Abstract
Background: Prior coronary artery bypass graft surgery (CABG) patients undergoing percutaneous coronary intervention (PCI) are often older and present with multiple comorbidities. Ticagrelor monotherapy after a short course of dual antiplatelet therapy (DAPT) has emerged as an effective bleeding-avoidance strategy among high-risk patients.
Aims: We aimed to examine the effects of ticagrelor with or without aspirin in prior CABG patients undergoing PCI within the TWILIGHT trial.
Methods: After 3 months of ticagrelor plus aspirin, patients were randomised to either aspirin or placebo, in addition to ticagrelor, for 12 months and compared by prior CABG status. The primary endpoint was Bleeding Academic Research Consortium (BARC) type 2, 3 or 5 bleeding. The key secondary endpoint was all-cause death, myocardial infarction (MI), or stroke.
Results: Out of 7,119 patients, a total of 703 (10.8%) patients had prior CABG within the randomised cohort. Prior CABG patients had more comorbidities and a higher incidence of BARC type 2, 3, or 5 bleeding and death, MI or stroke at 1 year after randomisation, compared with patients without prior CABG. Ticagrelor monotherapy was associated with significantly less BARC 2, 3, or 5 bleeding among prior CABG patients compared with DAPT (4.9% vs 9.6%, hazard ratio [HR] 0.50, 95% confidence interval [CI]: 0.28 to 0.90; pinteraction=0.676) and similar rates of death, MI or stroke (10.0% vs 8.7%, HR 1.14, 95% CI: 0.70 to 1.87; pinteraction=0.484). When comparing target vessel type, treatment effects were consistent among graft- and native-vessel interventions.
Conclusions: In high-risk patients with prior CABG, ticagrelor monotherapy reduced bleeding without compromising ischaemic outcomes compared with ticagrelor plus aspirin.
Introduction
Patients with previous coronary artery bypass graft (CABG) surgery are susceptible to the progression of coronary artery disease (CAD) and potential graft failure1. They are often older and frequently present with multiple comorbidities and anatomically complex CAD, which may complicate revascularisation. Thus, redo CABG surgery occurs infrequently2, and over the years percutaneous coronary intervention (PCI) has become the most common modality of repeat revascularisation in the setting of a favourable anatomy34. Nonetheless, PCI in patients with previous CABG surgery is associated with worse long-term outcomes compared with those without prior CABG, in particular when PCI is targeting a bypass graft567. Such risk is likely multifactorial and can be attributed to both differences in conduit pathophysiology as well as underlying patient complexity.
Dual antiplatelet therapy (DAPT) with aspirin and a P2Y12 inhibitor for 1 year post-PCI is currently the standard of care for preventing ischaemic complications after PCI8. The optimal antithrombotic therapy after PCI has rarely been investigated in patients with a previous CABG. Potent P2Y12 inhibitors, including ticagrelor and prasugrel, may represent alternative antiplatelet strategies to attenuate the thrombotic risk in prior CABG patients, and P2Y12 monotherapy has recently emerged as an effective bleeding-avoidance strategy among high-risk patients. The Ticagrelor With Aspirin or Alone in High-Risk Patients After Coronary Intervention (TWILIGHT) study demonstrated that after 3 months of DAPT, P2Y12 inhibitor monotherapy with ticagrelor reduced bleeding, compared with DAPT alone, without increasing ischaemic outcomes among patients undergoing PCI with contemporary drug-eluting stents (DES)9. We conducted a post hoc analysis of the TWILIGHT trial among patients with or without a previous CABG surgery to investigate the effects of ticagrelor monotherapy compared with ticagrelor plus aspirin in such patients.
Methods
Trial design and oversight
TWILIGHT was a randomised, double-blind, placebo-controlled trial conducted at 187 sites across 11 countries from July 2015 to July 2019. The trial design, rationale and principal results have been reported previously910. The Icahn School of Medicine at Mount Sinai sponsored the trial. AstraZeneca provided an investigator-initiated grant and supplied ticagrelor for the trial but did not contribute to the design, collection, analysis or interpretation of the data. The executive and steering committees were responsible for the trial conduct, integrity of data analysis and reporting of results. National regulatory agencies and institutional review boards or ethics committees of the participating sites approved the trial protocol. An independent data safety monitoring board provided external oversight to ensure the safety of the trial participants. All participants provided informed consent prior to enrolment.
Study population
Patients must have undergone successful PCI with at least 1 locally approved DES, at least 1 clinical and at least 1 angiographic criterion of high ischaemic or bleeding risk and had to be scheduled for discharge on ticagrelor plus aspirin to be eligible for enrolment. High-risk clinical features included being age 65 years or older, of female sex, and having troponin-positive acute coronary syndrome, confirmed vascular disease, diabetes mellitus on medications, or chronic kidney disease defined as an estimated glomerular filtration rate <60 mL/min/1.73 m2. High-risk angiographic criteria included multivessel CAD, a total stent length greater than 30 mm, a thrombotic target lesion, a bifurcation lesion treated with at least 2 stents, an obstructive left main or proximal left anterior descending coronary artery lesion, and a calcified target lesion treated with atherectomy. The key exclusion criteria included presentation with ST-segment elevation myocardial infarction, cardiogenic shock, prior stroke, treatment with oral anticoagulation, or having a contraindication to aspirin or ticagrelor.
Study regimen
Enrolled patients received open-label ticagrelor (90 mg twice daily) and enteric-coated aspirin (81 to 100 mg/day) during the 3 months after index PCI. At 3 months, patients who remained adherent to DAPT without a major bleeding or ischaemic event were randomised in a 1:1 fashion to either aspirin or matching placebo in addition to open-label ticagrelor. The treatment was continued for an additional 12 months, and follow-up was performed at 1 month after randomisation by telephone and at 6 and 12 months after randomisation in person. After 12 months of protocol-mandated therapy, patients were switched to a standard-of-care antiplatelet regimen at the discretion of the treating physician, followed by a last telephone follow-up 3 months later.
Endpoints
The primary endpoint was the first occurrence of Bleeding Academic Research Consortium (BARC) type 2, 3, or 5 bleeding between randomisation and 12 months after randomisation11. The key secondary ischaemic endpoint was a composite of death from any cause, myocardial infarction (MI), or stroke. Secondary bleeding endpoints included BARC type 3 or 5 bleeding, Thrombolysis in Myocardial Infarction (TIMI) major bleeding12, Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries (GUSTO) moderate, severe, or life-threatening bleeding13, or major bleeding as defined by the International Society of Thrombosis or Haemostasis (ISTH)14. MI was defined according to the third universal definition15, and stent thrombosis was classified according to the Academic Research Consortium16. All clinical events were adjudicated by an independent external committee blinded to treatment group assignments.
Statistical analyses
Baseline and clinical characteristics were compared by prior CABG status as well as by treatment arm and were summarised as means for continuous variables and counts and percentages for categorical variables. Treatment outcomes of ticagrelor monotherapy versus ticagrelor plus aspirin were evaluated by prior CABG status using Cox regression with formal interaction testing to assess for effect modification. An exploratory analysis was performed to evaluate treatment effects according to the type of target vessel (i.e., native coronary vessel vs venous or arterial graft PCI) among prior CABG patients. Patients who underwent both native coronary artery and venous or arterial graft PCI were included in the graft PCI subgroup.
