Abstract
Despite the use of conventional dual antiplatelet therapy (DAPT) after percutaneous coronary intervention (PCI), the risk of adverse events remains high among patients with increased thrombotic risk. Until recently, the optimal antiplatelet strategy to balance the ischaemic and bleeding risks in patients who are undergoing complex high-risk PCI has been unclear. The TAILored Versus COnventional AntithRombotic StratEgy IntenDed for Complex HIgh-Risk PCI (TAILORED-CHIP) trial is an investigator-initiated, multicentre, prospective randomised trial to evaluate the efficacy and safety of a time-dependent tailored antiplatelet therapy with an early (<6 months post-PCI) escalation (low-dose ticagrelor at 60 mg twice daily plus aspirin) and a late (>6 months post-PCI) de-escalation (clopidogrel monotherapy) in patients undergoing complex high-risk PCI as compared with standard DAPT (clopidogrel plus aspirin for 12 months). Eligible patients had to have at least one high-risk anatomical or procedural feature or clinical characteristic associated with an increased risk of ischaemic or thrombotic events. The primary endpoint was the net clinical outcome, a composite of death from any cause, myocardial infarction, stroke, stent thrombosis, urgent revascularisation, or clinically relevant bleeding (Bleeding Academic Research Consortium type 2, 3, or 5) at 12 months after randomisation. (ClinicalTrials.gov: NCT03465644)
The current guidelines recommend dual antiplatelet therapy (DAPT) including aspirin and a platelet receptor P2Y12 inhibitor as the standard of care for the prevention of atherothrombotic events in patients who have undergone percutaneous coronary intervention (PCI)12. In these guidelines, according to the clinical presentation and concomitant bleeding risk, different potencies and durations of DAPT are recommended.
In routine clinical practice, there are common clinical circumstances in which physicians are particularly concerned about the risk of thrombotic events, bleeding events, or both; therefore, the recommended potency or duration of DAPT after PCI or acute coronary syndromes (ACS) is still a moving target that is going beyond the traditional approach. In particular, complex high-risk PCI (complex high-risk and indicated PCI [CHIP] procedure) is rapidly increasing in contemporary PCI practice. Even with DAPT, the risk of adverse events remains unacceptably high among patients with increased thrombotic risk due to various anatomical features (e.g., complex and CHIP procedures involving the left main, multivessel PCI, complex bifurcation, diffuse long lesions, chronic total occlusion, and severely calcified lesions) and clinical factors (e.g., diabetes mellitus, chronic renal insufficiency, or depressed ventricular function)3456. However, the optimal antiplatelet strategy for such high-risk patients undergoing complex PCI is still undetermined.
It is well known that the risk of thrombotic or bleeding events after an ACS or PCI may substantially differ over time78; in general, thrombotic risk is higher in the early phase, but bleeding risk is higher in the late phase (Figure 1). In this context, temporal-dependent antiplatelet modulation (i.e., early escalation and late de-escalation) in high-risk patients who are undergoing a CHIP-PCI procedure could facilitate an ischaemic benefit in the early period and lower the risk of bleeding in the late period while preserving the ischaemic benefit. Therefore, the TAILored Versus COnventional AntithRombotic StratEgy IntenDed for Complex HIgh-Risk PCI (TAILORED-CHIP) trial will assess the potential benefit of temporal modulation (early escalation and late de-escalation) of antiplatelet therapy in patients undergoing complex high-risk PCI.
