
Abstract
Aims: Clopidogrel is the P2Y12 inhibitor of choice in patients who undergo PCI and have an indication for oral anticoagulation (OAC). Prediction of the bleeding risk is of major interest in this population. The aim of this analysis was to investigate whether an enhanced platelet inhibition by clopidogrel measured by platelet function testing (PFT) with the Multiplate Analyzer is associated with an increased bleeding risk in patients on triple antithrombotic therapy.
Methods and results: This investigation was performed in a cohort of 524 patients from the randomised ISAR-TRIPLE trial; 458 (87.4%) had PFT results available in the first 24 hours after PCI. Patients belonging to the lowest quintile according to PFT were considered as enhanced responders to clopidogrel. The primary endpoint was major bleeding according to TIMI criteria at nine months. The median of ADP-induced platelet aggregation in the whole population was 163 AU*min (107-241). Patients in the lowest quintile had values below 93 AU*min. These enhanced responders (92 patients) had a significantly higher risk of TIMI major bleeding (hazard ratio [HR] 3.13, 95% confidence interval [CI]: 1.38-7.09, p=0.01) and overall mortality (HR 3.42, 95% CI: 1.55-7.52, p=0.004) compared with the remaining patients (366 patients). No significant difference was observed for the secondary combined ischaemic endpoint (HR 1.27, 95% CI: 0.47-3.47, p=0.64).
Conclusions: Enhanced platelet inhibition delivered by clopidogrel is associated with an increased risk for major bleeding and death in patients on OAC who undergo PCI. These results support the use of PFT to identify patients with an increased risk for bleeding.
Introduction
A substantial number of patients (6-8%) undergoing percutaneous coronary intervention (PCI) with stent implantation have an indication for long-term oral anticoagulation (OAC)1,2. Clopidogrel is the P2Y12 inhibitor of choice for use in these patients.
The combination of OAC with dual antiplatelet therapy, often referred to as triple therapy, is associated with increased bleeding rates3. Thus, over recent years, several randomised trials have investigated different antithrombotic strategies in these patients. These studies showed that either shortening the duration of triple therapy4 or omission of aspirin5,6,7 was able to reduce bleeding events. Current guidelines recommend different treatment strategies for patients on OAC requiring antiplatelet therapy after PCI, depending on the individual risk for bleeding and ischaemic events2.
Assessing the bleeding risk in these patients is not trivial. The HAS-BLED score is able to assess the bleeding risk in patients with atrial fibrillation (AF) who receive OAC8. However, in patients with PCI who receive dual antiplatelet therapy (DAPT), the HAS-BLED score does not predict major bleeding events9 and, in patients with PCI and AF, a modified HAS-BLED score was also unable to predict bleeding events10.
Platelet function testing (PFT) in patients on DAPT after PCI can identify patients with an enhanced response to clopidogrel which is associated with an increased risk for bleeding events11. However, all major trials that investigated the association of PFT with bleeding events have excluded patients with OAC11,12. Thus, it remains unknown whether an enhanced response to clopidogrel is associated with an increased risk of bleeding in this high-risk group of patients.
The objective of the current analysis of the ISAR-TRIPLE trial4 was to assess whether an enhanced response to clopidogrel, measured by PFT, is associated with an increased risk of bleeding in patients requiring additional OAC after PCI.
Methods
STUDY POPULATION
The ISAR-TRIPLE trial was a prospective, randomised clinical trial investigating the effects of six weeks vs six months of clopidogrel treatment after drug-eluting stent implantation in patients receiving concomitant aspirin and OAC4. Outcomes4 as well as the design and rationale13 of the ISAR-TRIPLE trial were reported previously.
For the current post hoc analysis, patients from both treatment arms of the ISAR-TRIPLE trial were pooled; those who had undergone routine PFT in the first 24 hours after stent implantation at two centres in Germany (Klinikum rechts der Isar and Deutsches Herzzentrum, both in Munich) were included. This study complies with the Declaration of Helsinki. The local ethics committee approved the research protocol and informed consent was obtained from the subjects.