Bleeding and ischaemic outcomes were assessed in the intention-to-treat and per-protocol population, respectively. The Kaplan-Meier method was used to estimate the cumulative incidences of the primary and secondary endpoints. Patients who did not incur a primary endpoint between randomisation and 12 months were censored at the time of death, last known contact, or 365 days, whichever came first. A multivariable Cox proportional hazard model was used to generate hazard ratios (HR) with 95% confidence intervals (CI) for the association of prior CABG status with bleeding and ischaemic outcomes. Covariates for adjustment included baseline imbalances and known confounders: age, sex, diabetes, chronic kidney disease, hypertension, hypercholesterolaemia, smoking status and peripheral artery disease. Kaplan-Meier estimates were used to calculate absolute risk differences (ARD) and 95% CI for bleeding and ischaemic events. P-values were 2-sided and considered statistically significant if <0.05. Interaction p-values were calculated to determine whether differences between prior CABG and non-prior CABG patients were statistically significant, either on the multiplicative scale comparing HR or on the additive scale comparing ARD17. All analyses were performed using Stata version 16.0 (StataCorp).
Results
Patient characteristics
A total of 9,006 patients who underwent PCI were enrolled in the trial, and 7,119 were randomised at 3 months after PCI to either ticagrelor plus placebo or ticagrelor plus aspirin (Supplementary Figure 1). Patients were excluded for country-specific regulatory reasons (n=587), and 1 patient was excluded due to missing data on CABG status. Of the final cohort (n=6,531), 703 (10.8%) patients had previously undergone CABG surgery while 5,828 (89.2%) had not. Prior CABG patients were older, more frequently male, and more frequently white, compared with patients without prior CABG. They had a higher prevalence of almost all baseline comorbidities, including diabetes and chronic kidney disease, and had undergone more previous MI and PCI procedures (Table 1). Prior CABG patients were also more likely to present with stable CAD and require complex PCI. All prior CABG patients (100%) received native coronary-vessel PCI, of which 132 patients (18.8%) additionally received saphenous vein graft PCI and 30 patients (4.3%) arterial graft PCI (Table 2).
Among the treatment groups, the baseline and procedural characteristics were well-balanced (Supplementary Table 1, Supplementary Table 2). Finally, prior CABG patients were less adherent to both ticagrelor (72.2% vs 83.1%; p<0.001) as well as the study drug (79.9% vs 86.6%; p<0.001) upon conclusion of the trial period at 12 months after randomisation (Supplementary Figure 2). Reasons for non-adherence are listed in Supplementary Table 3 and Supplementary Table 4.
Table 1. Baseline clinical characteristics.
| Clinical parameters | OverallN=6,531 | Prior CABGN=703(10.8%) | No prior CABGN=5,828(89.2%) | p-value | |
|---|---|---|---|---|---|
| Age, years | 64.0±10.1 | 67.1±9.5 | 63.6±10.1 | <0.001 | |
| Female sex | 1,534 (23.5%) | 106 (15.1%) | 1,427 (24.5%) | <0.001 | |
| Non-white race | 1,609 (24.6%) | 109 (15.5%) | 1,500 (25.7%) | <0.001 | |
| BMI, kg/m2 | 28.9±5.6 | 29.9±5.4 | 28.7±5.6 | <0.001 | |
| Enrolling region | North America | 2,972 (45.5%) | 476 (67.7%) | 2,496 (42.8%) | <0.001 |
| Europe | 2,509 (38.4%) | 200 (28.4%) | 2,308 (39.6%) | ||
| Asia | 1,051 (16.1%) | 27 (3.8%) | 1,024 (17.6%) | ||
| Diabetes | 2,405 (36.8%) | 328 (46.7%) | 2,076 (35.6%) | <0.001 | |
| Diabetes treated with insulin | 650 (10.0%) | 120 (17.1%) | 530 (9.1%) | <0.001 | |
| Chronic kidney disease | 1,060 (16.9%) | 184 (26.8%) | 876 (15.7%) | <0.001 | |
| Anaemia | 1,207 (19.3%) | 154 (22.7%) | 1,053 (18.9%) | 0.016 | |
| Current smoker | 1,400 (21.4%) | 83 (11.8%) | 1,317 (22.6%) | <0.001 | |
| Hypercholesterolaemia | 4,239 (64.9%) | 612 (87.1%) | 3,626 (62.2%) | <0.001 | |
| Hypertension | 4,800 (73.5%) | 622 (88.5%) | 4,177 (71.7%) | <0.001 | |
| Peripheral arterial disease | 478 (7.3%) | 100 (14.2%) | 378 (6.5%) | <0.001 | |
| Previous MI | 1,937 (29.7%) | 317 (45.1%) | 1,619 (27.8%) | <0.001 | |
| Previous PCI | 2,838 (43.5) | 398 (56.6%) | 2,439 (41.8%) | <0.001 | |
| Previous major bleed | 54 (0.8%) | 14 (2.0%) | 40 (0.7%) | <0.001 | |
| Indication for PCI | Stable CAD | 2,411 (36.9%) | 309 (44.0%) | 2,102 (36.1%) | <0.001 |
| ACS | 4,120 (63.1%) | 394 (56.0%) | 3,725 (63.9%) | ||
| ACS: acute coronary syndrome; BMI: body mass index; CABG: coronary artery bypass graft; CAD: coronary artery disease; MI: myocardial infarction; PCI: percutaneous coronary intervention | |||||
Table 2. Baseline procedural characteristics.