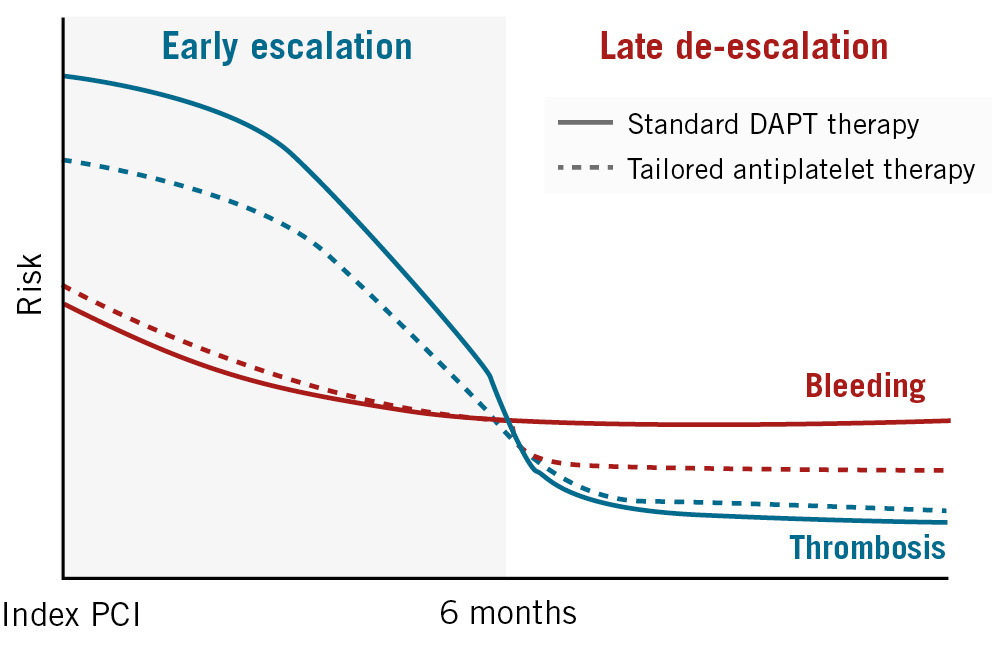
Figure 1. Risks of ischaemic and bleeding events after complex high-risk PCI. In the early period after a complex high-risk PCI, the benefits of intensive antiplatelet therapy generally outweigh the increased risk of bleeding. However, this benefit dissipates with additional time after such complex PCI procedures, favouring a therapeutic approach that considers the risks of both bleeding and ischaemic events. DAPT: dual antiplatelet therapy; PCI: percutaneous coronary intervention
Methods
Trial design
The TAILORED-CHIP trial (ClinicalTrials.gov: NCT03465644) is an investigator-initiated, multicentre, open-labelled, randoÂmised superiority trial. The primary objective of this trial is to evaluate the efficacy and safety of a tailored antiplatelet therapy with an early (<6 months post-PCI) escalation (low-dose ticagrelor [60 mg twice daily] plus aspirin [100 mg once daily]) and late (>6 months post-PCI) de-escalation (clopidogrel [75 mg once daily] monotherapy) strategy compared with standard DAPT (clopidogrel [75 mg once daily] plus aspirin [100 mg once daily] for 12 months) in patients undergoing complex high-risk PCI (Figure 2). Detailed information on ethics approval is provided in Supplementary Appendix 1.
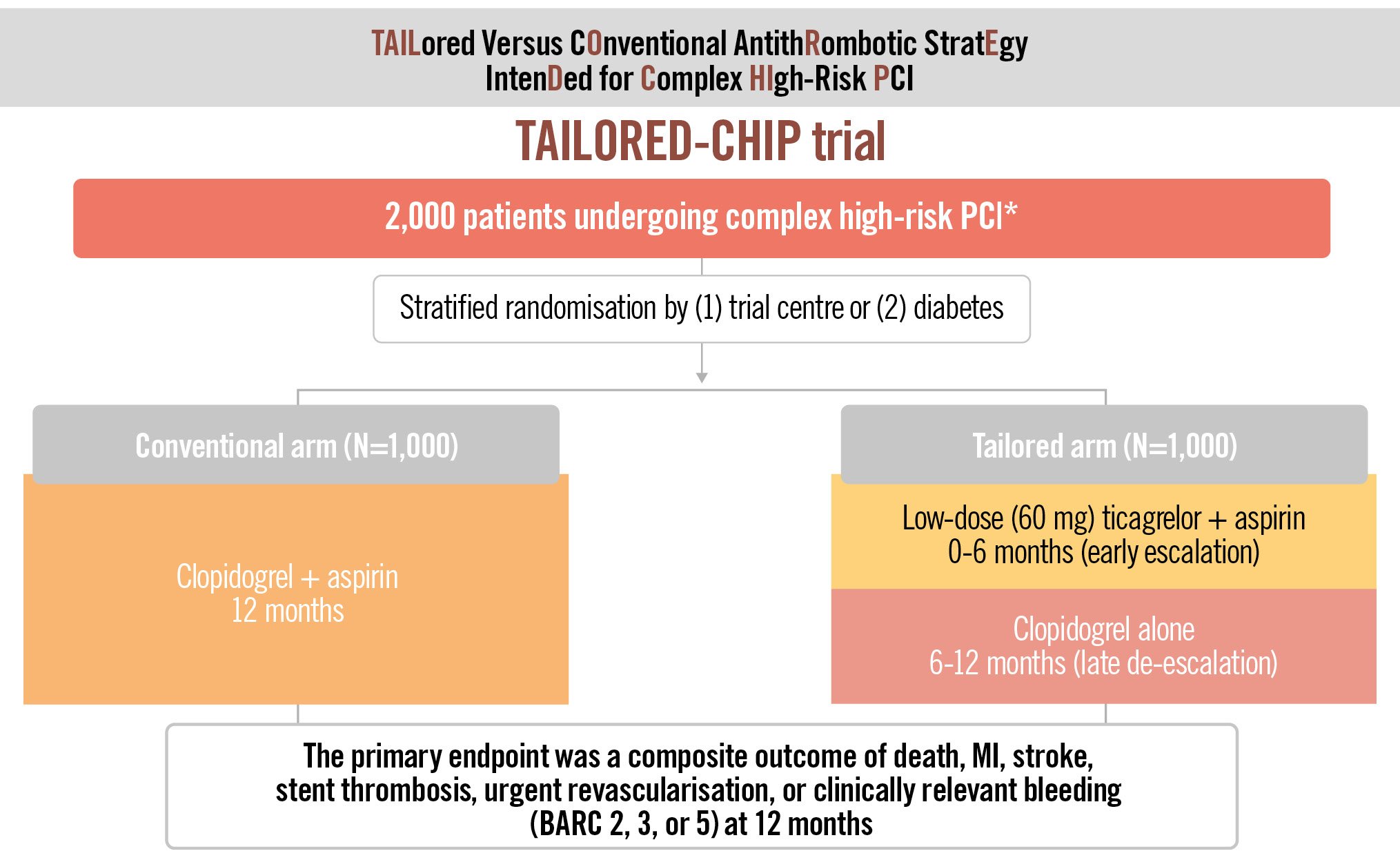