TREATMENT
Coronary angiography and PCI were performed according to conventional and local standards. A loading dose of 600 mg clopidogrel orally and 500 mg aspirin intravenously was administered before PCI. During the study duration patients received 75 mg clopidogrel once daily for six weeks or six months and 75 to 200 mg aspirin once daily according to local standards. Vitamin K antagonists (phenprocoumon or warfarin) were prescribed for all patients with the lowest recommended target international normalised ratio (INR) for the duration of the triple therapy.
BLOOD SAMPLING AND PLATELET FUNCTION TESTING
During the ISAR-TRIPLE trial PFT was performed according to clinical routine. Whole blood was obtained either from the remaining arterial sheath after PCI or from an antecubital vein within 24 hours after PCI following standard procedures. Adenosine diphosphate (ADP)-induced platelet aggregation was measured using the Multiplate® Analyzer (Roche Diagnostics, Rotkreuz, Switzerland) and quantified as area under the curve (AUC=AU×min) of aggregation units (AU), as previously described14. If multiple results were available from the same patient, the last value within a 24-hr time frame after PCI was chosen for the current study. According to the results obtained from the platelet function tests, patients were divided into quintiles14. We defined patients in the lowest quintile of PFT measurement (below 92 AU×min) as “enhanced responders” to clopidogrel.
STUDY ENDPOINTS AND DEFINITIONS
Outcomes were compared between patients with an enhanced response to clopidogrel (lowest quintile) versus the remaining patients (quintiles 2-5). The primary endpoint for the current analysis was Thrombolysis In Myocardial Infarction (TIMI) major bleeding at nine months after randomisation13. Secondary endpoints included bleeding according to the Bleeding Academic Research Consortium (BARC) criteria as well as mortality, cardiac death, myocardial infarction, definite stent thrombosis or ischaemic stroke.
FOLLOW-UP
Patients were evaluated by telephone call or physician office visits after six weeks, six months, and nine months. Events were adjudicated and classified by an event adjudication committee whose members were unaware of the assigned treatment.
STATISTICAL ANALYSIS
Variables are shown as mean±standard deviation (SD), numbers with percentages or median with interquartile range (IQR). Continuous data were analysed using the Student’s t-test or non-para-metric Wilcoxon rank-sum test depending on the distribution of the data. Categorical variables were compared using the chi-square test or Fisher’s exact test, as appropriate. All statistical tests were two-sided and a p-value <0.05 was considered statistically significant. Both unadjusted and adjusted risk estimates were calculated by Cox analysis. In multivariable analysis, the independent variables were enhanced response, female sex, duration of clopidogrel therapy (six weeks vs six months), indication for PCI (elective PCI vs ACS) and differences in the time interval between PCI and PFT. Log-rank optimisation was performed to determine a cut-off, which might have prognostic significance for the primary endpoint (TIMI major bleeding). The optimal cut-off was considered as the PFT value separating the population into two groups, the log-rank comparison of which revealed the highest chi-square statistic. The Kaplan-Meier method was used for building event curves. All statistical analyses were performed with the software R (version 2.15.0; R Foundation for Statistical Computing, Vienna, Austria) or the software package SPSS Statistics, Version 24 (IBM Corp., Armonk, NY, USA).
