group (p<0.001) and in the clopidogrel group (p=0.001) (Figure 4).
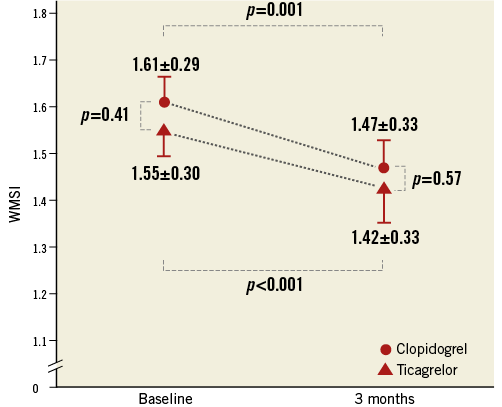
Figure 4. Infarct size estimated from the wall motion score index at baseline and three months later. A comparison of infarct size estimated by wall motion score index in STEMI patients with a 180 mg loading dose of ticagrelor or a 600 mg loading dose of clopidogrel.
Discussion
We hypothesised that, in patients who underwent primary PCI to treat STEMI, the greater platelet inhibition and increase in adenosine concentration associated with ticagrelor administration would lead to a reduction in microvascular injury compared to patients who received clopidogrel. This randomised study demonstrates that adjunctive ticagrelor treatment might be more effective in reducing microvascular injury, as estimated by the IMR immediately after primary PCI in STEMI patients. Administration of a ticagrelor loading dose before primary PCI reduced the IMR by 35% compared to patients who received clopidogrel in STEMI patients with grade 3 TIMI coronary flow on final angiography.
Ticagrelor exhibits greater and more rapid platelet inhibition than clopidogrel in stable patients1,2. Antiplatelet agents play a relevant role in protecting against thrombus microembolisation and reducing periprocedural ischaemia17,18. Greater platelet inhibition correlates with improved angiographic outcome, and correlates with increased levels of coronary flow after coronary intervention. The ARMYDA-6 MI trial has proven that aggressive antiplatelet treatment, defined as clopidogrel 600 mg, decreased the infarct size and improved the TIMI flow grade on final angiography when compared with clopidogrel 300 mg in STEMI patients19. Intravenous glycoprotein IIb/IIIa inhibitors showed more preserved microvascular integrity, as estimated by myocardial contrast echocardiography 48 hours after primary PCI in STEMI patients20. The INFUSE-AMI trial revealed that intracoronary abciximab diminished infarct size in patients with already normal coronary flow from a median of 17.4 to 14.4% (p=0.01)21. Although the degree of early platelet inhibition was suboptimal after a 180 mg loading dose of ticagrelor in STEMI patients compared with stable patients22, potent inhibition of platelet function can be expected with an LD of ticagrelor compared to an LD of clopidogrel even in STEMI patients. Recent studies have confirmed that there was high platelet reactivity (HPR; defined as >230 platelet reaction units) two hours after a 180 mg LD of ticagrelor in 42.3% of STEMI patients22, whereas there was HPR (defined as >235 platelet reaction units) at two hours after a 600 mg LD of clopidogrel in 64.5% of STEMI patients23.
Besides its potent inhibition of platelet function, ticagrelor increases adenosine levels by inhibiting adenosine re-uptake at tissues and inducing release of adenosine triphosphate (ATP) from human red blood cells4. Adenosine inhibits platelet aggregation and also dilates coronary microvessels through the activation of adenosine 2A receptors, which are strongly implicated in the regulation of coronary blood flow24,25. Recent in vivo studies have revealed that plasma adenosine concentration six hours after antiplatelet loading was more than twofold higher with ticagrelor than with clopidogrel (1.5 µm vs. 0.68 µm, p<0.01) in ACS patients5,26. In healthy volunteers without ischaemia, ticagrelor showed more adenosine-induced coronary blood flow velocity estimated by Doppler echocardiography when compared with clopidogrel27. These properties of adenosine may be particularly important for reducing microvascular injury in diseased vessels with ACS.
Finally, the CV-TIME trial has proven that, in STEMI patients, a ticagrelor loading dose before primary PCI significantly reduced IMR, which would correlate with microvascular injury, compared to patients who received clopidogrel. The IMR was 35% lower in the ticagrelor group compared to the clopidogrel group (22.1±18 vs. 34.4±18.8 U, p<0.01). However, the CV-TIME trial did not show any difference in angiographic outcomes in both groups as a secondary endpoint in our study. Recently, the PLATO angiographic substudy also revealed no differences between ticagrelor and clopidogrel in myocardial perfusion and coronary flow before or after PCI28. In STEMI patients, a 180 mg LD of ticagrelor may not be enough to show improvement in angiographic outcomes due to the comparative pharmacodynamic limitations of oral P2Y12 receptor blockers compared to IV glycoprotein IIb/IIIa inhibitors which showed improved angiographic outcome after coronary intervention in previous studies.
Until now, there have been no data on the role of the higher plasma adenosine and stronger inhibition of platelets from ticagrelor in the protection against microvascular injury in STEMI patients. In our randomised study in STEMI patients who received either ticagrelor or clopidogrel, IMR which would correlate with microvascular injury was significantly reduced in ticagrelor compared with clopidogrel.
Study limitations
A major limitation of this study is the small number of patients enrolled. This study did not have sufficient power to assess the relationship between the level of microvascular injury and infarct size. Also, infarct size was not evaluated by cardiac MR, the gold standard for infarct size measurement. Therefore, we could not determine whether the reduction in microvascular injury seen with the administration of a loading dose of ticagrelor had any effect in decreasing the size of the infarct. In addition, no analysis of platelet function was carried out, which would have allowed assessment of the relationship between platelet inactivation and the level of microvascular injury. Third, the CV-TIME trial was highly selective, enrolling only STEMI patients who had successful primary PCI and showed TIMI grade 3 flow. Consequently, values of IMR both in the ticagrelor and in the clopidogrel group were relatively low compared with previous IMR studies6. Even if the difference of microvascular injury between the ticagrelor and clopidogrel groups is pronounced, a relatively preserved microvascular function seems not to make a difference on infarct burden between the ticagrelor and clopidogrel groups.
Conclusions
In patients with STEMI treated by primary PCI, a 180 mg LD of ticagrelor might be more effective in reducing microvascular injury than a 600 mg LD of clopidogrel, as demonstrated by IMR immediately after primary PCI.
| Impact on daily practice The index of microcirculatory resistance (IMR) measured using a pressure-temperature sensor-tipped guidewire is a novel measure of microvascular function and a good predictor of microvascular damage in patients with STEMI. Ticagrelor is a non-thienopyridine direct P2Y12 receptor blocker and has previously been shown to increase adenosine levels by inhibiting adenosine re-uptake at the tissue level, so stimulating vasodilation. In patients with STEMI, a 180 mg loading dose of ticagrelor might be more effective at reducing microvascular injury, as measured by IMR immediately after primary PCI. |
Acknowledgements
We are grateful to the numerous colleagues who provided clinical and research support in treating and following the patients included in this trial. This work was not supported by any external funding.
Conflict of interest statement
The authors have no conflicts of interest to declare.