Figure 2. Study flow diagram of the TAILORED-CHIP trial. *Complex high-risk PCI: left main PCI, chronic total occlusion, bifurcation with 2 stents implanted, severe calcification, diffuse long lesion (lesion length ≥30 mm), multivessel PCI (≥2 vessels stented), ≥3 stents implanted, ≥3 lesions treated, total stent length >60 mm, diabetes, CKD (creatinine clearance <60 mL/min) or severe LV dysfunction (EF <40%). BARC: Bleeding Academic Research Consortium; CKD: chronic kidney disease; EF: ejection fraction; LV: left ventricular; MI: myocardial infarction; PCI: percutaneous coronary intervention
Study population
For enrolment in the TAILORED-CHIP trial, eligible patients had to have at least 1 high-risk anatomical or procedural feature or clinical characteristic associated with an increased risk of ischaemic or thrombotic events356910. Anatomical or procedural criteria for high-risk CHIP-PCI included an unprotected left main PCI, a complex bifurcation PCI requiring a 2-stent technique, a chronic total occlusion, a severely calcified lesion, a diffuse long lesion (lesion length ≥30 mm), a multivessel PCI (≥2 major epicardial vessels being stented), or a complex PCI requiring ≥3 planned stents, ≥3 lesions treated, or a total stent length >60 mm. The clinical criteria for a high thrombotic risk were medically treated diabetes mellitus, chronic renal dysfunction (creatinine clearance <60 ml/min), or severe left ventricular dysfunction (ejection fraction <40%). Detailed lists of the inclusion and exclusion criteria are presented in Table 1.
Table 1. Inclusion and exclusion criteria.