Results
PATIENTS
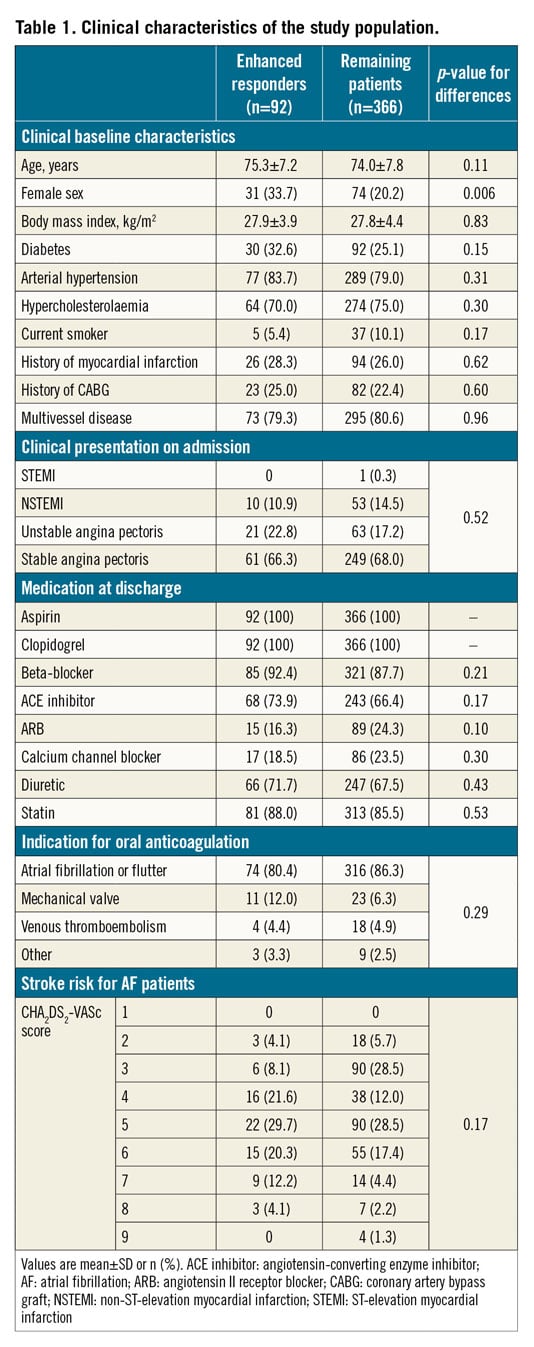
For the current analysis, 524 patients from the ISAR-TRIPLE trial who had been recruited at the two centres in Germany were included. PFT results within 24 hours after PCI were available in 458 out of 524 patients (87.4%) (Figure 1). The median of ADP-induced platelet aggregation as measured by the Multiplate Analyzer was 163 AU*min (107-241). In the group of enhanced responders, the median of ADP-induced platelet aggregation was 50 AU*min (30-73) and 190 AU*min (138-272) in the remaining patients (Supplementary Figure 1). The mean time between PCI and blood sampling for PFT was 12.7 (±8.5) vs 8.9 (±8.4) hrs.
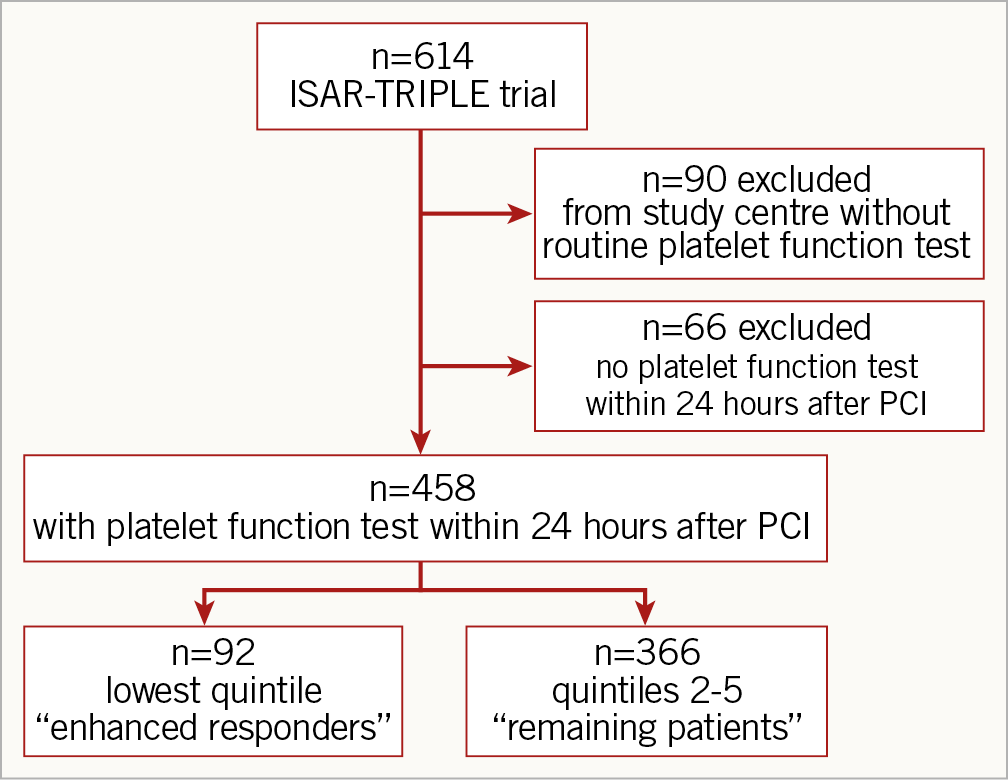
Figure 1. Study flow chart.
