Abstract
Aims: The aim of this study was to evaluate the impact of a novel antiplatelet regimen in patients with increasing total stent length (TSL).
Methods and results: This is a post hoc analysis of the GLOBAL LEADERS trial, a prospective, multicentre, open-label, randomised trial, investigating the impact of the experimental strategy (one-month dual antiplatelet therapy [DAPT] followed by 23-month ticagrelor monotherapy) versus the reference regimen (12-month DAPT followed by 12-month aspirin monotherapy) in patients with a Biolimus A9-eluting stent (BES). The primary endpoint was the composite of all-cause death and new Q-wave myocardial infarction (MI), and the secondary endpoint was Bleeding Academic Research Consortium (BARC) type 3 or 5 bleeding at two years. To investigate the association between total stent length and outcomes, groups were compared in quartiles according to TSL; the fourth quartile group was at significantly higher ischaemic risk at two years. In that stratum (TSL ≥46 mm), the experimental strategy significantly reduced the risk of the primary endpoint (hazard ratio [HR] 0.67, 95% confidence interval [CI]: 0.49-0.90; pinteraction=0.043), while demonstrating a similar risk of BARC type 3 or 5 bleeding (HR 0.99, 95% CI: 0.66-1.49; pinteraction=0.975).
Conclusions: Ticagrelor monotherapy could potentially balance ischaemic and bleeding risks, thereby achieving a net clinical benefit in patients with a TSL ≥46 mm with a BES.
Introduction
An increase in total lesion length or number of lesions treated results in the need for longer total stent length (TSL), which has been associated with an increased risk of major adverse cardiovascular events (MACE) after percutaneous coronary intervention (PCI) with bare metal stents (BMS)1. Whilst first-generation drug-eluting stents (DES) significantly reduced neointimal hyperplasia and subsequently improved clinical outcomes as compared with BMS, TSL still remained a significant predictor of target lesion revascularisation (TLR) and stent thrombosis (ST)2. Since the advent of the second-generation DES, clinical outcomes in patients with increasing TSL have improved significantly, and TSL is no longer associated with a higher risk of ST3,4,5,6,7.
To date, data on the effect of different antiplatelet regimens in patients who received longer stents are limited. A prior pooled patient-level analysis from six randomised controlled trials (RCT) has demonstrated that, compared with an abbreviated dual antiplatelet therapy (DAPT) regimen (three or six months), prolonged (>12 months) DAPT significantly reduced MACE (a composite of cardiac death, myocardial infarction [MI], and definite or probable ST) in patients who underwent complex PCI, where one of the criteria was a TSL >60 mm8. However, an increased risk of bleeding according to Bleeding Academic Research Consortium (BARC) definition (type 3 or 5) was documented8. Given that bleeding is associated with impaired quality of life, morbidity, and mortality, so-called “aspirin-free” strategies (an abbreviated DAPT followed by potent P2Y12 monotherapy) have recently been proposed, aiming to reduce an excess of bleeding risk mainly related to the addition of aspirin while maintaining a potent anti-ischaemic efficacy9,10. Two recent RCT, STOPDAPT-2 and SMART-CHOICE, showed that, compared to 12-month DAPT, one- or three-month DAPT followed by P2Y12 inhibitor monotherapy was superior for bleeding and non-inferior for the composite ischaemic endpoint at one-year follow-up. The aim of this study is to evaluate the impact of one-month DAPT followed by 23-month ticagrelor monotherapy versus 12-month DAPT followed by 12-month aspirin monotherapy on two-year clinical outcomes in patients with increasing TSL.
Methods
STUDY DESIGN
This study is a post hoc analysis of the GLOBAL LEADERS trial, a prospective, multicentre, open-label, RCT (NCT01813435). Details of the study design and protocol have been reported previously11. In summary, the trial randomised patients undergoing PCI by default with BES (BioMatrix™; Biosensors, Morges, Switzerland) in a 1:1 ratio to either (i) the experimental strategy consisting of one-month DAPT (aspirin and ticagrelor) followed by 23-month ticagrelor monotherapy, or (ii) the reference regimen consisting of 12-month DAPT (aspirin and either ticagrelor for acute coronary syndrome [ACS] or clopidogrel for stable coronary artery disease [CAD]) followed by 12-month aspirin monotherapy.
The trial was approved by the institutional review board at each centre and followed the ethical principles of the Declaration of Helsinki. All the patients provided written informed consent prior to participation in the trial.
TOTAL STENT LENGTH
In the present analysis, nominal stent length is used to calculate TSL as per patient. During the trial, available stent diameters were 2.25 to 4.0 mm with a stent length of 8, 11, 14, 18, 24, 28, 33, and 36 mm. To evaluate the association between stent length and outcomes, groups are compared in quartiles according to a given TSL (quartile 1: 8 to 17 mm; quartile 2: 18 to 27 mm; quartile 3: 28 to 45 mm; quartile 4: ≥46 mm), as in previous studies2,12. Given suboptimal outcomes in patients with increasing TSL, a longer and more potent antiplatelet regimen may be useful13. Thus, clinical outcomes are further assessed to determine whether the experimental strategy could improve outcomes in patients with long stenting as compared with the reference regimen.
STUDY ENDPOINTS
The primary endpoint was the composite of all-cause death or new Q-wave MI at two years. Deaths from any cause were ascertained without adjudication. Q-wave MI was centrally adjudicated and defined in compliance with the Minnesota classification (new major Q-QS wave abnormalities) or by the appearance of a new left bundle branch block in conjunction with abnormal biomarkers. The key secondary endpoint was bleeding according to the BARC criteria (type 3 or 5) up to two years. Other secondary endpoints included individual components of the primary endpoint, any stroke, any MI, any revascularisation, and definite ST.