| Inclusion criteria: the subject had to meet all of the following criteria to be eligible for treatment in the study: | |
|---|---|
| 1. | The subject was >19 years of age |
| 2. | The subject was scheduled for PCI with a contemporary DES |
| 3. | Patients must have at least one of any features of complex high-risk anatomical, procedural, or clinical-related factors |
| 3-1 Lesion- or procedure-related factors: left main PCI, chronic total occlusion, bifurcation lesion requiring 2-stent technique, severe calcification, diffuse long lesion (lesion length ≥30 mm), multivessel PCI (≥2 vessels requiring stent implantation), complex PCI requiring implantation of ≥3 stents, ≥3 lesions to be treated, or predicted total stent length for revascularisation >60 mm | |
| 3-2 Clinical factors: medically treated diabetes, chronic kidney disease (defined as a creatinine clearance <60 mL/min), or severe LV dysfunction (LVEF <40%) | |
| 4. | The patient or guardian agreed to the study protocol and the schedule of clinical follow-up and provided informed, written consent, as approved by the appropriate institutional review board/ethics committee of the respective clinical site |
| Exclusion criteria: subjects were excluded from the study if any of the following criteria were met: | |
| 1. | Enzyme-positive acute myocardial infarction (NSTEMI or STEMI) |
| 2. | Contraindications to aspirin or P2Y12 inhibitors (ticagrelor or clopidogrel) |
| 3 | Use of glycoprotein IIb/IIIa inhibitors at the time of randomisation |
| 4. | Cardiogenic shock |
| 5. | Treatment with only BMS or balloon angioplasty during the index procedure |
| 6. | Requirement for chronic oral anticoagulation (warfarin or NOACs) |
| 7. | Active bleeding or extremely high risk for major bleeding (e.g., active peptic ulcer disease, gastrointestinal pathology with a high risk for bleeding, malignancies with a high risk for bleeding) |
| 8. | History of intracranial haemorrhage or an intracranial aneurysm |
| 9. | Planned surgery within 180 days |
| 10. | Severe liver disease (ascites and/or coagulopathy) or dialysis-dependent renal failure at screening |
| 11. | Platelet count <80,000 cells/mm3 or haemoglobin level <10 g/dL |
| 12. | At risk of bradycardia (subjects with sinus node dysfunction or atrioventricular block >2nd degree but without a permanent pacemaker) |
| 13. | Use of a strong cytochrome P450 3A inhibitor or inducer within 2 weeks of the date of enrolment: ketoconazole, clarithromycin, nefazodone, ritonavir, atazanavir, rifampin/rifampicin, rifabutin, dexamethasone, phenytoin, carbamazepine, phenobarbital |
| 14. | Pregnant and/or lactating women |
| 15. | Concurrent medical condition with a life expectancy of less than 1 year |
| 16. | Active participation in another investigational study of a drug or device that has not completed the primary endpoint or follow-up period |
| 17. | Inability to provide written informed consent or to participate in long-term follow-up |
| BMS: bare metal stent; DES: drug-eluting stent; LV: left ventricular; LVEF: left ventricular ejection fraction; NOAC: non-vitamin K antagonist oral anticoagulant; NSTEMI: non-ST-segment elevation myocardial infarction; PCI: percutaneous coronary intervention; STEMI: ST-segment elevation myocardial infarction | |
Randomisation, trial regimen and rationale
Eligible patients were randomly assigned in a 1:1 ratio to receive either a tailored antiplatelet therapy (early 6-month escalation therapy with low-dose ticagrelor plus aspirin and then late 6-month de-escalation therapy with clopidogrel monotherapy) or standard 12-month DAPT (clopidogrel plus aspirin). Randomisation was performed after diagnostic coronary angiography and before the time of the index PCI procedure. Randomisation was conducted with an Interactive Web Response System (IWRS) with the use of randomly permuted block sizes of 4 or 6, with stratification according to the presence or absence of diabetes and the participating centre.
In the tailored antiplatelet group, the choice of low-dose (60 mg twice daily) ticagrelor as the early escalation regimen was based on supportive pharmacodynamic data from the Comparison of Low-Dose, Standard-Dose Ticagrelor and Clopidogrel for Inhibition of Platelet Reactivity in Patients With Acute Coronary Syndromes (OPTIMA) trial11 and clinical data from the Prevention of Cardiovascular Events in Patients With Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin–Thrombolysis in Myocardial Infarction 54 (PEGASUS-TIMI 54) trial12. In OPTIMA, low-dose (60 mg twice daily) ticagrelor demonstrated better efficacy of platelet inhibition than clopidogrel, but its efficacy was similar to that of standard-dose ticagrelor in patients with ACS and PCI11. In the substudy of PEGASUS-TIMI 54, ticagrelor at 60 mg twice daily achieved high peak and trough platelet inhibitor levels, similar to ticagrelor dosed at 90 mg twice daily13. PEGASUS-TIMI 54 demonstrated that low-dose (60 mg twice daily) ticagrelor showed a similar magnitude of efficacy but a better safety profile (a lower rate of bleeding and dyspnoea) than standard-dose (90 mg twice daily) ticagrelor in patients who had a prior myocardial infarction (MI)12; based on this trial, a ticagrelor dose of 60 mg twice daily is now approved for long-term use in patients with a history of MI.