Clinical and demographic baseline characteristics are shown in Table 1. Details concerning antithrombotic therapy are provided in Supplementary Appendix 1.
OUTCOMES
The primary endpoint (TIMI major bleeding) occurred in 10.0% (9 patients) of the enhanced responders group as compared with 3.3% (12 patients) of the remaining patients (p=0.01) at nine months (Figure 2A, Table 2). This difference remained statistically significant in the multivariable analysis (adjusted p=0.01).
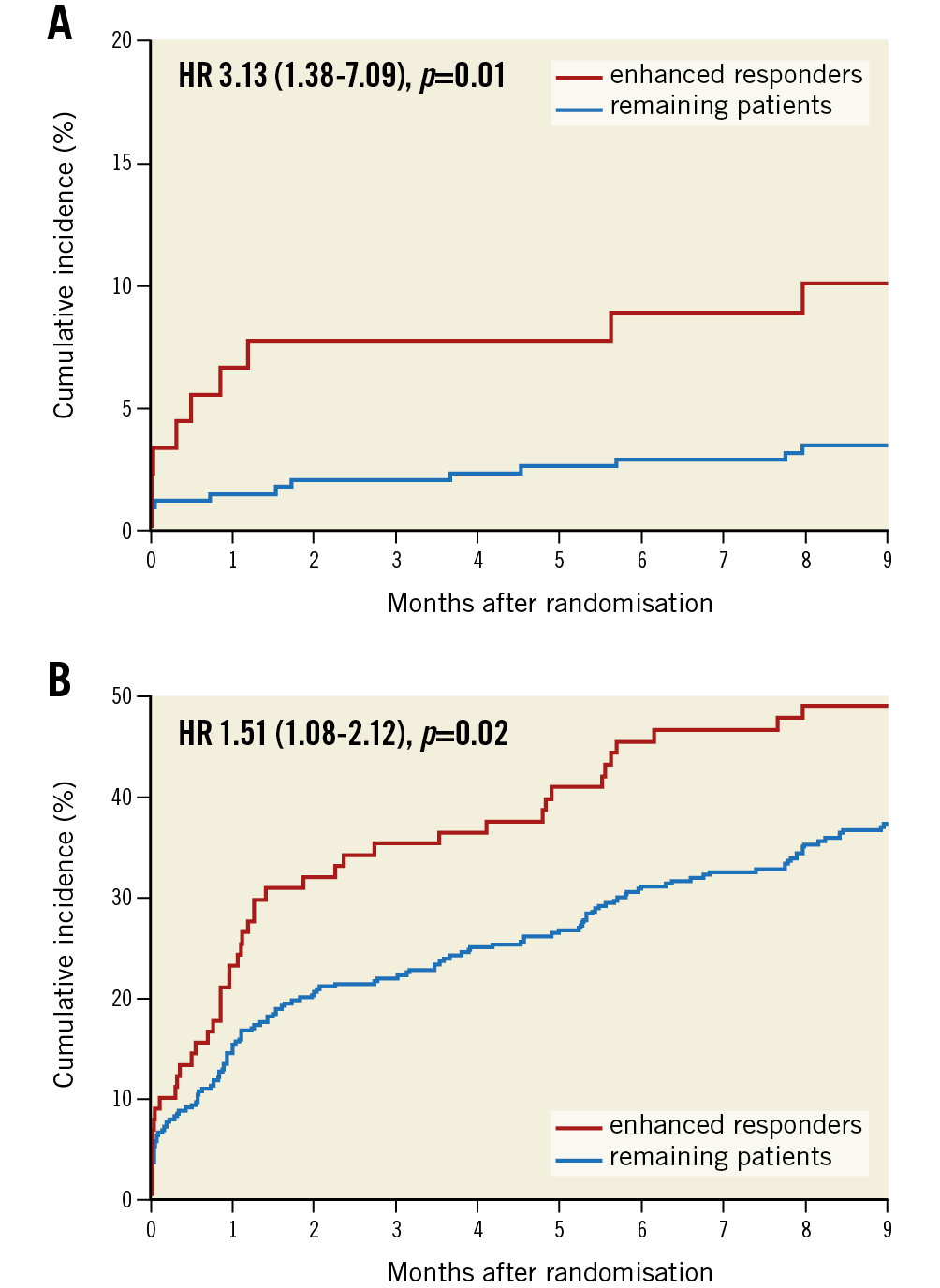
Figure 2. Cumulative incidence of the primary and secondary bleeding endpoints. Kaplan-Meier analysis of the primary endpoint (TIMI major bleeding) (A) and secondary bleeding endpoint (BARC types 1 to 5 bleeding) (B) at nine months. HR: hazard ratio
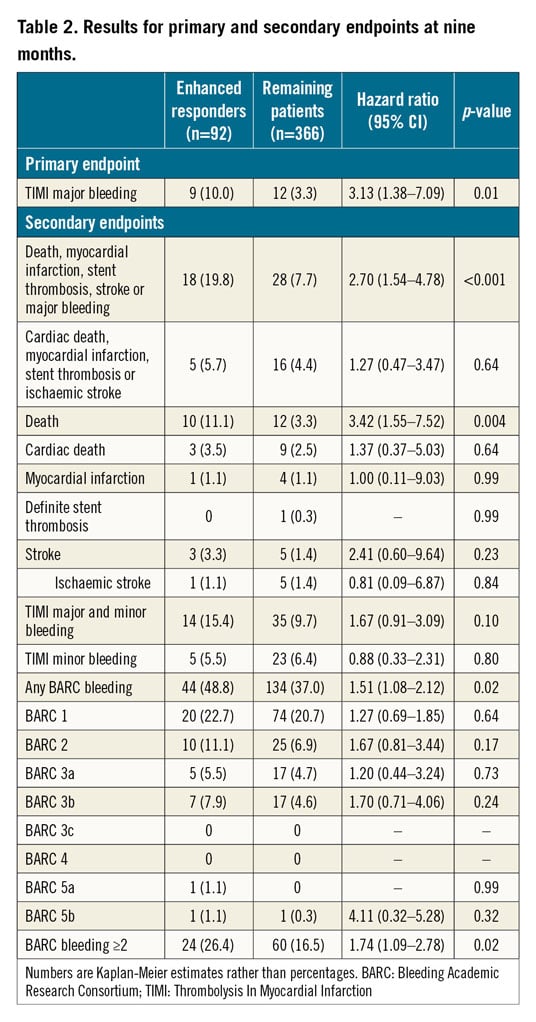
For secondary endpoints, we observed significantly more cumulative bleeding events, defined as any BARC bleeding (BARC 1-5), in the group of enhanced responders as compared to the remaining patients (48.8% vs 37%; p=0.02) at nine months (Figure 2B, Table 2). Most of the bleedings were localised in the nose and skin or were gastrointestinal and access-site bleeds (Supplementary Table 1).
There was no significant difference in the incidence of ischaemic events (cumulative incidence of cardiac death, myocardial infarction, definite stent thrombosis, or ischaemic stroke) between the groups (5.7% vs 4.4%; p=0.64) at nine months (Figure 3A, Table 2). However, total mortality was higher in patients with an enhanced response (11.1% vs 3.3%; p=0.004) at nine months (Figure 3B, Table 2). Details of the patients who died during the study are provided in Supplementary Table 2.