In addition, patient-oriented cardiovascular events (POCE) and net adverse clinical events (NACE) were explored up to two years according to the Academic Research Consortium (ARC)-2 definition. POCE is the composite of all-cause death, any stroke (ischaemic and haemorrhagic), any MI (periprocedural or spontaneous with ST-elevation MI [STEMI] or non-ST-elevation MI [NSTEMI]), and any revascularisation (repeat PCI or coronary artery bypass grafting [CABG] surgery in target or non-target vessel). The third universal definition of MI was the recommended criterion to report MI. NACE is the composite of POCE and BARC type 3 or 5 bleeding. Composite endpoints were analysed hierarchically. Individual components of the composite endpoints as well as definite ST according to Academic Research Consortium (ARC) definition were reported non-hierarchically. The endpoints were site-reported with the exception of the primary endpoint, all-cause death and new Q-wave MI, which was assessed by an independent electrocardiogram (ECG) core lab.
STATISTICAL ANALYSIS
The analyses were performed according to the intention-to-treat principle. The cumulative incidence of clinical events during two-year follow-up was calculated using the Kaplan-Meier method and compared using the log-rank test. Hazard ratios (HR) with 95% confidence intervals (CI) were estimated using an unadjusted Cox regression model. The treatment effect of the experimental versus the reference strategy between the subgroups was estimated using an unadjusted Cox regression model. To confirm whether the treatment effect of the experimental strategy versus the reference regimen was significantly modified according to the longer TSL, we performed a multivariate analysis based on a Cox proportional hazards regression model for the primary endpoint through the inclusion of randomised treatment-by-TSL ≥46 mm interaction term as well as the traditional covariates - age, hypertension, diabetes, current smoker, previous MI, previous PCI, and clinical presentation (ACS versus stable CAD)12.
In addition, the one-year landmark analysis was reported using the pre-specified time point of one year (at the time of the planned cessation of a P2Y12 inhibitor in the reference strategy). The pre-specified stratified analysis according to clinical presentation (stable CAD or ACS) was performed since a different P2Y12 inhibitor in the reference group was used according to clinical presentation (i.e., clopidogrel for stable CAD or ticagrelor for ACS)11.