| Procedural characteristics | OverallN=6,531 | Prior CABGN=703(10.8%) | No prior CABGN=5,828(89.2%) | p-value | |
|---|---|---|---|---|---|
| Radial artery access | 4,628 (70.9%) | 249 (35.4%) | 4,378 (75.1%) | <0.001 | |
| Multivessel CAD | 4,069 (62.3%) | 618 (87.9%) | 3,451 (59.2%) | <0.001 | |
| Target vessel | Native vessel | 6,532 (100%) | 703 (100%) | 5,828 (100%) | |
| Left main | 289 (4.4%) | 87 (12.4%) | 202 (3.5%) | <0.001 | |
| LAD | 3,639 (55.7%) | 217 (30.9%) | 3,421 (58.7%) | <0.001 | |
| LCx | 2,099 (32.1%) | 315 (44.8%) | 1,784 (30.6%) | <0.001 | |
| RCA | 2,297 (35.2%) | 247 (35.1%) | 2,050 (35.2%) | 0.983 | |
| Venous bypass graft | 132 (2.0%) | 132 (18.8%) | |||
| Arterial bypass graft | 30 (0.5%) | 30 (4.3%) | |||
| Number of vessels treated | 1.3±0.5 | 1.2±0.5 | 1.3±0.5 | 0.024 | |
| Number of lesions treated | 1.5±0.7 | 1.5±0.8 | 1.5±0.7 | 0.155 | |
| Lesion morphology† | Moderate/severe calcification | 940 (14.4%) | 125 (17.8%) | 815 (14.0%) | 0.007 |
| Bifurcation | 790 (12.1%) | 61 (8.7%) | 729 (12.5%) | 0.003 | |
| Total occlusion | 367 (5.6%) | 51 (7.3%) | 316 (5.4%) | 0.046 | |
| Thrombotic | 724 (11.1%) | 43 (6.1%) | 680 (11.7%) | <0.001 | |
| Total stent length, mm‡ | 38.9±23.5 | 35.7±23.0 | 39.3±23.5 | <0.001 | |
| Minimum stent diameter, mm | 2.8±0.5 | 2.9±0.5 | 2.8±0.5 | 0.107 | |
| Complex PCI§ | 2,072 (31.7%) | 356 (50.6%) | 1,716 (29.4%) | <0.001 | |
| ≥3 vessels treated | 169 (2.6%) | 20 (2.8%) | 149 (2.6%) | 0.649 | |
| ≥3 lesions treated | 633 (9.7%) | 59 (8.4%) | 574 (9.8%) | 0.218 | |
| Total stent length >60 mm | 1,031 (15.8%) | 95 (13.5%) | 936 (16.1%) | 0.080 | |
| Bifurcation with at least 2 stents implanted | 219 (3.4%) | 10 (1.4%) | 209 (3.6%) | 0.003 | |
| Atherectomy device use | 240 (3.7%) | 42 (6.0%) | 198 (3.4%) | <0.001 | |
| Left main PCI | 289 (4.4%) | 87 (12.4%) | 202 (3.5%) | <0.001 | |
| Surgical bypass graft | 161 (2.4%) | 158 (22.5%) | |||
| Chronic total occlusion as target lesions | 367 (5.6%) | 51 (7.3%) | 316 (5.4%) | 0.046 | |
| †Lesion morphology assessed by operators. ‡Stent length calculated by operators. §Complex PCI is defined as having any of the following PCI characteristics: ≥3 vessels treated, ≥3 lesions treated, total stent length >60 mm, bifurcation with at least 2 stents implanted, atherectomy device use, left main PCI, surgical bypass graft or chronic total occlusion as target lesions. CABG: coronary artery bypass graft; CAD: coronary artery disease; LAD: left anterior descending; LCx: left circumflex; PCI: percutaneous coronary intervention; RCA: right coronary artery | |||||
Bleeding outcomes
The primary endpoint of BARC 2, 3, or 5 bleeding occurred more frequently among patients with versus without prior CABG (7.2% vs 5.2%, HR 1.38, 95% CI: 1.02 to 1.87; p=0.036); however, after multivariable adjustment, bleeding differences were no longer significant (adjusted HR 1.32, 95% CI: 0.96 to 1.82; p=0.082) (Supplementary Figure 3). BARC 3 or 5 bleeding also occurred more frequently in prior CABG patients compared with those without prior CABG (Supplementary Figure 4). Among prior CABG patients, BARC 2, 3, or 5 bleeding occurred significantly less often in ticagrelor plus placebo compared with ticagrelor plus aspirin (4.9% vs 9.6%, HR 0.50, 95% CI: 0.28 to 0.90; p=0.02) (Figure 1A), with an ARD of –4.7% (95% CI: –8.6% to –0.8%) (Supplementary Table 5). Among patients without prior CABG, BARC 2, 3, or 5 bleeding also occurred less often in ticagrelor plus placebo patients compared with DAPT (3.8% vs 6.6%; HR 0.57, 95% CI: 0.45 to 0.72; p<0.01), without heterogeneity of treatment effects among patients with or without prior CABG (pinteraction=0.676; multiplicative scale).
