Abstract
Background: The impact of intense low-density lipoprotein cholesterol (LDL-C) reduction using a proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor on profiles of platelet reactivity has yet to be explored.
Aims: Our aim was to investigate the effects of the PCSK9 inhibitor, evolocumab, on platelet reactivity in patients with atherosclerotic cardiovascular disease (ASCVD) on clopidogrel treatment.
Methods: This was a prospective, randomised, double-blind, placebo-controlled pharmacodynamic study in patients with ASCVD on clopidogrel treatment and with LDL-C levels ≥70 mg/dL despite a maximally tolerated statin dose. Patients were stratified according to levels of platelet reactivity using VerifyNow P2Y12 reactivity units (PRU) into high platelet reactivity (HPR; PRU >208) or normal platelet reactivity (NPR; PRU >85 and ≤208). Each cohort was randomised to receive evolocumab 420 mg or placebo. The primary endpoint was the difference in PRU at 30 days.
Results: A total of 84 patients (HPR, n=37 [19 evolocumab vs 18 placebo]; NPR, n=47 [22 evolocumab vs 25 placebo]) were included. Evolocumab significantly reduced LDL-C compared to placebo at 14 (p<0.001) and 30 (p=0.001) days. At 14 days, PRU levels were significantly lower with evolocumab compared to placebo in the HPR (218.2±29.7 vs 246.6±35.2; p=0.017), but not in the NPR cohort (141.2±42.8 vs 148.2±41.7; p=0.578). At 30 days, there were no significant differences in PRU in the HPR (219.3±38.3 vs 240.9±51.8; p=0.161) or NPR (141.5±54.3 vs 158.6±40.8; p=0.229) cohorts.
Conclusions: Compared to placebo, evolocumab in adjunct to statin therapy did not significantly reduce platelet reactivity at 30 days in ASCVD patients on clopidogrel treatment despite intense LDL-C reduction. ClinicalTrials.gov: NCT03096288.
Introduction
Clopidogrel is the most broadly used oral platelet P2Y12 receptor inhibitor in patients with atherosclerotic cardiovascular disease (ASCVD) manifestations1. Despite its established efficacy, pharmacodynamic (PD) studies have shown that approximately 30-40% of clopidogrel-treated patients have an impaired response and persist with high on-treatment platelet reactivity (HPR)23. Importantly, HPR is a marker of thrombotic risk, underscoring the need to optimise PD response profiles in clopidogrel-treated patients23. Lipid-lowering treatment with statins has been shown to reduce platelet reactivity among clopidogrel-treated patients4567. Although the exact biological mechanism(s) involved in the modulation of platelet function induced by statin therapy remain elusive, there is accumulating evidence that circulating lipoproteins may affect platelet function89. In particular, low-density lipoprotein cholesterol (LDL-C), very low-density lipoprotein (VLDL), and especially oxidised LDL-C are atherogenic lipoproteins known to increase platelet activation89. Hence, reduction of these circulating lipoproteins may potentially modulate platelet function and PD response profiles to antiplatelet drugs.
Proprotein convertase subtilisin/kexin type 9 (PCSK9) plays an essential role in regulating cholesterol homeostasis by modulating LDL-C receptor degradation, thus reducing cellular uptake10. Evolocumab is a fully human monoclonal antibody that inhibits PCSK9, leading to a marked reduction in LDL-C and a lower incidence of cardiovascular events1011. Whether such a reduction in cardiovascular events is due to solely LDL-C reduction or can also be attributed to other mechanisms, such as modulating thrombotic processes, is unknown. Of note, elevated circulating levels of PCSK9 have been associated with increased platelet reactivity12. However, the effects on platelet reactivity associated with intense LDL-C lowering induced by evolocumab remain unexplored. The aim of this study was to explore the impact of evolocumab on the PD effects of clopidogrel in patients with ASCVD.