Continuous variables were reported as mean±standard deviation (SD) or median and interquartile range (IQR) and were compared using the Student’s t-test or Mann-Whitney U test. Categorical variables were reported as percentages and numbers and were compared using the chi-square test or Fisher’s exact test as appropriate. No adjustment was performed for multiple testing due to the post hoc nature of the analysis14. All tests were two-sided and a p-value of <0.05 was considered to be statistically significant. All statistical analyses were performed using SPSS Statistics, Version 25 (IBM Corp., Armonk, NY, USA).
Results
PATIENTS
Between July 2013 and November 2015, at 130 hospitals in 18 countries (Europe, Asia, Brazil, Australia and Canada), the GLOBAL LEADERS trial randomised a total of 15,991 patients, of whom 15,450 (96.6%) were included in this analysis. This cohort was subsequently divided into quartiles according to TSL per patient (Figure 1, Figure 2). The cumulative frequency of TSL is presented in Supplementary Figure 1.
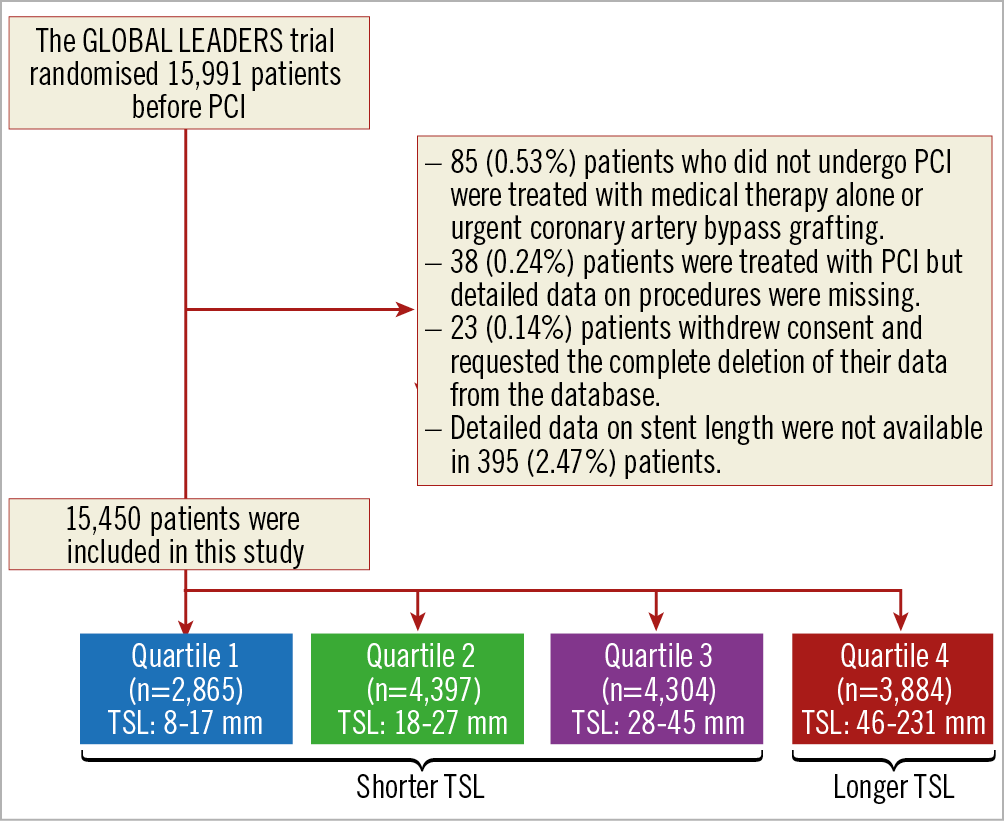
Figure 1. Patient flow diagram of the present study. PCI: percutaneous coronary intervention; TSL: total stent length
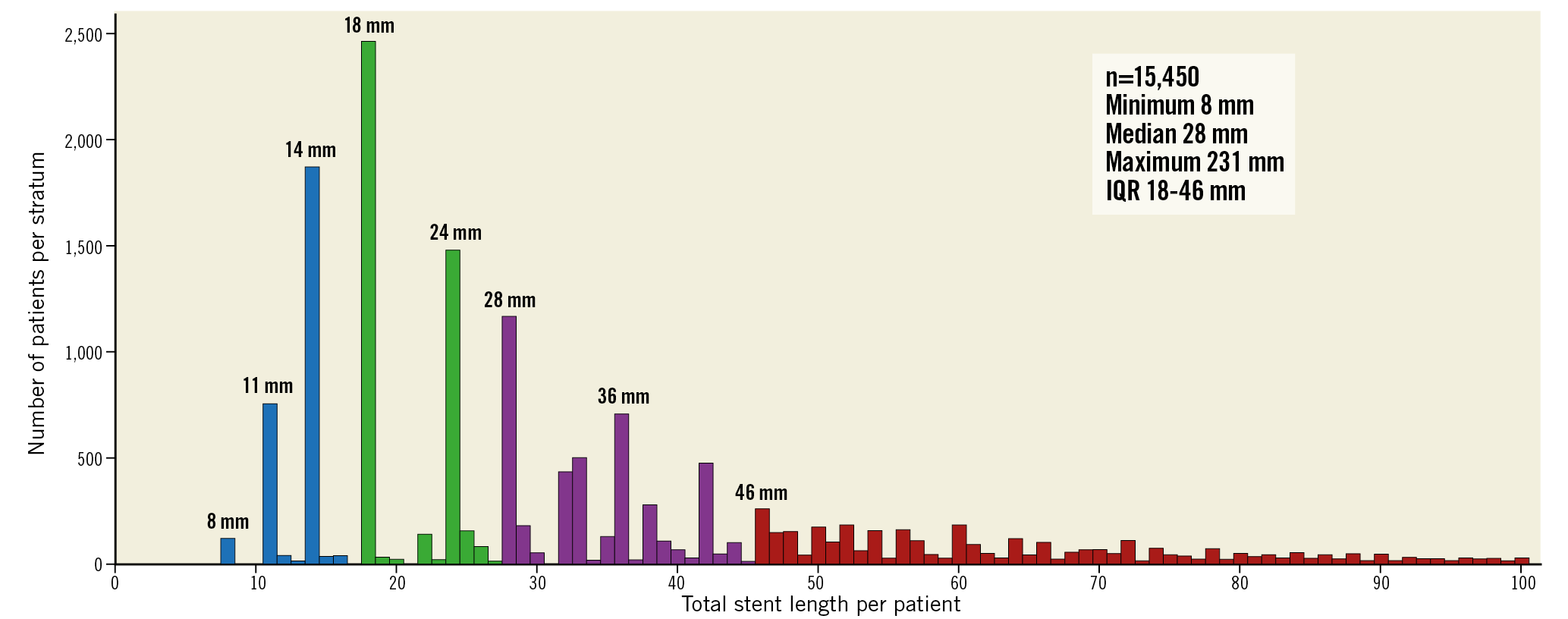
Figure 2. Distribution of total stent length per patient. The distribution of the total stent length depends on the available nominal stent lengths of BioMatrix stent (8, 11, 14, 18, 24, 28, 33, and 36 mm). Colours indicate the quartile 1 (blue): 8 to 17 mm; quartile 2 (green): 18 to 27 mm; quartile 3 (purple): 28 to 45 mm; and quartile 4 (red): ≥46 mm. Data are not shown in patients with total stent length >100 mm.
BASELINE CHARACTERISTICS AND CLINICAL OUTCOMES ACCORDING TO TSL
Baseline characteristics according to quartile are presented in Supplementary Table 1. Patients in quartile 4 were more likely to be male and had a higher prevalence of diabetes and hypercholesterolaemia and a lower prevalence of previous PCI. In terms of angiographic variables, patients in this group were more likely to receive multivessel PCI and less likely to undergo direct stenting. They also had a greater number of treated lesions with a higher prevalence of bifurcation and a greater number of stents implanted, which resulted in a greater TSL per patient.
Two-year clinical outcomes according to quartile are presented in Figure 3 and Supplementary Table 2. There was a non-significant higher risk of the primary endpoint according to quartile (log-rank p=0.073). Increasing TSL resulted in a higher risk of POCE (log-rank p<0.001). The risk of BARC type 3 or 5 bleeding was numerically higher according to quartile (log-rank p=0.077).