There was a significant reduction in major (i.e., BARC 3 or 5) bleeding among prior CABG patients receiving ticagrelor plus placebo compared with DAPT (1.1% vs 4.5%, HR 0.25, 95% CI: 0.08 to 0.76; p=0.015) (Figure 1B), with an ARD of –3.3% (95% CI: –5.8% to –0.9%) (Supplementary Table 5). Patients without a prior CABG also had fewer BARC 3 or 5 bleeding events in the ticagrelor plus placebo group compared with DAPT (0.9% vs 1.7%, HR 0.54, 95% CI: 0.34 to 0.88; p=0.012). Ticagrelor monotherapy was also associated with significant reductions in other secondary bleeding endpoints compared with DAPT (Figure 2). Relative treatment effects of ticagrelor plus placebo versus DAPT were consistent among patients with or without prior CABG in terms of BARC 3 or 5 bleeding (pinteraction=0.211; multiplicative scale) and other secondary bleeding endpoints. The ARD was significantly larger in the prior CABG group with respect to ISTH major bleeding (pinteraction=0.046; additive scale) (Supplementary Table 5).
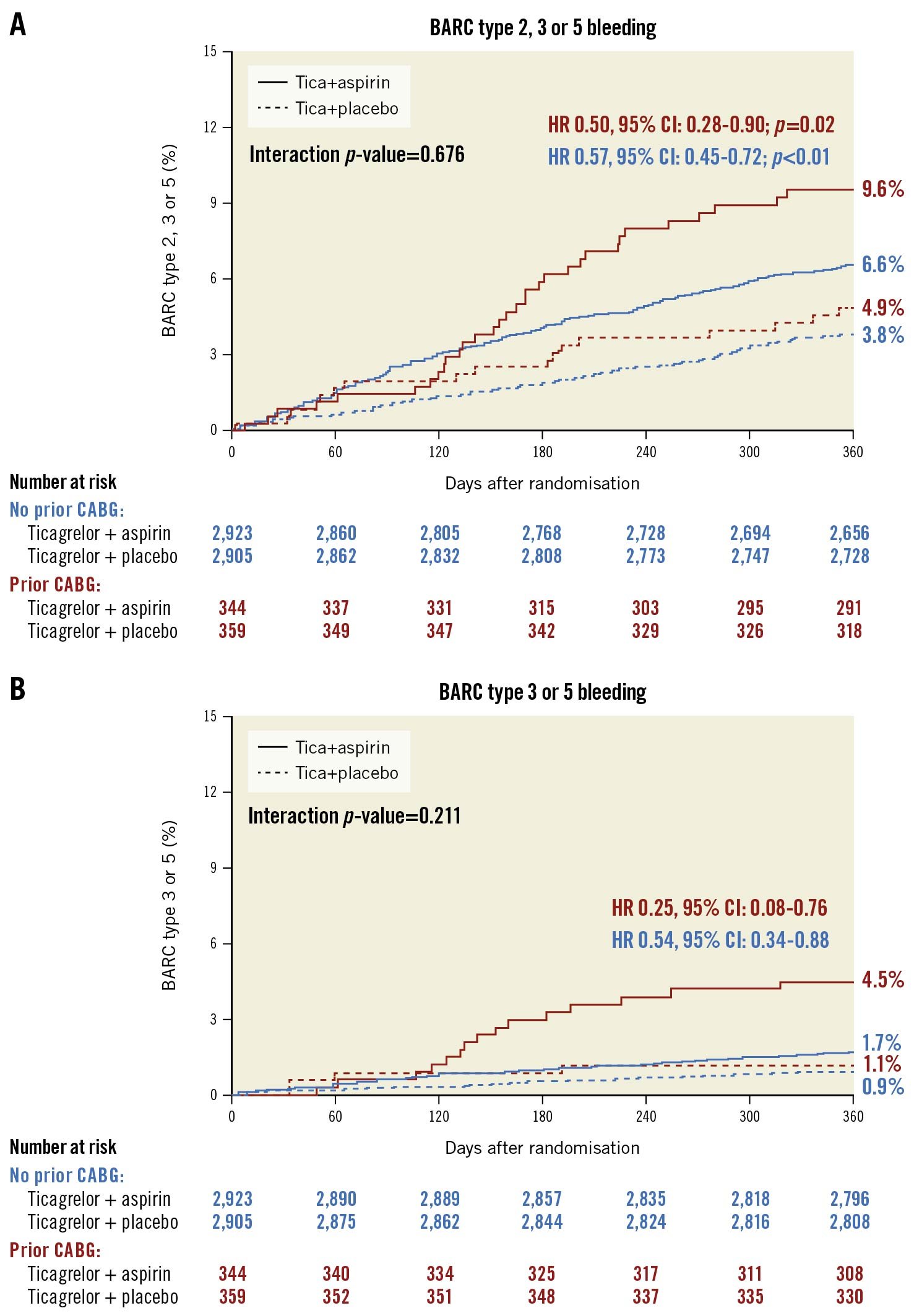
Figure 1. Rates of BARC bleeding at 1 year after randomisation by prior CABG status and treatment arm. Kaplan-Meier curves with bleeding rates at 1 year of (A) BARC 2, 3 or 5 bleeding, and (B) BARC 3 or 5 bleeding for ticagrelor plus placebo versus ticagrelor plus aspirin in patients with and without prior CABG. BARC: Bleeding Academic Research Consortium; CABG: coronary artery bypass graft; CI: confidence interval; HR: hazard ratio; Tica: ticagrelor
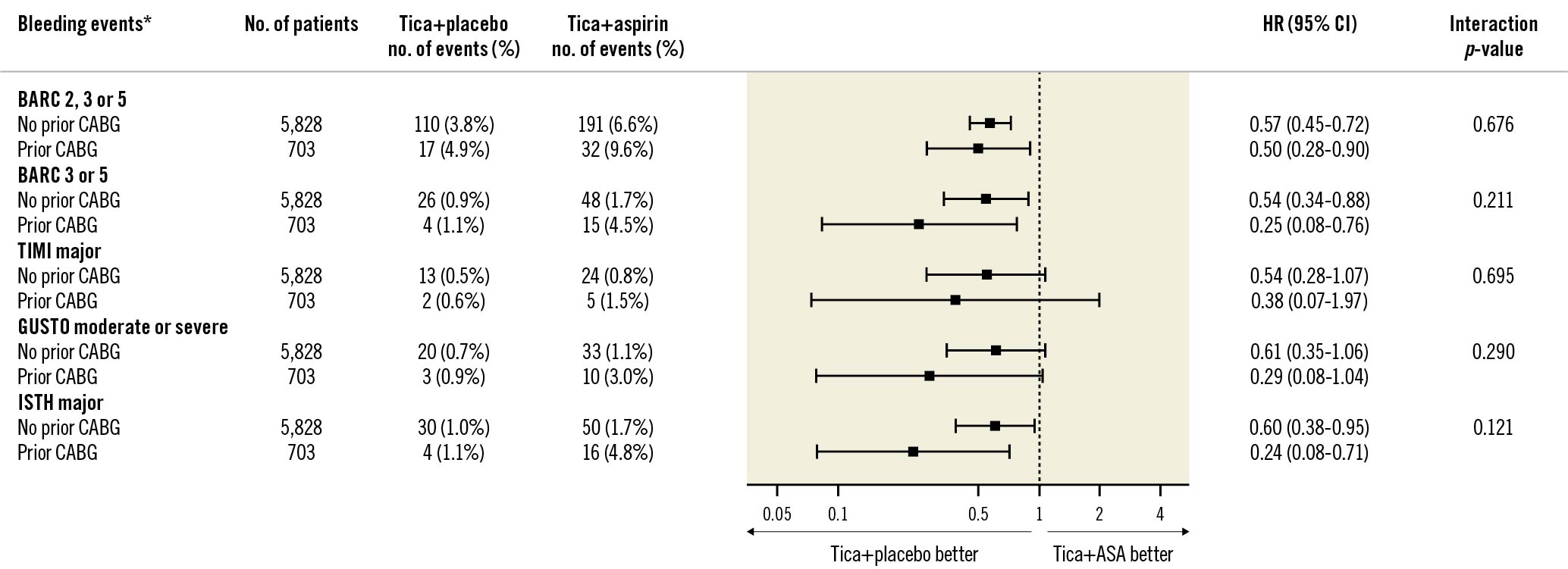
Figure 2. Bleeding events by prior CABG status at 1 year after randomisation. Forest plot illustrating outcomes of ticagrelor plus placebo versus ticagrelor plus aspirin according to prior CABG status. The interaction p-value is for the interaction test between randomised treatment assignment and prior CABG status. *Bleeding events were analysed in intention-to-treat cohort. ASA: aspirin; BARC: Bleeding Academic Research Consortium; CABG: coronary artery bypass graft; CI: confidence interval; GUSTO: Global Utilization of Streptokinase and TPA for Occluded Arteries; HR: hazard ratio; ISTH: International Society on Thrombosis and Haemostasis; Tica: ticagrelor; TIMI: Thrombolysis in Myocardial Infarction; TPA: tissue plasminogen activator
Ischaemic outcomes
The key secondary ischaemic composite endpoint of death, MI or stroke occurred more frequently in patients with prior CABG compared with those without prior CABG (9.4% vs 3.3%, adjusted HR 2.20, 95% CI: 1.63 to 2.98; p<0.001) (Supplementary Figure 5). Cardiovascular death, MI or ischaemic stroke also occurred more frequently in prior CABG patients compared with those without prior CABG (Supplementary Figure 6). Among prior CABG patients, there were no significant differences between ticagrelor plus placebo versus DAPT in the composite of death, MI, or ischaemic stroke (10.0% vs 8.7%, HR 1.14, 95% CI: 0.70 to 1.87; p=0.550) (Figure 3A), with an ARD of 1.3% (95% CI: –3.0% to 5.7%) (Supplementary Table 6). Patients without prior CABG, furthermore, had similar outcomes in the composite of death, MI or stroke events among both the ticagrelor monotherapy and DAPT groups (3.2% vs 3.4%, HR 0.93, 95% CI: 0.70 to 1.24; p=0.618) and relative treatment effects were consistent among patients with or without prior CABG (pinteraction=0.484; multiplicative scale).