Methods
Study design and participants
This was a prospective, randomised, double-blind, placebo-controlled PD study assessing the effects of evolocumab on platelet reactivity in patients with ASCVD treated with clopidogrel (ClinicalTrials.gov: NCT03096288). The study was performed at the University of Florida Health – Jacksonville (Jacksonville, FL, USA). Patients were screened for eligibility at the outpatient clinics of our healthcare system. Details on study inclusion and exclusion criteria are reported in the Supplementary Appendix 1. In brief, patients with ASCVD were screened and required to meet all of the following study entry criteria: a) ≥18 years old; b) treated with clopidogrel (75 mg once daily [od]), with or without low-dose aspirin (81 mg od), as per the standard of care, for at least 30 days; c) have PD profiles of clopidogrel response consistent with either normal (NPR) or high platelet reactivity according to consensus definitions3; d) fasting LDL-C ≥70 mg/dL or non-high-density lipoprotein cholesterol ≥100 mg/dL after ≥2 weeks of optimised stable lipid-lowering therapy with a maximally tolerated statin dose. Key exclusion criteria included treatment with any oral anticoagulant, treatment with any antiplatelet agent other than aspirin or clopidogrel in the prior 14 days, use of PCSK9 inhibitors in the prior 90 days or history of a serious hypersensitivity reaction to evolocumab. The study complied with the Declaration of Helsinki and was approved by the Western Institutional Review Board. All patients gave their written informed consent.
ASCVD was defined as a history of acute coronary syndromes (ACS), stable or unstable angina, coronary or other arterial revascularisation, stroke, transient ischaemic attack, or peripheral artery disease (PAD) presumed to be of atherosclerotic origin. HPR was defined as P2Y12 reactivity units (PRU) >208 by VerifyNow P2Y12 and NPR was defined as PRU between 85 and 208, in line with consensus definitions3. Patients meeting study entry criteria were stratified according to their baseline PRU into two cohorts: HPR and NPR.