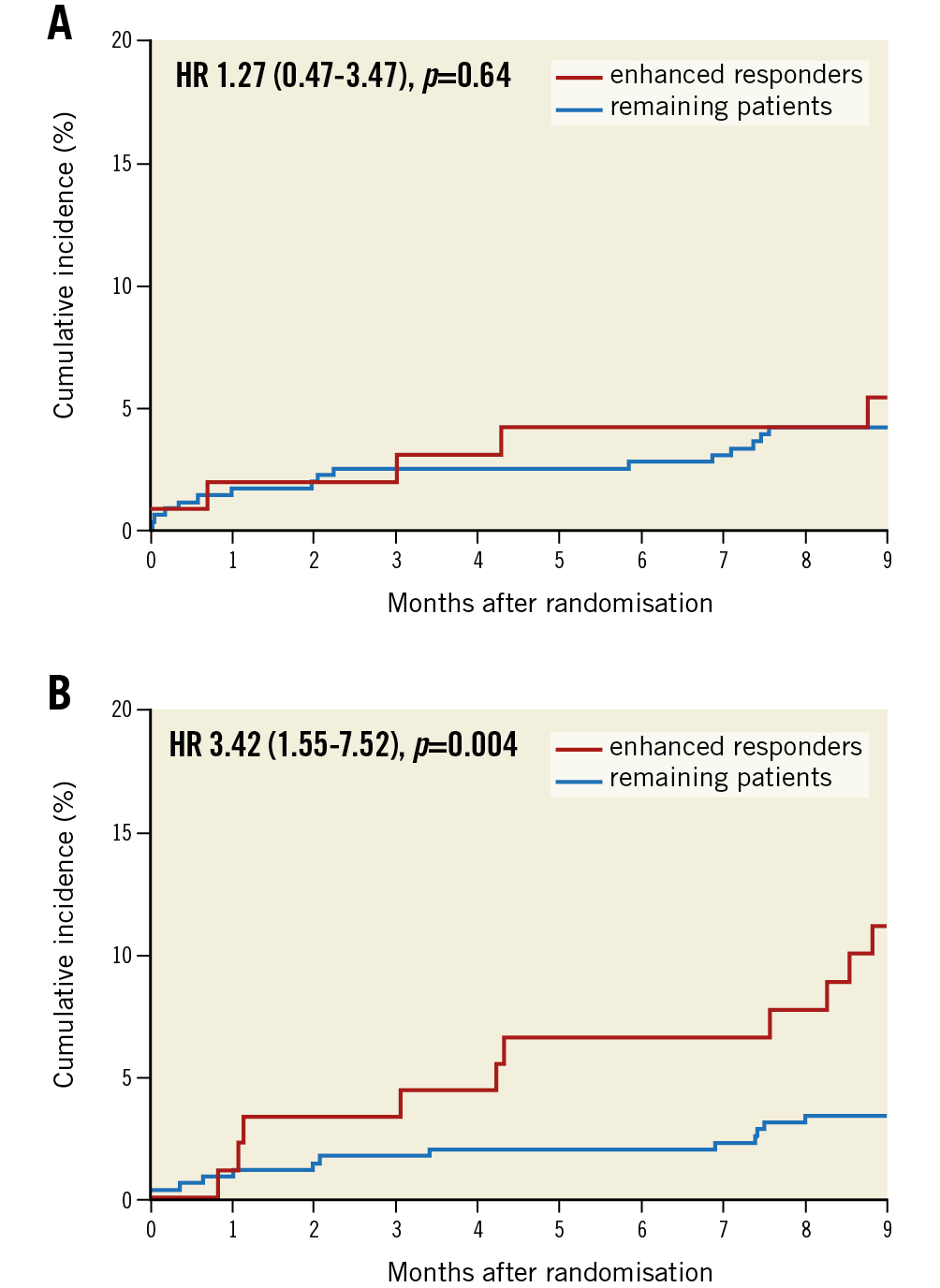
Figure 3. Cumulative incidence of the secondary ischaemic endpoint and death. Kaplan-Meier analysis of the combined ischaemic endpoint (A) and death (B) at nine months. HR: hazard ratio
The secondary net clinical outcome (a composite of death, myocardial infarction, definite stent thrombosis, stroke, or TIMI major bleeding) was reported in 19.8% of the enhanced responders and 7.7% of the remaining patients (p<0.001) at nine months (Table 2).