Moreover, no treatment-related differences were noted for the composite of cardiovascular death, MI, or ischaemic stroke (8.9% vs 8.4%, HR 1.05, 95% CI: 0.63 to 1.74; p=0.811) (Figure 3B), nor for the individual components (Figure 4). Relative treatment effects of ticagrelor plus placebo versus ticagrelor plus aspirin were consistent among patients with or without prior CABG in terms of cardiovascular death, MI or ischaemic stroke (pinteraction=0.720; multiplicative scale) and other secondary ischaemic endpoints (Figure 4). There were no significant differences between ARD of ischaemic outcomes among patients with and without prior CABG (Supplementary Table 6).
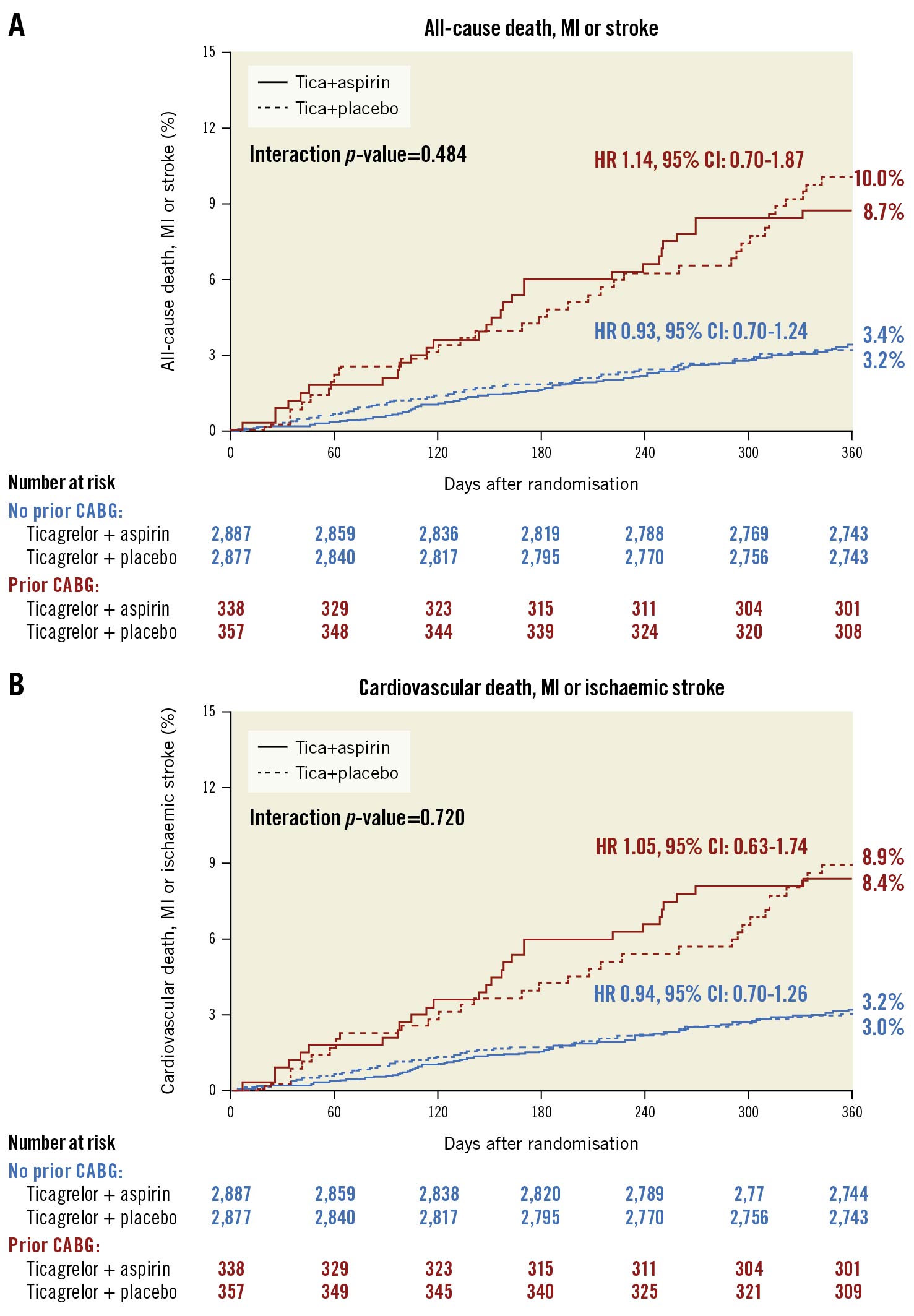
Figure 3. Rates of all-cause death, MI or stroke, and cardiovascular death, MI or ischaemic stroke at 1 year after randomisation by prior CABG status and treatment arm. Kaplan-Meier curves with ischaemic rates at 1 year of (A) death, MI, or ischaemic stroke, and (B) cardiovascular death, MI, or ischaemic stroke for ticagrelor plus placebo versus ticagrelor plus aspirin in patients with and without prior CABG. CABG: coronary artery bypass graft; CI: confidence interval; HR: hazard ratio; MI: myocardial infarction; Tica: ticagrelor
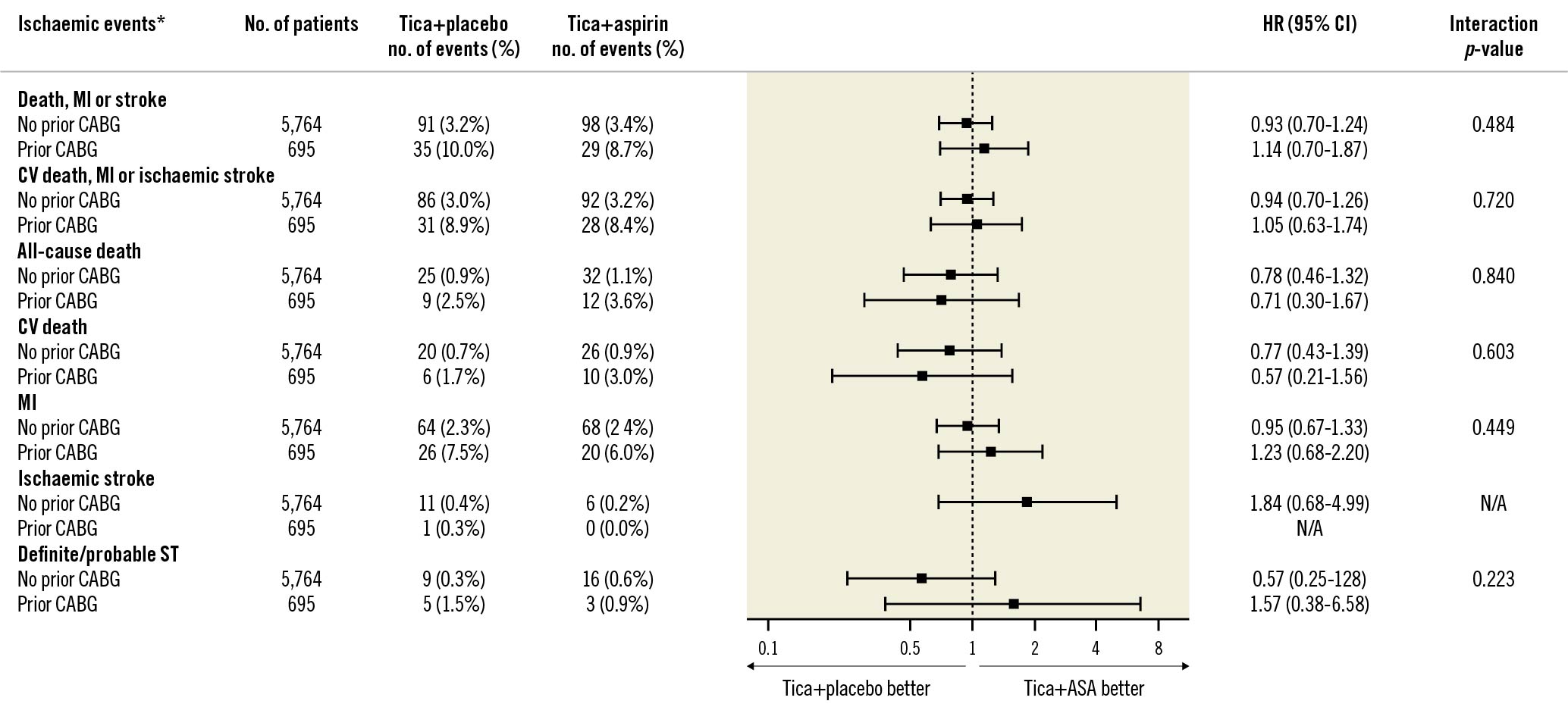
Figure 4. Ischaemic events by prior CABG status at 1 year after randomisation. Forest plot illustrating outcomes of ticagrelor plus placebo versus ticagrelor plus aspirin according to prior CABG status. The interaction p-value is for the interaction test between randomised treatment assignment and prior CABG status. *Ischaemic events were analysed in per-protocol cohort. ASA: aspirin; CABG: coronary artery bypass graft; CI: confidence interval; CV: cardiovascular; HR: hazard ratio; MI: myocardial infarction; ST: stent thrombosis; Tica: ticagrelor
Exploratory target vessel analysis
After stratification by the target vessel of the intervention, treatment effects of ticagrelor plus placebo compared with ticagrelor plus aspirin in the primary endpoint of BARC 2, 3, or 5 bleeding remained consistent among graft- and native-vessel interventions (pinteraction=0.998; multiplicative scale), without any signs of treatment heterogeneity in other secondary bleeding outcomes (Supplementary Figure 7). There were, furthermore, no significant differences in the treatment effects between ticagrelor plus placebo compared with ticagrelor plus aspirin among graft- and native-vessel interventions for the composite endpoint of death, MI, or stroke or other ischaemic outcomes (Supplementary Figure 8).
Discussion
The main findings of this post hoc analysis of the TWILIGHT trial, which examined ticagrelor monotherapy compared with ticagrelor plus aspirin in patients with and without a previous CABG surgery, include the following: 1) patients with prior CABG had an increased risk of both major bleeding and ischaemic events compared with patients without prior CABG; 2) ticagrelor monotherapy reduced bleeding events compared with ticagrelor plus aspirin without a concomitant increase in ischaemic events, irrespective of prior CABG status; 3) the treatment effect of aspirin withdrawal compared with continuation remained consistent among bleeding and ischaemic endpoints regardless of the target vessel of choice. A summary of the findings of this study is presented in the Central Illustration.
Although there has been substantial interest in comparing PCI to CABG in various types of CAD, data investigating secondary PCI in prior CABG patients are scarce. Yet PCI has become increasingly common among patients with a history of a previous CABG surgery, representing roughly 18% of all procedures within the US National Cardiovascular Data Registry6. Contemporary practices have largely been extrapolated from PCI in those without a prior CABG, yet baseline and procedural characteristics differ substantially. In our study, prior CABG patients were older, had numerous comorbidities, and had more extensive and challenging coronary anatomy, with ~50% of interventions classified as