
There have been truly incredible advances in clinical and interventional cardiology over recent decades. We have gone from an era where we really did not have effective therapies for many cardiac conditions to an era where the pharmacological and interventional armamentarium has become so rich that selecting the proper combination becomes the issue. In addition, specific problems have emerged in interventional cardiology regarding the understanding and delivery of “best pharmacological care” in patients treated with devices. There is a myriad of possible combinations of drugs and devices in terms of drug selection, drug combinations, dosing, timing of initiation, and duration of therapy. While cardiology has a culture of randomised clinical trials and evidence-based guidelines, not all combinations have or can be tested formally, guidelines cannot address the diversity of the potential clinical scenarios, and evidence is often either unavailable, partial, or very complex and confusing. Therefore, clinicians quite frequently have to rely on best clinical judgement. Previous informal polling of participants at the EuroPCR congress has shown that the interaction of drugs and devices is a topic that has not yet been fully addressed. The example of the optimal use of antithrombotic agents in patients undergoing interventions is a case in point: there is ongoing debate regarding the type of antithrombotic therapy to be used during and after interventions in terms of agent selection, combination, dosing and duration. However, similar problems will soon emerge regarding other classes of agent. An example is the ongoing debate regarding the integration and specific roles of intervention and drug therapy in patients with stable coronary artery disease. Tomorrow, with the advent of potent anti-inflammatory interventions or of plaque-stabilising lipid interventions, new choices will need to be discussed.
This year, EuroPCR aims to address this unmet need by incorporating into the scientific programme a new session format, namely “PCR clinical algorithms”.
These sessions are case-based short educational sessions, each devoted to addressing a single common clinical conundrum in the field of drug-device interactions. They will be led by recognised international experts, involve interactive presentation of clinical scenarios and aim to end with the provision of a simple “PCR clinical algorithm”, in the form of a single slide and single page guidance. The sessions and algorithms are not evidence reviews (in areas where evidence can be lacking or contradictory and confusing). They aim to be highly practical and brief. PCR clinical algorithms are delivered and endorsed by each individual expert and do not represent a guideline or the view of any official body (including PCR). Importantly, they are not sponsored by industry. They represent the personal views of an individual expert, at a given point in time (the time of the presentation) as evidence often accrues continuously and can modify views and recommendations over a short period of time. These sessions, slides and clinical algorithms will be available on the EuroPCR website, which will, over time, become a repository for clinical guidance on drug-device interactions.
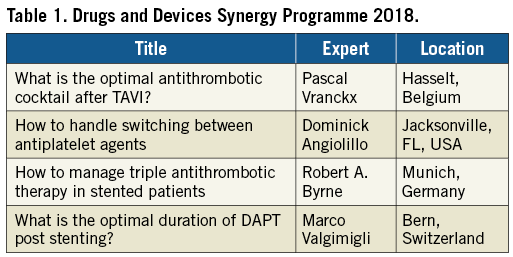
In 2018, the EuroPCR programme includes four sessions (Table 1), led by world-recognised experts in the field. Based on the experience of this year and on the feedback of participants, the scope and number of these sessions may eventually be expanded. Their goal will be, first and foremost, to address the unmet needs of the interventional community with respect to their understanding and delivery of best pharmacological care in patients treated with devices and to address the drug-device interactions in a practical way to enhance patient benefit. We look forward to your attendance!
Conflict of interest statement
P.G. Steg discloses the following relationships: research grants from Bayer, Merck, Sanofi, and Servier, and speaker’s or consulting fees from Amarin, Amgen, AstraZeneca, Bayer/Janssen, Boehringer-Ingelheim, Bristol-Myers-Squibb, Lilly, Merck, Novartis, Pfizer, Regeneron, Sanofi, and Servier. W. Wijns has received institutional research grants from stent manufacturing companies and speaker fees from Abbott Vascular, Biotronik, MiCell, and MicroPort; is cofounder of Argonauts, an innovation facilitator; and past Board member of Cardio3 BioSciences, now Celyad (cell-based cancer therapy).