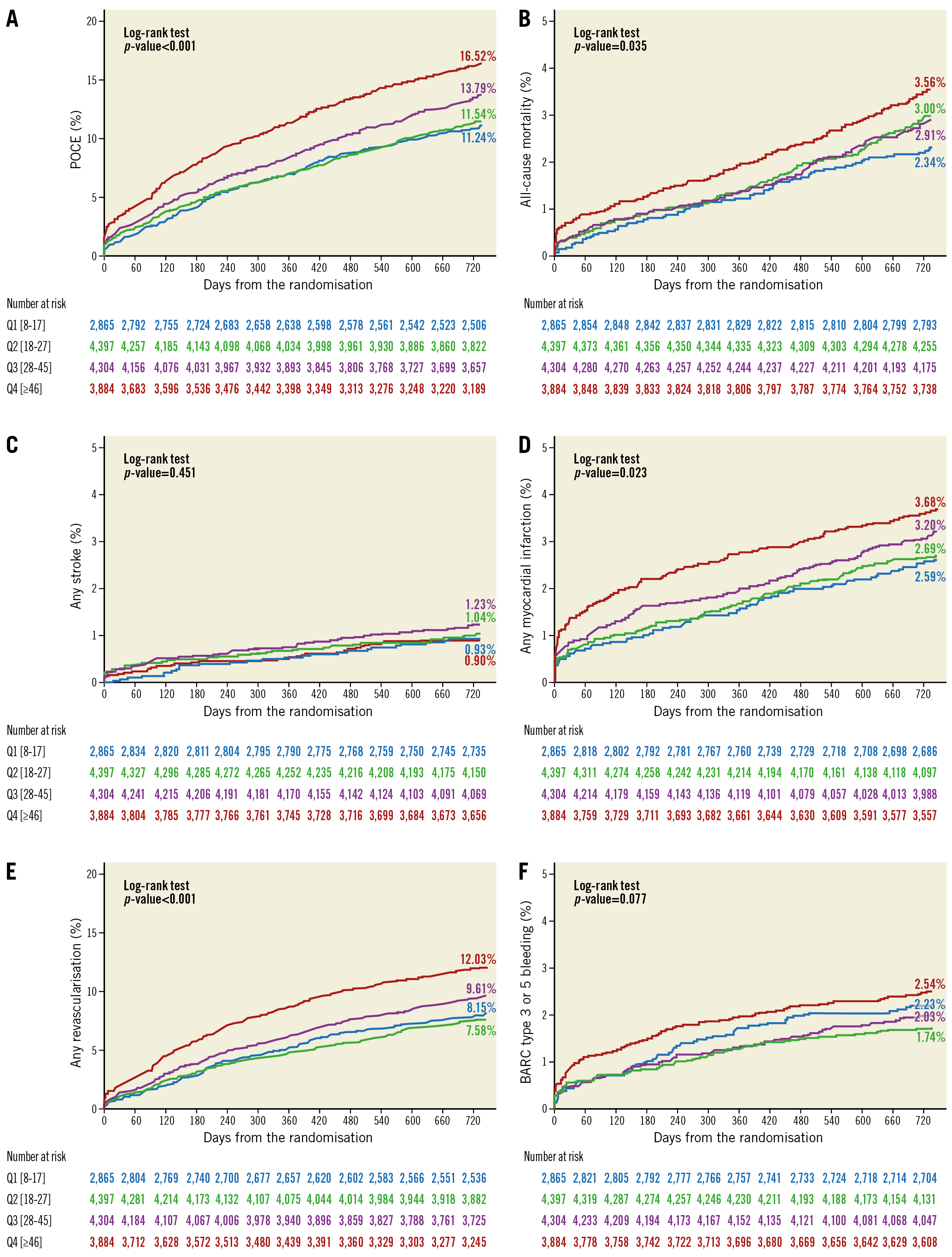
Figure 3. Clinical outcomes in quartiles according to TSL. A) POCE. B) All-cause mortality. C) Any stroke. D) Any MI. E) Any revascularisation. F) BARC type 3 or 5 bleeding.
IMPACT OF THE EXPERIMENTAL STRATEGY IN PATIENTS WITH LONGER TSL
Baseline characteristics stratified according to antiplatelet regimen in patients with longer TSL (defined as a TSL ≥46 mm) are presented in Table 1. All baseline characteristics with the exception of diabetes and the type of stent implanted were statistically similar between groups.
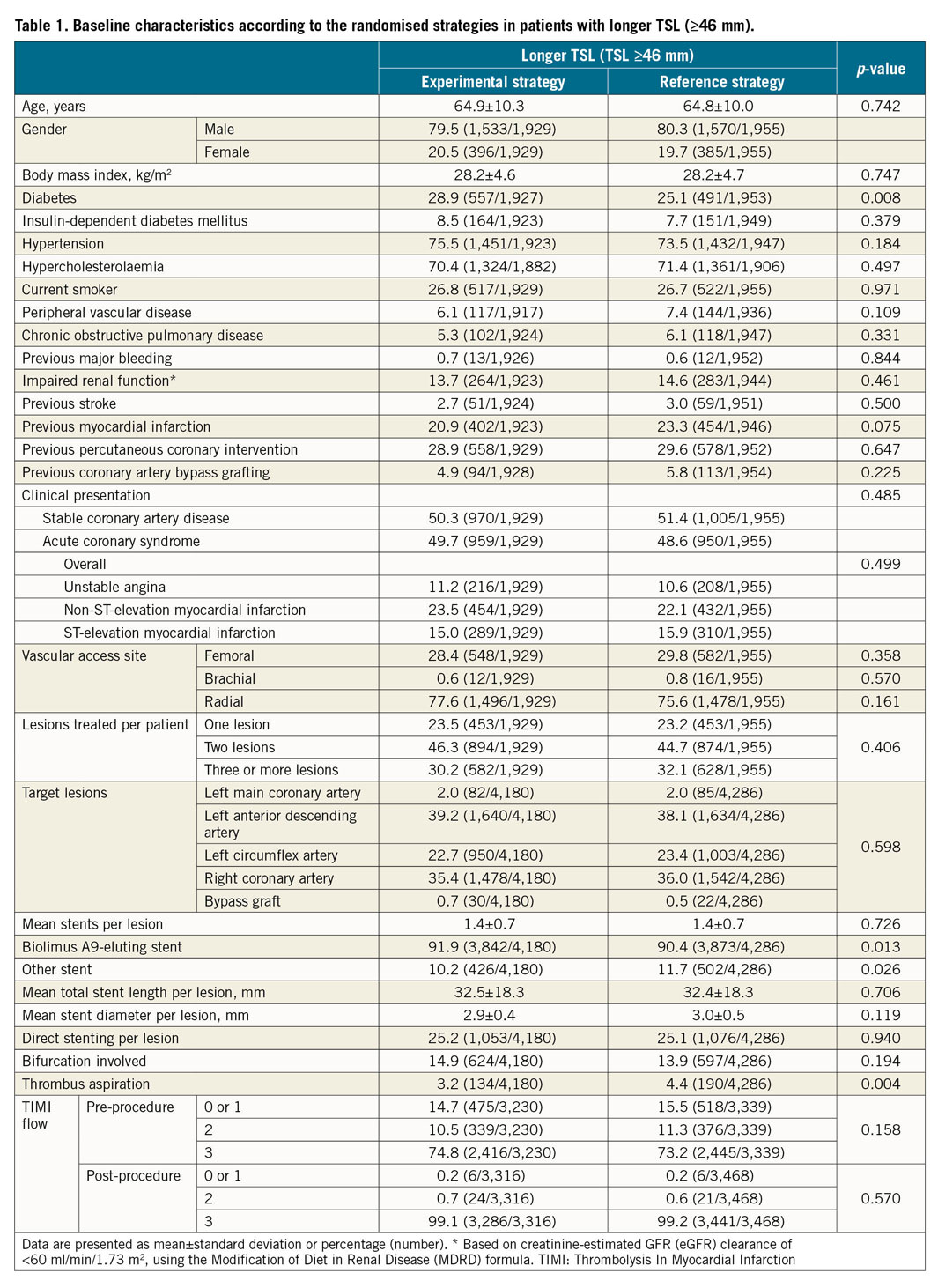
Two-year efficacy and safety outcomes according to the randomised treatment allocation in patients with longer TSL are presented in Figure 4 and Supplementary Table 3. The treatment effect of the experimental strategy versus the reference regimen is presented in Figure 5. The experimental strategy led to a significantly