complex PCI. As a result, we found that prior CABG patients suffered significantly more ischaemic events compared with patients without prior CABG, consistent with previous reports57, in addition to higher rates of major bleeding. However, compared with those without prior CABG, the risk of ischaemic events among prior CABG patients was substantially greater than the increased risk of bleeding (+6.1% vs +2.0% on the absolute scale). Furthermore, there was no significant difference in bleeding events after multivariate adjustment. Such differences highlight the highly complex ischaemic risk of PCI and emphasise the potential for more potent antiplatelet therapy, such as ticagrelor after PCI in patients with a previous CABG surgery.
Data examining different antiplatelet strategies in patients with a previous CABG are scarce. After index CABG, ticagrelor plus aspirin provided higher 1-year vein graft patency rates in the DACAB trial18 as well as reduced rates of cardiovascular and total mortality in a subgroup analysis of the PLATO trial19, when compared with clopidogrel plus aspirin. However, studies examining ticagrelor monotherapy after index CABG surgery have not illustrated improved 1-year clinical outcomes compared with aspirin alone, the latter of which remains the mainstay therapy after elective CABG surgery1820. After PCI however, potent P2Y12 inhibitors do improve clinical outcomes in both low- and high-risk patients compared with clopidogrel, yet, when combined with aspirin, they do come at the expense of increased bleeding21. P2Y12 monotherapy after PCI and 3 months of DAPT has since been examined in multiple randomised studies with favourable results22. Most studies, however, were open-label and only included low-risk patients, not all studies utilised potent P2Y12 inhibitors, such as ticagrelor, and none specifically examined the effects in prior CABG patients. TWILIGHT preferentially enrolled patients at high ischaemic and/or bleeding risk and, although lower than in real-world practice, included a particularly high number of prior CABG patients for a non-dedicated CABG study (703 patients at ~10% of the total population) and compared with other antithrombotic drug trials. Consistent with the main trial results, our subanalysis extends the benefit of potent P2Y12 monotherapy to the prior CABG population by demonstrating a reduction in bleeding events while preserving similar thrombotic effect. Furthermore, the larger absolute reduction in ISTH major bleeding compared with non-prior CABG patients may even indicate an added benefit of ticagrelor monotherapy in high-risk prior CABG patients, although, given the limited power of this post hoc analysis, such findings should only be considered as hypothesis-generating.
Moreover, despite an almost 3-fold increase in ischaemic risk after PCI among prior CABG patients, withdrawal of aspirin did not increase ischaemic events, highlighting the fact that “dropping aspirin” remains a safe approach to reduce bleeding while retaining similar protection against ischaemic events, even for patients at extremely high ischaemic risk. This was furthermore notable given the lower medication adherence in the prior CABG group (Supplementary Figure 2). Although this is partially due to higher adverse events, poor adherence to optimal medical therapy is indeed a multifactorial recognised problem among the CABG population23. Regardless, the 2020 ESC guidelines have proposed that the novel strategy of ticagrelor monotherapy after 3 months of DAPT may be considered, based on balancing the bleeding risk with the perceived trade-off of increased ischaemic risk (class IIa, level of evidence A)24. However, our prior CABG analysis adds to multiple other investigations among high ischaemic risk populations that demonstrate the benefits of potent P2Y12 monotherapy in patients at high thrombotic risk compared with standard DAPT252627. Overall, our study demonstrates that ticagrelor monotherapy may be an acceptable, effective alternative in prior CABG patients at high ischaemic risk.
