
Most interventional cardiologists would agree that the contemporary outcomes of percutaneous coronary intervention (PCI) are so respectable not only because of important developments in stent technologies, but also as the consequence of remarkable progress in antithrombotic pharmacotherapy1. The landscape of antithrombotic drugs for PCI is quite extensive, with thousands of potential strategic permutations based on drug choice, timing of administration, dose and prescribed duration. It is fair to admit that this abundance represents a clinical opportunity but also a threat when it comes to determining which of the disparate pharmacological approaches for a given condition is ultimately associated with net benefit after accounting for patients’ individual circumstances.
Most interventional cardiologists would also agree that, in the era of evidence-based medicine, randomised clinical trials (RCTs) are the undisputed gold standard to advance clinical practice. In the field of antithrombotic pharmacotherapy, RCTs are typically conducted under the leadership of academic physicians, with or without the economic support of pharmaceutical companies. This support may be provided in the form of an unrestricted grant, where the industry plays little or no role in the design, conduct and analysis of the study, which can still be flagged as “physician-driven” (PD). Conversely, other studies are clearly flagged as “industry-driven” (ID) because the sponsor plays a major role from conception to dissemination of the results.
Not surprisingly, in the 2017 focused update on dual antiplatelet therapy for coronary artery disease from the European Society of Cardiology, most class I recommendations with level of evidence A or B are supported by ID-RCTs2. Such “high-budget” studies are conducted in large cohorts encompassing several thousands of patients to validate the clinical indication for a novel or existing drug. From the industry perspective, a return on the investment occurs if the study is positive and the new indication is firstly cleared by regulatory authorities and endorsed by guidelines task forces, then launched onto the market and finally embraced by the medical community. Many antiplatelet drugs have successfully found their way into clinical practice following this long process, including clopidogrel for ACS following the CURE trial and, subsequently, prasugrel and ticagrelor following the TRITON and PLATO trials3-5. Yet, translating the conclusions of an ID-RCT of antithrombotic pharmacology into broad acceptance from the medical community is not an automatic process – ask the vendors of vorapaxar, the platelet PAR-1 antagonist tested in the TRA-2P trial, or those of rivaroxaban tested at the 2.5 mg dose in the ATLAS ACS 2 trial6,7. In this uncertain landscape of antithrombotic opportunities, the journey of ticagrelor 60 mg for secondary prevention has just begun, with the drug still unavailable in many countries three years after the publication of the PEGASUS trial8. Similar hurdles are now faced by rivaroxaban, once again used at the dose of 2.5 mg for the new indication of antithrombotic prevention in stable atherosclerosis tested in the COMPASS trial9.
From the physician’s perspective, ID-RCTs are not immune from concerns. In fact, these studies are designed to provide a “black or white” answer that will play a major role in influencing the destiny of the drug for the tested indication. The study design is intended to maximise the chances of success, which is reflected by the careful selection of patients to be included, endpoints to be investigated and control group to be confronted. Many would argue that in some cases the final population of an ID-RCT does not entirely reflect the typical characteristics of patients observed in clinical practice (an issue of generalisability), or would argue that the study question does not always focus on what really matters to patients and physicians. Indeed, there is an inevitable trade-off between the medical validity of the study design and the chance for a study to be conceived by the industry or even funded in the form of an unrestricted grant. For example, many doctors would be interested in a large-scale head-to-head comparison of prasugrel or ticagrelor, but a similar study goes beyond the interest of the drug manufacturers, which leaves us at best with smaller PD-RCTs that lack sufficient power to be conclusive10.