A late DAPT de-escalation approach (switching to clopidogrel monotherapy) may be attractive for reducing late bleeding risk while preserving the ischaemic benefit after PCI with contemporary safer drug-eluting stent (DES) platforms1415. Reducing the duration of aspirin therapy may allow for more prolonged use of potent P2Y12 inhibitors while avoiding aspirin-related bleeding risk, particularly with respect to gastrointestinal toxicity16. At the time of switching from ticagrelor to clopidogrel at 6 months, because ticagrelor has a relatively fast offset of action and to avoid any significant gap in platelet inhibition, the use of a 600 mg loading dose of clopidogrel should be considered when de-escalating from ticagrelor; at 24 hours from the last dose of ticagrelor, a 600 mg loading dose of clopidogrel should be given to all patients in the tailored-strategy arm17.
In the conventional DAPT group, enrolled patients were prescribed clopidogrel (75 mg once daily) and aspirin (100 mg once daily) for 12 months after the index PCI. In both groups, adherence was assessed with manual pill counts, and non-adherence was classified according to the underlying reason18. After 12 months of protocol-mandated therapy, patients were switched to a standard-of-care antiplatelet regimen at the discretion of their treating physician.
PCI procedure and post-PCI subsequent care
The PCI procedure was performed using standard techniques. Detailed information on PCI procedures and post-PCI subsequent care are described in Supplementary Appendix 2.
Trial endpoints and follow-up
The primary endpoint of this trial is a net clinical outcome, which is a composite of death from any cause, MI, stroke, stent thrombosis, urgent revascularisation, or clinically relevant bleeding (Bleeding Academic Research Consortium [BARC] type 2, 3, or 5) at 12 months after randomisation. The key secondary endpoints include individual components of the primary composite endpoint, a composite of ischaemic clinical endpoints (all-cause death, MI, stroke, stent thrombosis, or unplanned revascularisation), a composite of hard clinical endpoints (all-cause death, MI, or stroke), and safety outcomes (major bleeding, clinically relevant non-major bleeding, fatal bleeding, or any major or minor bleeding). Detailed lists and definitions of all the primary and secondary clinical endpoints are summarised in Table 2 and Supplementary Table 1. All trial endpoints are adjudicated by a clinical events committee (CEC) whose members are unaware of the trial-group assignments (detailed information is described in Supplementary Appendix 3).
After randomisation, trial follow-up assessments are routinely conducted at baseline, 1 month, 3 months, 6 months, and 12 months, with additional evaluations for routine clinical care scheduled as required.
Table 2. Primary and secondary endpoints.
| Primary endpoints* |
|---|
| Net clinical outcomes – composite of all-cause death, myocardial infarction, stroke, stent thrombosis, urgent revascularisation, or clinically relevant bleeding (BARC 2, 3, or 5) at 12 months after randomisation |
| Secondary outcomes* |
| Individual components of the primary composite endpoint |
| Efficacy outcomes |
| Death (any, cardiovascular, or non-cardiovascular causes) |
| Myocardial infarction (any, periprocedural, or spontaneous) |
| Stroke (any, ischaemic, or haemorrhagic) |
| Stent thrombosis |
| Repeat revascularisation (any, target vessel, or non-target vessel) |
| Composite of ischaemic clinical endpoints (all-cause death, myocardial infarction, stroke, stent thrombosis, or urgent revascularisation) |
| Composite of hard clinical endpoints (all-cause death, myocardial infarction, or stroke) |
| Safety outcomes† |
| BARC major bleeding (type 3 or 5 bleeding) |
| TIMI major or minor bleeding |
| GUSTO moderate or severe bleeding |
| ISTH major bleeding |
| Any major or minor bleeding |
| *Detailed definitions of the primary and secondary clinical endpoints are available in Supplementary Table 1. †Although bleeding events were assessed primarily using BARC criteria, bleeding events were also adjudicated according to different criteria including TIMI, GUSTO, or ISTH. BARC: Bleeding Academic Research Consortium; GUSTO: Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries; ISTH: International Society of Thrombosis and Haemostasis; TIMI: Thrombolysis in Myocardial Infarction |
Sample size calculation
We hypothesised that a tailored antiplatelet strategy would be superior to a conventional DAPT strategy with respect to the primary endpoint of net clinical benefit. On the basis of the reported rates of ischaemic and bleeding events in complex high-risk PCI and DAPT-related trials (EXCEL, DAPT, and a pooled meta-analysis of randomised controlled trials [RCTs])6919, we assumed a 1-year event rate of the primary composite endpoint of 14% in the conventional DAPT group. We estimated that enrolment of 2,000 patients would provide the study with 80% power to detect a relative reduction of 30% in the primary composite endpoint in the tailored antiplatelet group compared with those in the conventional DAPT group, assuming an attrition rate of 5% (e.g., follow-up loss or non-compliance) at an alpha significance level of 0.05.