reduced risk of the primary endpoint (3.79% vs 5.68%, HR 0.66, 95% CI: 0.49-0.89; p=0.006, pinteraction=0.043) in favour of the longer TSL group. In addition, the experimental treatment had a significant risk reduction in POCE (14.75% vs 18.26%; HR 0.79, 95% CI: 0.67-0.92; p=0.003, pinteraction=0.017) in patients with TSL ≥46 mm. The risk of BARC type 3 or 5 bleeding was statistically similar between the two regimens (2.53% vs 2.55%; HR 0.99, 95% CI: 0.66-1.49; p=0.963, pinteraction=0.975), resulting in a significantly reduced risk of NACE (16.25% vs 19.80%; HR 0.80, 95% CI: 0.69-0.93; p=0.004, pinteraction=0.025) in patients with longer TSL.
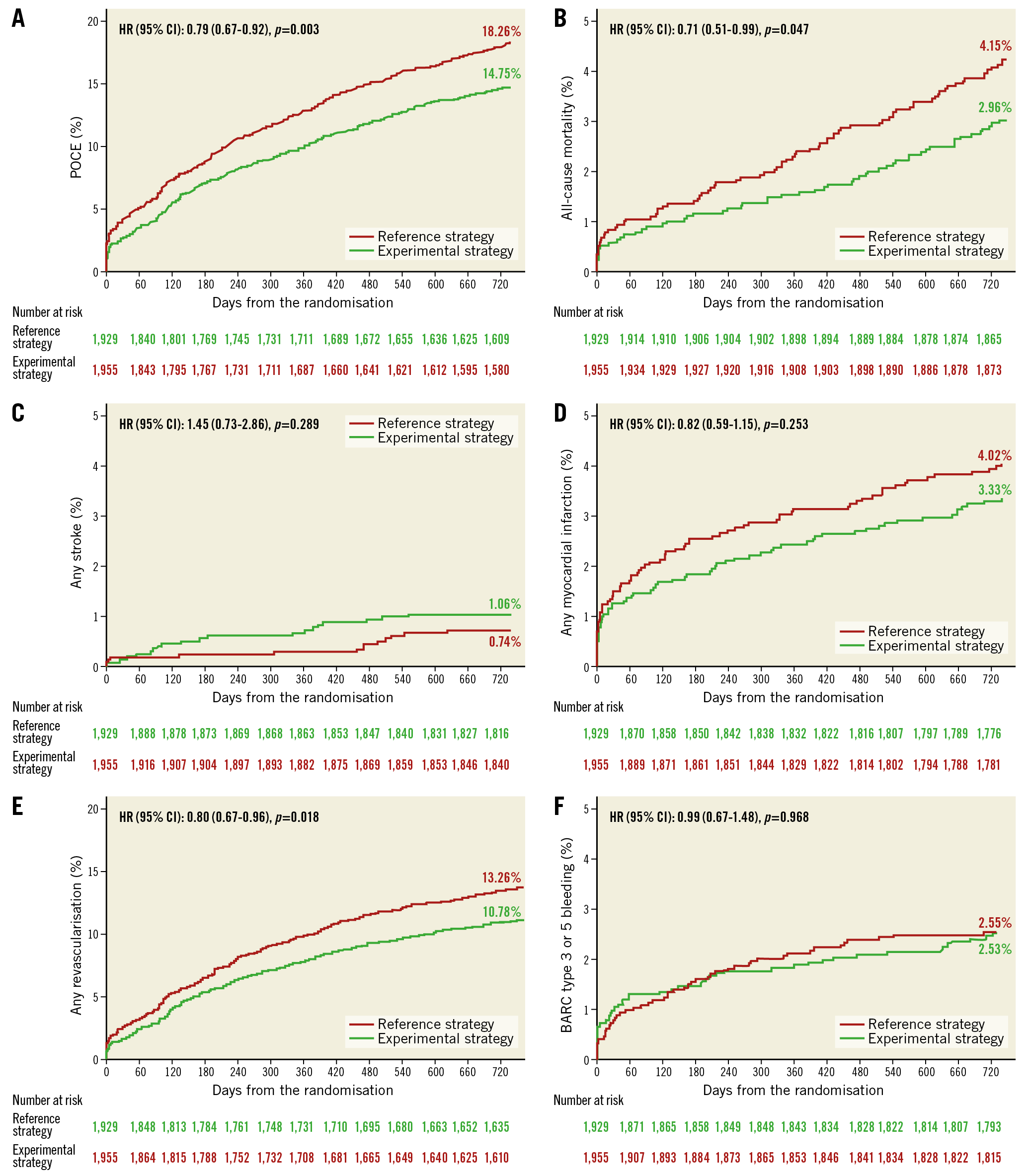
Figure 4. Clinical outcomes of the experimental strategy versus the reference regimen in patients with longer TSL. A) POCE. B) All-cause mortality. C) Any stroke. D) Any MI. E) Any revascularisation. F) BARC type 3 or 5 bleeding.