In addition, we performed a log-rank optimisation to determine a cut-off which maximised discrimination for the primary endpoint (TIMI major bleeding). This analysis revealed a cut-off of 49 AU*min. According to this cut-off, 46 patients were defined as enhanced responders and 412 as the remaining patients. The primary endpoint occurred in 15.4% (7 patients) of the enhanced responders as compared with 3.5% (14 patients) of the remaining patients (HR 4.79; 95% CI: 1.93 to 11.87; p=0.006 [adjusted]) at nine months.
For further evaluation we performed a sensitivity analysis considering only the first six weeks of the study. During this period all patients in the ISAR-TRIPLE trial were prescribed the same antithrombotic regimen consisting of a triple therapy of ASA, clopidogrel and OAC. The results from this analysis are in concordance with the results at the nine-month endpoint, with an even higher relative risk of TIMI major bleeding (7.6% vs 1.4%, HR 5.67; 95% CI: 1.80 to 17.9; p=0.003). Results for the secondary endpoints of the sensitivity analysis are provided in Supplementary Appendix 2.
Discussion
This analysis from the ISAR-TRIPLE cohort investigated for the first time the impact of an enhanced response to clopidogrel measured by PFT on the risk of bleeding events in patients on OAC who underwent PCI followed by DAPT with ASA and clopidogrel. The main finding is that an enhanced response to clopidogrel is associated with an increase in major bleeding events and a higher incidence of death.
Enhanced responders had a threefold higher risk of TIMI major bleeding as compared to the remaining patients, which is in the same range as previously reported for patients who were solely receiving DAPT11. Any BARC bleeding and BARC bleeding ≥2 was also significantly increased while there was no difference in TIMI minor bleeding. This observation has been seen before in a population treated solely with DAPT15; an explanation is still lacking.
PFT measurements after PCI were performed as part of the clinical routine. The Multiplate Analyzer, which was used for PFT, is one of four methods for assessing ADP-induced platelet aggregation that have been shown to predict clinical outcomes in large numbers of patients after PCI16. In our patient cohort the median ADP-induced platelet aggregation was 163 AU*min. This is lower than the value of 225 AU*min, which has been seen previously for patients receiving solely DAPT without OAC14. In the prospective studies that investigated the association of PFT with bleeding events using the Multiplate Analyzer, blood sampling for PFT was performed peri-interventionally immediately before PCI11,15. In the ISAR-TRIPLE trial, the timing of PFT was not defined by the study protocol but was performed within clinical routine. Antiplatelet therapy was not intensified in patients receiving OAC as it is known that an intensified antiplatelet therapy is associated with an increased risk of bleeding17. Instead, we re-assessed the antiplatelet effects of clopidogrel in some patients, resulting in multiple PFT values. In these patients the last PFT within 24 hours after PCI was chosen for the current analysis. It has been previously reported that antiplatelet effects of clopidogrel are time-dependent18, and PFT assessed 24 hours after PCI yields significantly lower values than when measured peri-interventionally19,20. In summary, the increased time interval of blood sampling for PFT after PCI in the ISAR-TRIPLE trial led to overall lower aggregation values in our cohort as compared to other studies11. Thus, the cut-off of 190 AU*min, that has been associated with increased bleeding events in patients treated with DAPT11, was not applicable to this population.
Several studies show that major bleeding events in patients after PCI contribute substantially to the overall mortality21. In line with this, we observed an approximately threefold higher overall mortality in patients with an enhanced response to clopidogrel, which is similar to the increase in major bleeding events observed in these patients. However, whether this association is causal cannot be concluded from the current data. Further studies are needed.