Statistical analysis plan
Details regarding the statistical methods are provided in Supplementary Appendix 4. All endpoint analyses will be performed according to the intention-to-treat principle of all randomised patients at the time of the first event. Cumulative event curves will be generated using the Kaplan-Meier method and compared with the log-rank test. Statistical comparisons (a test of superiority) of the two randomised groups will be based on a time-to-first-event analysis using the Cox proportional hazards model. Relative risks will be expressed as hazard ratios with associated 95% confidence intervals and will be derived from the Cox model. Absolute differences and 95% confidence intervals for primary and key secondary endpoints at 1 year will be calculated with Kaplan-Meier estimates and Greenwood standard errors20. To evaluate the consistency of results among clinically relevant subgroups, prespecified subgroup analyses will be performed. Several prespecified sensitivity analyses of the primary outcome will be conducted, including other methods to analyse recurrent events21.
Landmark analyses will be performed according to prespecified landmark points at 6 months post-PCI, at which time the tailored antiplatelet strategy will be changed in the experimental arm (from low-dose ticagrelor plus aspirin to clopidogrel alone); the relative risks will be calculated separately for events up to the landmark point from randomisation and for events occurring after the landmark point up to 12 months. We will also estimate the difference in the restricted mean event-free time analyses over 12 months. The restricted mean event-free survival time is the mean time that a patient is free from an outcome event, adjusted for loss to follow-up, and reflects the area under the survival curve22.
Trial organisation
Details regarding the organisation of the trial are provided in Supplementary Appendix 5. An independent data safety monitoring board is responsible for monitoring safety during the trial and thus will periodically review the safety data according to a dedicated charter and make recommendations based on safety analyses, protocol deviation, and clinical follow-up reports.
Recruitment status
Between February 2019 and January 2024, 2,018 patients from 24 participating sites in South Korea were enrolled and randomised in the TAILORED-CHIP trial. Follow-up of the last enrolled patient will be completed in January 2025, and the primary results of the TAILORED-CHIP trial are expected to be available by mid or late 2025.
Discussion
To our knowledge, TAILORED-CHIP is the first large-scale RCT to investigate the potential role of temporal antiplatelet modulation with early escalation and late de-escalation in high-risk patients undergoing CHIP procedures. The TAILORED-CHIP trial mainly targets temporal (i.e., time-dependent) modulation of escalation and de-escalation strategies after complex high-risk PCI. We assumed that efficacy and safety outcomes might be optimised according to the strategy used, with an early (<6 months post-PCI) escalation approach being more effective in reducing ischaemic events without a relevant increase in bleeding than standard DAPT therapy and a late (>6 months post-PCI) de-escalation approach being more effective in reducing bleeding risk without any trade-off in efficacy.