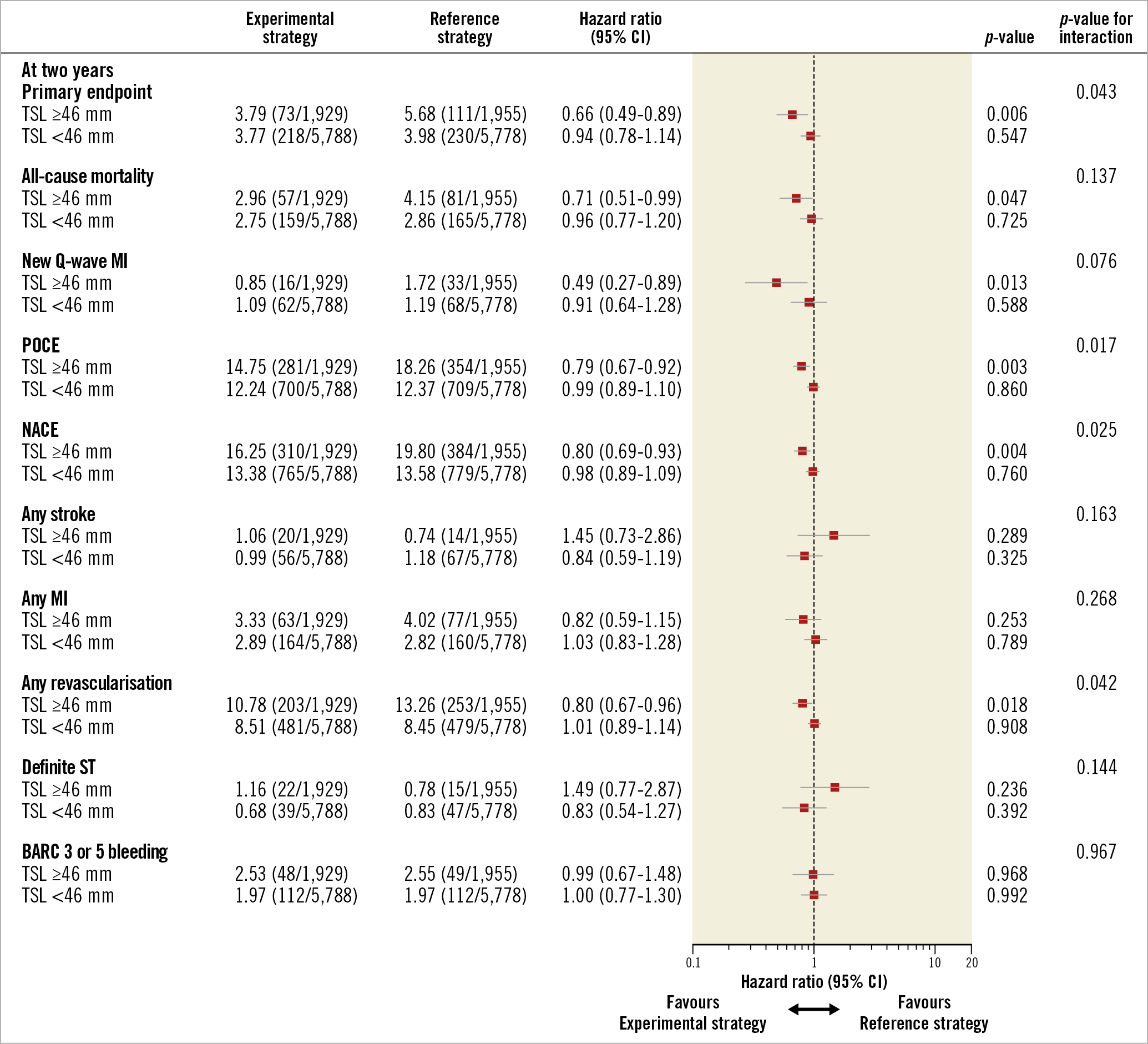
Figure 5. The treatment effect of the experimental strategy versus the reference regimen stratified by TSL. The favourable treatment effect of the experimental strategy was observed in terms of POCE, NACE, and any revascularisation at two years in favour of patients with TSL ≥46 mm.