Outcomes after PCI in prior CABG patients additionally differ based on the target vessel selection. Bypass graft PCI represents ~25% of interventions and has a particularly high thrombotic risk, with friable tissue present in venous grafts known to cause thrombosis and embolise after PCI, leading to worse outcomes and complications, such as slow-flow and no-reflow, compared with native vessel interventions628. Guidelines, therefore, recommend the bypassed native coronary vessel as the preferred intervention site with PCI, if accessible4. Indeed, 100% of included patients underwent at least 1 native coronary vessel intervention with 18.8% of such patients undergoing additional saphenous vein graft (SVG)-PCI, in alignment with contemporary practice. Extended DAPT therapy has been investigated after venous graft stents with favourable results, potentially decreasing ischaemic events compared with shorter standard durations of DAPT29. A prolonged DAPT duration, however, lacks both standardisation and prospective evaluation and is additionally associated with an increased bleeding risk30. This risk may be further amplified in the prior CABG population where multiple high-bleeding risk characteristics are common. The treatment effects of ticagrelor monotherapy among bleeding and ischaemic endpoints were consistent with the main TWILIGHT results when stratified by graft- and native-vessel interventions in our sensitivity analysis. Although hazard ratios among bleeding endpoints did not reach statistical significance, this is likely due to smaller sample sizes and lack of power within the target vessel groups, and a non-significant trend among bleeding endpoints was notably present. Overall, our study may justify potent ticagrelor monotherapy as a safe and effective alternative to consider in order to attenuate the thrombotic risk associated with graft PCI.
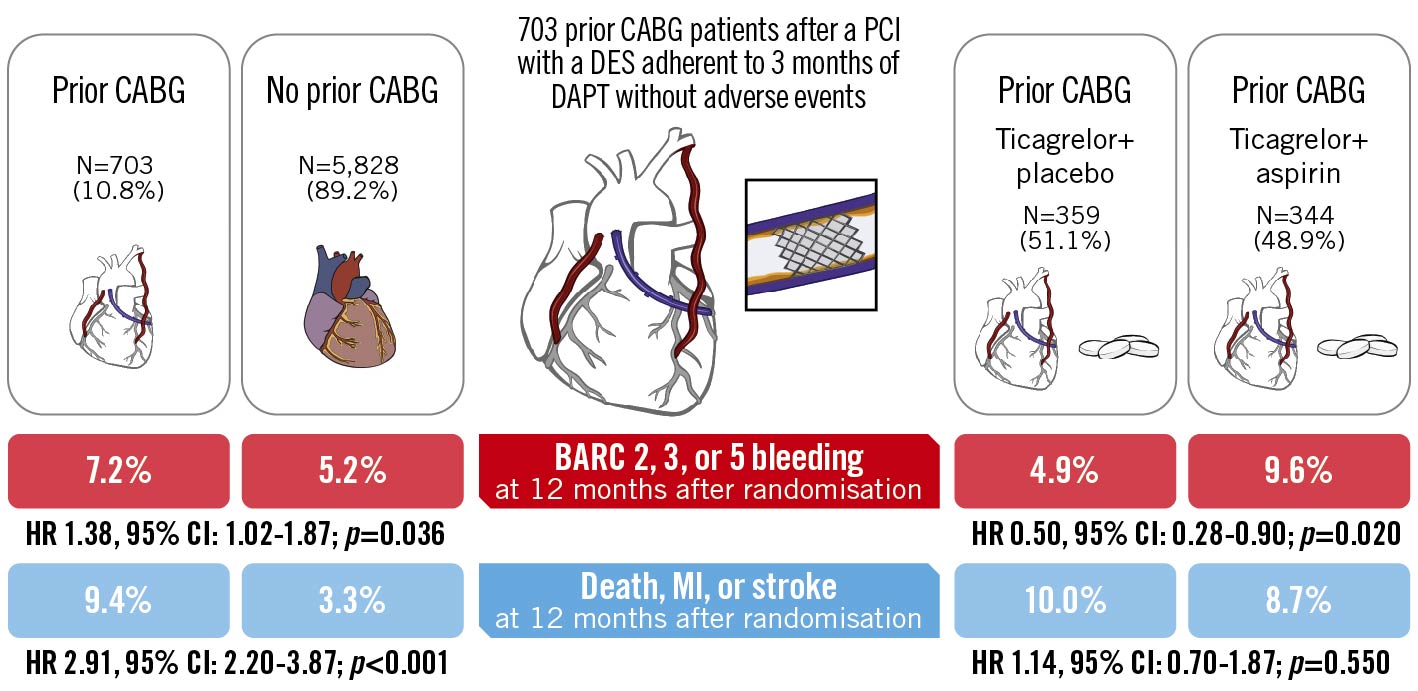
Central Illustration. Ticagrelor with or without aspirin after PCI in patients with a previous CABG surgery. Prior CABG patients who were event-free after 3 months after PCI with DES and adherent to DAPT with ticagrelor were randomised to ticagrelor plus placebo or ticagrelor plus aspirin. At 12 months after randomisation, prior CABG patients suffered significantly more bleeding and ischaemic events. However, patients receiving ticagrelor monotherapy, compared with ticagrelor plus aspirin, significantly reduced clinically relevant Bleeding Academic Research Consortium (BARC) type 2, 3, or 5 bleeding outcomes without increasing ischaemic events of all-cause death, MI, or stroke. CABG: coronary artery bypass graft; CI: confidence interval; DAPT: dual antiplatelet therapy; DES: drug-eluting stent(s); HR: hazard ratio; MI: myocardial infarction; PCI: percutaneous coronary intervention
Limitations
As a post hoc analysis, our study has some important limitations. Randomisation was not stratified by prior CABG status and multiplicity was not accounted for. The study is, furthermore, post hoc in nature and did not prospectively collect information on the time from prior CABG, number of prior CABG surgeries, patency of grafts at the time of PCI, or details on specific types of vein or arterial grafts. In addition, our analysis had a lower incidence of prior CABG patients compared to real-world US registries. Findings should, therefore, only be considered as hypothesis-generating and warrant further investigation. The study was underpowered to draw definitive conclusions regarding rare events such as stent thrombosis and stroke. Despite multivariable adjustment, significant differences in baseline characteristics within the prior CABG population may still have confounded the study results. Our study was also limited by the patient population of the TWILIGHT trial, which included predominantly white, male, high-risk patients that tolerated 3 months of DAPT without adverse events. The results do not apply to P2Y12 inhibitors other than ticagrelor, nor to patients excluded from the trial such as those presenting with ST-elevation MI and end-stage renal disease, both common in patients with a previous CABG. Finally, it is notable that no patients included underwent SVG-PCI alone, as a venous culprit conduit may serve as the only accessible occlusion during urgent PCI. Our results cannot be extrapolated to such situations, which should be further investigated.