Recently, in the antithrombotic pharmacology field we observed an interest in conducting “strategy” PD-RCTs to address practical medical questions that go beyond the scope of pharmaceutical companies to position and characterise the efficacy and safety of their drugs. The goal of these smaller-sized studies is more relevant to how to optimise the use of established drugs in clinical daily routine. These studies do not have the power to warrant an immediate change of practice, but the impact on subsequent research can be profound. For example, the WOEST trial, with only 573 patients, has convinced many physicians that the paradigm of triple antithrombotic therapy is not inescapable for patients with a concurrent indication to oral anticoagulation and antiplatelet therapy11. Interestingly, this hypothesis has been validated subsequently in ID-RCTs such as the PIONEER-AF and RE-DUAL PCI trials12-14. Another paradigmatic example deals with the emerging concept of “antiplatelet de-escalation”, which has recently been pushed by two relatively small PD-RCTs15,16. Both the TOPIC and TROPICAL-ACS trials suggested that, in selected post-ACS patients, de-escalation by switching DAPT from newer P2Y12 blockers to clopidogrel could be safe with respect to preventing bleeding events and without an apparent increase in ischaemic complications, although the latter cannot be formally concluded from the small sample sizes. Notably, the budgets of these studies were not comparable to those of the foundational ID-RCTs of clopidogrel, prasugrel and ticagrelor, but the focus was not on revisiting current indications, but rather on exploring new avenues in the use of established drugs.
Understandably, there is now an accruing debate around the position of “strategy” PD-RCTs in evidence-based medicine. Some critics note that many of these studies have severe methodological limitations (e.g., lack of power for efficacy, lower methodological standards), cannot confute the conclusions of ID-RCTs, and may even be misleading. For example, although most PD-RCTs of shorter dual antiplatelet therapy duration concluded that a shorter term is at least as safe as a longer term, the only ID-RCT powered for efficacy reached the opposite conclusion1.
We believe that both ID- and PD-RCTs are necessary to advance science and practice in antithrombotic pharmacotherapy, and we see them as complementary rather than contradictory (Figure 1). ID-RCTs are essential to define the merits and side effects of novel or existing drugs. However, there are study questions that are not relevant to the interest of the industries and need the agility of smart PD-RCTs in order to be addressed. For example, bivalirudin has been downgraded in clinical guidelines following the results of a PD-RCT built around a modern hypothesis (e.g., no bleeding advantage versus heparin in the era of glycoprotein IIb/IIIa inhibitor use for bail-out only)17. Another PD-RCT conducted using a registry platform further validated this concept, concurrently demonstrating that PD-RCTs can also be affordable on a large scale despite being industry-independent18. The independence of PD-RCTs should also be valued as a strength, because the only objective of such studies is to promote new approaches without the economically driven restrictions that somehow influence the study design and patient selection. Finally, in strategy PD-RCTs, implementation of the study results in clinical practice can be quicker than in regulatory ID-RCTs, because the drugs are already available and used by physicians. The main implication of PD-RCTs will be to keep using the same drugs but differently in certain patients (e.g., aspirin drop or de-escalation in patients at high risk of bleeding). This aspect is close to daily practice in that it does not propose a new “standard” but only an alternative strategy for selected scenarios. Appreciating that a synergy of strengths rather than a dichotomy exists between ID- and PD-RCTs is essential to shift the “one-size-fits-all” paradigm of antithrombotic pharmacotherapy to the more contemporary concept of “personalised medicine”.
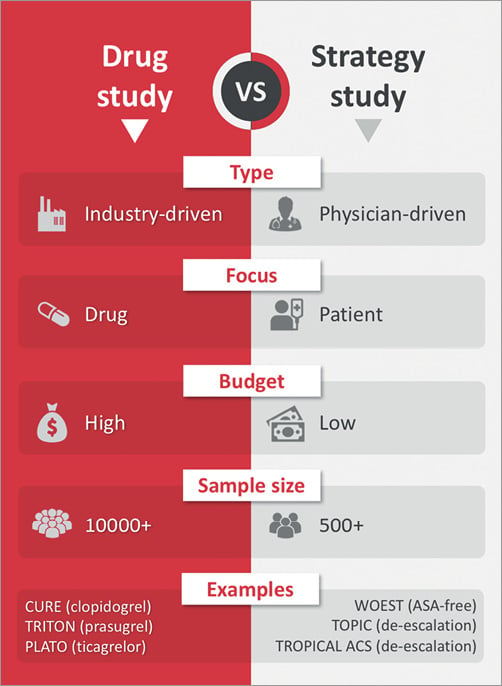
Figure 1. Side-by-side comparison of industry-driven and patient-driven randomised clinical trials.
Conflict of interest statement
T. Cuisset received honoraria and/or consulting fees from AstraZeneca, Medtronic and Sanofi-Aventis. D. Capodanno received speaker’s honoraria from AstraZeneca, Bayer, Pfizer, and Sanofi-Aventis.