The multivariable analysis confirmed that there was a significant interaction of the experimental strategy versus the reference regimen according to TSL ≥46 mm in terms of the primary endpoint (pinteraction=0.047).
Based on a landmark analysis at one year, ticagrelor monotherapy, when compared with aspirin monotherapy, had no incremental benefit with respect to any ischaemic and bleeding endpoints in the second year (Supplementary Table 3).
STRATIFIED ANALYSIS ACCORDING TO CLINICAL PRESENTATION
In stable CAD patients with longer TSL, the experimental treatment had a numerically lower risk of the primary endpoint (3.92% vs 5.48%, HR 0.71, 95% CI: 0.47-1.07, p=0.103, pinteraction=0.342) and a significant risk reduction in POCE (14.64% vs 18.73%; HR 0.76, 95% CI: 0.61-0.95; p=0.014, pinteraction=0.056). However, its anti-ischaemic efficacy was achieved at the expense of a numerically higher risk of BARC type 3 or 5 bleeding (2.63% vs 1.52%; HR 1.74, 95% CI: 0.92-3.31; p=0.088, pinteraction=0.360) (Supplementary Table 4).
Conversely, in ACS patients with longer TSL, the experimental treatment had a significantly lower risk of the primary endpoint (3.66% vs 5.90%, HR 0.61, 95% CI: 0.40-0.93, p=0.023, pinteraction=0.055) and a numerically lower risk of POCE (14.86% vs 17.75%; HR 0.82, 95% CI: 0.66-1.03; p=0.083, pinteraction=0.143). The risk of BARC type 3 or 5 bleeding was numerically lower in the experimental strategy (2.43% vs 3.67%; HR 0.66, 95% CI: 0.39-1.12; p=0.127, pinteraction=0.554), which led to a significantly lower risk of NACE (16.32% vs 19.97%; HR 0.80, 95% CI: 0.64-0.98; p=0.036, pinteraction=0.131) (Supplementary Table 5).
Discussion
The main findings of this study can be summarised as follows.
(1) There was a non-significant higher risk of the primary endpoint according to quartile, whereas increasing TSL resulted in a greater risk of POCE, which was driven by all-cause death, any MI, and any revascularisation.
(2) In patients with a TSL ≥46 mm, the experimental strategy with ticagrelor monotherapy, when compared to the reference regimen, significantly reduced the risk of the primary endpoint as well as POCE with a similar risk of BARC type 3 or 5 bleeding, thereby achieving a significant net clinical benefit at two years. The benefits with the experimental strategy were largely confined to the first year of treatment and ACS patients who underwent long stenting.
Numerous RCT and large registries have shown that newer generations of DES have significantly reduced the risk of restenosis and the need for repeat revascularisation. Nevertheless, even with a second-generation DES, post-stenting reference segment plaque burden has been associated with edge restenosis. Hence, the preferred strategy is full coverage of atherosclerotic lesions, resulting in stents with longer lengths. As of today, there have been a few studies investigating the effect of increasing TSL with a second-generation DES on clinical outcomes3,4,5,6,7. Three studies have reported that longer stent lengths were no longer associated with a significant increase in MACE, TLR, and ST in patients who received a second-generation DES3,4,5. However, these studies had relatively small to medium sample sizes (n=730, 1,181 and 2,111, respectively) compared to the present study which included the largest cohort (n=15,450). In the present analysis, the longer TSL group had a significantly higher risk of ARC-2-defined POCE, driven by all-cause death, any MI, and any revascularisation (Figure 3). The pathophysiological foundation for this association may reside in the following facts. First, longer and more stents implanted may increase the likelihood of stent size mismatch, stent underexpansion, malapposition, and overlapping, all of which may lead to incomplete endothelialisation and an enhancement of the stent-related ischaemic risk15. Second, patients who require more and longer stents represent a more advanced state of CAD, which often results in incomplete revascularisation with a subsequently increased risk of recurrent thrombotic events and mortality. Third, patients who undergo long stenting tend to have more cardiovascular and non-cardiovascular comorbidities with a greater probability of natural plaque progression followed by thrombotic events (i.e., non-stent-related ischaemic risk)8.
This limited efficacy of PCI in patients who required a longer TSL reaffirms the need for a dedicated Heart Team approach regarding the best revascularisation modality, either PCI or CABG. Specifically, the risk of repeat revascularisation and recurrent MI should be weighed against the risk of stroke. Once PCI is considered as the preferred revascularisation strategy, every possible effort should be made to achieve optimal outcomes. One potential strategy in patients with long lesions is intravascular ultrasound-guided PCI16. Moreover, when patients receive longer stents, irrespective of the type of DES, optimal medical therapy for secondary prevention remains of paramount importance.
Currently, the ESC guidelines support a personalised approach regarding antiplatelet therapy after PCI13. In particular, prolonged (>12 months) DAPT may be a preferred strategy in patients with stent-driven ischaemic risks such as long stenting13. However, due to the systemic effect of an antiplatelet therapy, this anti-ischaemic efficacy is achieved at the expense of a significantly higher risk of bleeding8, suggesting that an optimal antiplatelet regimen that can balance ischaemic and bleeding risk is warranted in this high ischaemic risk population. In the present study, the experimental strategy demonstrated a more potent anti-ischaemic efficacy without a trade-off in the risk of major bleeding in patients with long stenting. This negative result of bleeding was the amalgam between patients with stable CAD and ACS treated with different types of antiplatelet regimen. Specifically, in the experimental treatment group, patients with ACS had a state of high platelet reactivity17, which could be neutralised by a single potent antiplatelet inhibition with ticagrelor even in the absence of aspirin, whereas in patients with stable CAD, in whom the platelet reactivity was assumed to be normal17, the use of ticagrelor as monotherapy could lower the level of homeostasis excessively, leading to a borderline excess of bleeding. Conversely, in the reference treatment group, ACS patients received a combination of ticagrelor and aspirin, and thereby platelet reactivity was certainly normalised, while in stable patients receiving a less potent antiplatelet therapy, namely clopidogrel, the risk of bleeding was not excessive even in conjunction with aspirin. Thus, the real benefit of ticagrelor monotherapy was confined to ACS patients with long stenting.