Conclusions
Prior CABG patients suffer from higher bleeding and ischaemic complications after PCI compared with patients without a previous CABG. Ticagrelor monotherapy, however, reduced the risk of clinically relevant bleeding events after PCI without increasing ischaemic events compared with ticagrelor plus aspirin among high-risk patients that tolerated 3 months of DAPT, irrespective of prior CABG status. These findings support ticagrelor monotherapy as a safe bleeding-avoidance strategy that could be considered after PCI among patients with a previous CABG surgery.
Impact on daily practice
PCI is a common modality for secondary revascularisation in prior CABG patients but is associated with a higher rate of bleeding and thrombotic complications, compared with PCI in those without prior CABG. After PCI and an initial 3 months of DAPT, ticagrelor monotherapy significantly reduced bleeding in prior CABG patients compared with traditional DAPT without an increase in ischaemic events. Based on this post hoc analysis of the TWILIGHT trial, ticagrelor monotherapy may be a safe and effective antithrombotic strategy after PCI for high ischaemic risk patients with a previous CABG surgery.
Acknowledgements
The authors thank Marielle Mahan for her expert assistance with the Central illustration.
Funding
This research was conducted with support from AstraZeneca Pharmaceuticals LP.
Conflict of interest statement
D. Angiolillo has received payment for consulting fees and/or honorarium from Abbott, Amgen, Aralez, AstraZeneca, Bayer, Biosensors, Boehringer Ingelheim, Bristol Myers Squibb, Chiesi, Daiichi Sankyo, Eli Lilly, Haemonetics, Janssen, Merck, PhaseBio, PLx Pharma, Pfizer, Sanofi, and The Medicines Company; individual payments for participation in review activities from CeloNova and St. Jude Medical; and institutional payments for grants from Amgen, AstraZeneca, Bayer, Biosensors, CeloNova, CSL Behring, Daiichi Sankyo, Eisai, Eli Lilly, Gilead, Idorsia, Janssen, Matsutani Chemical Industry Co., Merck, Novartis, Osprey Medical, Renal Guard Solutions, and the Scott R. MacKenzie Foundation. G. Dangas reports institutional research grants from Abbott Laboratories, AstraZeneca, Bayer, Boston Scientific, and Daiichi Sankyo. U. Baber has received honoraria from Amgen and AstraZeneca. C. Briguori reports receiving a grant from the Icahn School of Medicine at Mount Sinai. D. Cohen reports receiving grants from Abbott Vascular, Boston Scientific, and Svelte; and consulting fees from Medtronic, Abbott Vascular, Boston Scientific, Svelte, MyoKardia, and Bristol Myers Squibb. J. Escaned reports receiving consulting fees and lecture fees from Abbott, Philips, and Boston Scientific; and lecture fees from Abiomed, Terumo, Medtronic, and Biosensors. C.M. Gibson has received grants from SCAD Alliance, Johnson & Johnson, Janssen, and CSL Behring; has received personal fees from Bayer, AstraZeneca (during the conduct of the study), Angel Medical Corporation, CSL Behring, Janssen, Johnson & Johnson, the Boston Clinical Research Institute, the Cardiovascular Research Foundation, Gilead Sciences, WebMD, UpToDate in Cardiovascular Medicine, Somahlution, Boston Scientific, the Duke Clinical Research Institute, Medtelligence, Microport, PERT Consortium, Caladrius Biosciences, CeleCor Therapeutics, Eidos Therapeutics, Microdrop, MD Magazine, the Society for Cardiovascular Angiography and Interventions, Revance Therapeutics, Pfizer, Dyad Medical, CytoSorbents, AMAG Pharmaceuticals, Bristol Myers Squibb, Amarin, PhaseBio, Bioclinica, Inari, MedImmune, PLx Pharma, Merck, Paratek, Anthos Therapeutics, PHRI, SmartMedics, EXCITE International ($0 Received), NovoNordisk, MedTrace, and the Cardiovascular Clinical Science Foundation; has received nonfinancial support from the Baim Institute; and has equity from nference, Absolutys, and Dyad Medical. K. Huber reports payment fees from AstraZeneca and Bayer. M. Krucoff reports grants and/or personal fees from Abbott Vascular, Biosensors, Boston Scientific, CeloNova, Medtronic, OrbusNeich, and Terumo. V. Kunadian has received personal fees/honoraria from Bayer, AstraZeneca, Abbott, Amgen, and Daiichi Sankyo. S. Marx reports research funding from the National Institutes of Health (not related to this work, payments made to Columbia University) and participates on a clinical event adjudications committee for Cardiovascular Research Foundation and the Mount Sinai School of Medicine. R. Mehran reports institutional research payments from Abbott, Abiomed, Alleviant Medical, AM-Pharma, Applied Therapeutics, Arena, AstraZeneca, BAIM, Bayer, Beth Israel Deaconess, Biosensors, Biotronik, Boston Scientific, Bristol Myers Squibb, CardiaWave, CellAegis, CeloNova, CERC, Chiesi, Concept Medical, CSL Behring, Cytosorbents, DSI, Duke University, Element Science, Faraday, Humacyte, Idorsia, Insel Gruppe AG, Magenta, Medtronic, Novartis, OrbusNeich, Philips, RenalPro, Vivasure, and Zoll; personal fees from CineMed Research, and WebMD; consulting fees paid to the institution from Abbott, Janssen, Medtronic, and Novartis; holds equity <1% in Applied Therapeutics, Elixir Medical, STEL, and CONTROLRAD (spouse); is on the Scientific Advisory Board for AMA, ACC (BOT Member), SCAI (Women in Innovations Committee Member); is an Associate Editor for JAMA; and is a faculty member at CRF (no fee). S. Mehta reports research grants from AstraZeneca and Boston Scientific. D. Moliterno reports grant support from AstraZeneca during the course of the study. He, furthermore, participated on a Data Safety Monitoring Board or Advisory Board DSMB for Janssen Pharmaceuticals. E.M. Ohman reports research grants from Chiesi and Abiomed; and consulting fees from Cytokinetics, Cara Therapeutics, Milestone, Otsuka, Pfizer, and XyloCor. G. Sardella reports receiving consulting fees from Abbott, Shockwave, Boston Scientific, and Balmed; and payment or honoraria for lectures, presentations, speaker’s bureau, manuscript writing or educational events from Abbott, Alvimedica, Shockwave, Medtronic, and Biosensors. S. Sharma has received speakers bureau fees from Abbott Vascular, Boston Scientific, and Cardiovascular Systems. P.G. Steg reports grants and personal fees from Amarin, Sanofi, Servier and Bayer; personal fees from Amgen, AstraZeneca, BMS, Boehringer Ingelheim, Idorsia, NovoNordisk, PhaseBio, Pfizer, and Novartis. In addition, he is the coinventor on a patent for the use of alirocumab to reduce risk after ACS (royalties to Sanofi). The other authors have no relationships relevant to the contents of this paper to disclose.
Supplementary data
To read the full content of this article, please download the PDF.