Although there is a paucity of data supporting potent P2Y12 agents such as ticagrelor and prasugrel in a broad population inclusive of both patients with ACS and chronic coronary syndromes, their use is increasing across the diverse clinical spectrum of patients undergoing PCI23. Given increasing clinician familiarity with these P2Y12 agents, administrative data indicate that ticagrelor and prasugrel are prescribed off-label in 1 in 3 patients with non-ACS indications24. Until recently, relatively few studies have been conducted on the escalation antiplatelet strategy using ticagrelor in contemporary PCI settings. In the Effect of Ticagrelor on Health Outcomes in Diabetes Mellitus Patients Intervention Study (THEMIS), ticagrelor added to aspirin reduced cardiovascular death, MI, and stroke, although with increased major bleeding in diabetic patients with stable coronary artery disease and a history of previous PCI; overall, ticagrelor provided a favourable net clinical benefit25. In the Ticagrelor With Aspirin or Alone in High-Risk Patients After Coronary Intervention (TWILIGHT) trial18, 35.2% of enrolled patients underwent PCI for non-ACS (silent ischaemia or stable angina). In the Assessment of Loading With the P2Y12 Inhibitor Ticagrelor or Clopidogrel to Halt Ischemic Events in Patients Undergoing Elective Coronary Stenting (ALPHEUS) trial, ticagrelor was not superior to clopidogrel in reducing periprocedural myocardial necrosis within 48 hours after elective PCI and did not cause an increase in major bleeding2627.
Time-dependent antiplatelet regimens (i.e., early escalation, late de-escalation) may be reasonable in this complex, high-risk patient population to achieve a balance between timely and sufficient platelet inhibition and an acceptable bleeding risk. Until recently, several therapeutic strategies to decouple thrombotic and haemorrhagic risks have been tested1528. In particular, P2Y12 inhibitor monotherapy was found to preserve ischaemic protection while limiting bleeding risk compared with DAPT after complex PCI14. Our rationale for late de-escalation of clopidogrel monotherapy is supported by recent relevant RCTs2930. The TicAgrelor Versus CLOpidogrel in Stabilized Patients With Acute Myocardial Infarction (TALOS-AMI) trial supports the uniform unguided de-escalation antiplatelet strategy of switching from ticagrelor to clopidogrel, which was superior to the ticagrelor-based DAPT strategy in stabilised patients with acute MI29. In addition, the Harmonizing Optimal Strategy for Treatment of Coronary Artery Stenosis-EXtended Antiplatelet Monotherapy (HOST-EXAM) trial supported the idea that clopidogrel monotherapy was superior to aspirin monotherapy in preventing future adverse clinical events, including both the thrombotic composite endpoint and any bleeding in patients who underwent PCI, and successfully maintained the intended duration of DAPT30.
Limitations
This trial may have some limitations. First, the open-label design has a potential for bias in outcome reporting and ascertainment. However, all endpoints have a standardised definition and were specifically adjudicated by an independent CEC. Second, the trial sample size was calculated by estimating the occurrence of a net adverse clinical benefit; thus, the efficacy and safety of antiplatelet therapy is a bivariate outcome, and summarising it in a unidimensional variable could be misleading. Third, the trial is underpowered to provide reliable information on hard ischaemic endpoints, such as death, MI, or stent thrombosis. Fourth, the study population is exclusively East Asian patients. Finally, we did not routinely perform platelet function testing or genotyping during the study. Thus, whether a similar strategy would have resulted in a different outcome in a population of patients with poor responses to clopidogrel with high platelet reactivity is unknown.
Conclusions
The TAILORED-CHIP trial has the unique feature of testing early escalation using a potent P2Y12 inhibitor of low-dose ticagrelor and late de-escalation with less potent P2Y12 monotherapy of clopidogrel after complex high-risk PCI. The impending results of this trial will provide novel and clinically meaningful insights on the potential role of temporal modulation of antiplatelet therapy in high-risk patients who are undergoing CHIP procedures.
Funding
TAILORED-CHIP is an investigator-initiated trial with funding from the CardioVascular Research Foundation (CVRF; Seoul, Republic of Korea) and the Chong Kun Dang Pharmaceutical Co., Ltd (Seoul, Republic of Korea). The sponsors have played no role in the design of the trial, in the collection or analysis of the data, in the interpretation of the trial results, nor in the writing of the manuscript.
Conflict of interest statement
D.-Y. Kang reports speaker fees from Abbott, Daiichi Sankyo, Viatris, Boryung, and Daewoong Pharmaceutical. S.-J. Park reports research grants and speaker fees from Abbott, Medtronic, Daiichi Sankyo, Chong Kun Dang Pharmaceutical, Daewoong Pharmaceutical, and Edwards Lifesciences. D.-W. Park reports research grants and speaker fees from Chong Kun Dang Pharmaceutical, Abbott, Medtronic, Daiichi Sankyo, Edwards Lifesciences, and Daewoong Pharmaceutical. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