Limitations
The present results need to be interpreted bearing in mind the following limitations. First, this substudy was not predefined in the protocol of the trial. Together with the inherent limitations of sub-analyses including multiple testing14, the study findings should be considered as hypothesis-generating only and call for confirmatory randomised trials. Second, we did not collect the anatomic SYNTAX score in the whole population, precluding outcome assessment stratified according to the SYNTAX score. Third, data on overlapping were not available in our data set. However, a previous study has reported that overlap of second-generation DES was no longer associated with a higher risk of ischaemic events as compared to first-generation DES18. Fourth, secondary endpoints were site-reported, since the trial did not have a clinical adjudication committee for serious adverse events. However, seven on-site monitoring visits were performed in each participating centre, and 20% of reported events were checked according to source documents. In addition, the trial was monitored for event under-reporting and event definition consistency.
Conclusions
Patients with long stenting (defined as a TSL ≥46 mm) were associated with an increased risk of ischaemic events. In these patients, compared to the standard of care, one-month DAPT followed by 23-month ticagrelor monotherapy resulted in a significant reduction in the primary endpoint and POCE with a similar risk of BARC type 3 or 5 bleeding, thereby maximising a significant net clinical benefit at two years. The real benefits of the experimental strategy seem to be related to ACS patients with long stenting.
|
Impact on daily practice The present study included the largest cohort (n=15,450) treated by default with Biolimus A9-eluting stents. When patients were divided into quartiles according to TSL per patient, the fourth quartile group (TSL ≥46 mm) had a significantly higher risk of POCE, predominantly driven by all-cause death, any MI, and any revascularisation. In that stratum (TSL ≥46 mm), the experimental strategy, when compared to the reference regimen, had a significantly reduced risk of the primary endpoint as well as POCE without trade-off in the risk of BARC type 3 or 5 bleeding, thereby achieving a significantly lower risk of NACE at two years. These significant benefits were mainly confined to the first year of the treatment and to ACS patients. |
Guest Editor
This paper was guest edited by Alec Vahanian, MD, PhD; Department of Cardiology, Hôpital Bichat-Claude Bernard, and University Paris VII, Paris, France.
Funding
The GLOBAL LEADERS trial was supported by resources from AstraZeneca, Biosensors, and The Medicines Company.
Conflict of interest statement
R. Modolo reports research grants from the Sao Paulo Research Foundation (FAPESP grant number 2017/22013-8) and Biosensors. L. Holmvang reports being a local primary investigator in the GLOBAL LEADERS trial and institutional research contracts with AstraZeneca, MicroPort and Biosensors. M. Haude reports institutional grants/research support from Abbott and Biotronik, and honoraria or consultation fees from Biotronik, Cardiac Dimensions, OrbusNeich, and Philips. C. Hamm reports personal fees from AstraZeneca. H.P. Stoll is a full-time employee of Biosensors International. J.G.P. Tijssen reports personal fees from Cardialysis, for DSMB membership of the GLOBAL LEADERS trial during the conduct of the study. R.J. de Winter reports grants from AstraZeneca. M. Valgimigli reports grants and personal fees from Abbott, AstraZeneca and Terumo, and personal fees from Chiesi, Bayer, Daiichi Sankyo, Amgen, Alvimedica, Biosensors and Idorsia, and grants from Medicure, outside the submitted work. P. Vranckx reports personal fees from AstraZeneca, The Medicines Company, Bayer Health Care, Terumo, Daiichi Sankyo and CSL Behring. S. Windecker reports research and educational grants to the institution from Abbott, Amgen, BMS, Bayer, Boston Scientific, Biotronik, Cardinal Health, CardioValve, CSL Behring, Daiichi Sankyo, Edwards Lifesciences, Johnson & Johnson, Medtronic, Querbet, Polares, Sanofi, Terumo, Sinomed. He serves as unpaid member of the steering/executive group of trials funded by Abbott, Abiomed, Amgen, BMS, Boston Scientific, Biotronik, Cardiovalve, Edwards Lifesciences, MedAlliance, Medtronic, Polares, Sinomed, V-Wave and Xeltis, but has not received personal payments by any pharmaceutical company or device manufacturer. He is also member of the steering/executive committee group of several investigator-initiated trials that receive funding by industry without impact on his personal remuneration. G. Steg reports grants and personal fees from Bayer/Janssen, Merck, Sanofi, and Amarin, personal fees from Amgen, Bristol Myers Squibb, Boehringer-Ingelheim, Pfizer, Novartis, Regeneron, Lilly, and AstraZeneca, and grants and personal fees from Servier, outside the submitted work. P.W. Serruys reports personal fees from Biosensors, Micel Technologies, Sinomedical Sciences Technology, Philips/Volcano, Xeltis, and HeartFlow, outside the submitted work. The other authors have no conflicts of interest to declare. The Guest Editor is a consultant for Edwards Lifesciences.
Supplementary data
To read the full content of this article, please download the PDF.