Three randomised clinical trials have investigated different antiplatelet and OAC combinations and have shown that ASA can be omitted from the therapy without a significant increase in ischaemic events5,6,7. However, concerns about ischaemic events still prevail and omittance of an antiplatelet drug (e.g., ASA) from the therapy is currently recommended only in patients at high risk for bleeding and low risk for ischaemic events2. Assessing the bleeding risk in these patients is complex. Established risk scores, such as the HAS-BLED score, failed to predict major bleeding events in patients with PCI who receive DAPT alone9 or in combination with OAC10. Our results support the use of PFT in patients undergoing PCI and having an indication for OAC to identify those at increased risk of bleeding. The results from the log-rank optimisation suggest that especially patients with very low PFT values are at high risk for major bleeding events. In these patients several different options to de-escalate antithrombotic therapy exist2: one option may be to eliminate ASA early from the treatment or even omit it completely. Other options include adapting the choice and dosage of the oral anticoagulant or shortening the duration of clopidogrel therapy.
Study limitations
The ISAR-TRIPLE trial was only powered to detect fairly large differences in events. For this analysis patient numbers were further limited due to availability of PFT. Thus, our analysis might lack statistical power, especially for differences of ischaemic events; these results should be interpreted with caution. Moreover, the exclusion of patients due to lack of availability of PFT could have led to a selection bias, although the event rates for the primary as well as the secondary endpoints were comparable among patients with and without PFT (data not shown). Another limitation is that only a single platelet function assay was used (Multiplate) and PFT was performed within clinical routine. Thus, blood sampling was not standardised in all patients, rendering established cut-off-values for bleeding not applicable to this study population. Moreover, this might also limit the predictability of bleeding events. Unfortunately, the HAS-BLED score is not available in our population because in the year 2008, when the ISAR-TRIPLE protocol was designed, this score was not routinely used to stratify the bleeding risk in patients with atrial fibrillation.
In the ISAR-TRIPLE trial patients received vitamin K antagonists. These possess a less favourable safety profile compared to non-vitamin K oral anticoagulants (NOAC)22, and current guidelines recommend the use of NOAC over vitamin K antagonists in patients with non-valvular AF requiring anticoagulation and antiplatelet therapy2. Whether the association between enhanced response to clopidogrel with major bleeding and death observed in this analysis can be transferred to patients receiving NOAC instead of a vitamin K antagonist or to patients who are treated with dual antithrombotic therapy warrants further investigation.
Conclusions
In this analysis, an enhanced response to clopidogrel was associated with higher rates of major bleeding and death in patients on OAC who require antiplatelet therapy after PCI. These results support the use of PFT to identify patients who are at increased risk for bleeding and adverse outcomes. Prospective clinical trials are needed to evaluate the clinical effect of a tailored therapy based on PFT in these patients.
|
Impact on daily practice Enhanced response to clopidogrel measured by platelet function testing is associated with an increased risk for bleeding in patients on triple antithrombotic therapy (consisting of aspirin, clopidogrel and oral anticoagulation). These patients should be closely monitored for bleeding events and de-escalation of the antithrombotic therapy may be considered. |
Conflict of interest statement
J. Mehilli has received modest lecture fees from Abbott Vascular, Boston Scientific, Biotronik, Lilly/Daiichi Sankyo, Edwards Lifesciences, and Terumo, and grants from Abbott Vascular and Edwards Lifesciences. S. Schüpke is the recipient of the Else-Kröner-Memorial Stipendium. D. Sibbing has received speaker fees and honoraria for consulting from Eli Lilly, Daiichi Sankyo, Bayer Vital, AstraZeneca, Verum Diagnostica, and Roche Diagnostics, and grants from Roche Diagnostics. M. Maeng has received fees for advisory board membership and/or lectures from Novo Nordisk, AstraZeneca, Bayer, and Boehringer-Ingelheim, and grants from Volcano, Boston Scientific, and Biosensors. H. Schunkert has received honoraria from AstraZeneca, Bayer Vital, MSD Sharp & Dohme, Novartis, Servier, Genzyme, and Sanofi-Aventis, consulting fees from MSD Sharp & Dohme, Amgen, and AstraZeneca, and grants from AstraZeneca, Bayer Vital, MSD Sharp & Dohme, Novartis, Boehringer-Ingelheim, Sanofi-Aventis, Pfizer, and Daiichi Sankyo. N. Sarafoff has received speaker fees from Boehringer-Ingelheim, and a travel grant from Bayer Vital. The other authors have no conflicts of interest to declare.