After providing written informed consent, patients in each cohort were randomly assigned in a 1:1 fashion to receive a single subcutaneous administration of either evolocumab 420 mg or placebo (0.9% sodium chloride). Blood sampling for PD testing was conducted at 3 timepoints: baseline (before administration of randomised treatment), 14±2 days, and 30±2 days after randomised drug administration. At each timepoint, blood was collected before the morning dose of clopidogrel to measure trough levels of platelet reactivity (defined as 24±2 hrs after the last maintenance dose of clopidogrel). A flow diagram of the study design is illustrated in Figure 1A.
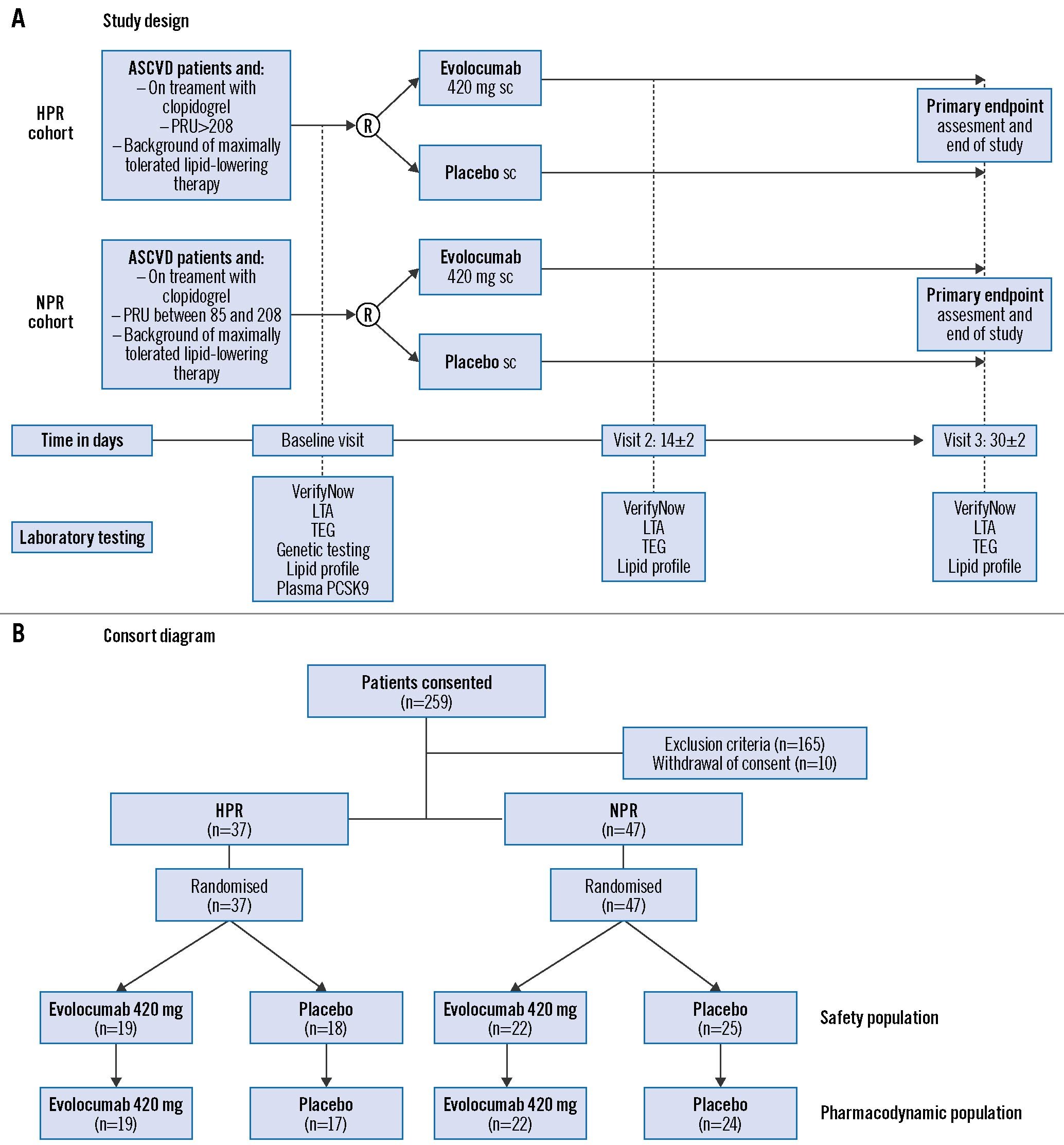
Figure 1. Study design and consort diagram. A) Study design and (B) consort diagram. ASCVD: atherosclerotic cardiovascular disease; HPR: high platelet reactivity; LTA: light transmission aggregometry; NPR: normal platelet reactivity: PCSK9: proprotein convertase subtilisin/kexin type 9; PRU: P2Y12 reactivity unit; R: randomisation; sc: subcutaneous; TEG: thromboelastography; S3: SAPIEN 3; TAV: transcatheter aortic valve; THV: transcatheter heart valve
Blood sampling and laboratory assessments
Peripheral venous blood samples were drawn through a short venous catheter inserted into a forearm vein and collected in citrate, ethylenediaminetetraacetic acid, and serum tubes as appropriate for assessments. The first 2-4 mL of blood were discarded to avoid spontaneous platelet activation. PD assessments were conducted using three different assays: the VerifyNow PRU system (Werfen) with results reported in PRU, light transmission aggregometry (LTA; Chrono-Log) following arachidonic acid (AA; 1 mM), collagen (3μg/ml), adenosine diphosphate (ADP; 5 μM and 20 μM), and thrombin receptor-activating peptide (TRAP; 15 μM) stimuli with results reported as maximum platelet aggregation (MPA%), and the thromboelastography coagulation analyser (TEG) 6s system (Haemonetics) using ADP and kaolin (thrombin pathway) as stimuli with results reported as maximum clot amplitude (MA, mm)131415. At baseline, cytochrome P450 2C19 (CYP2C19) genotyping was performed with Spartan RX rapid genotyping (Spartan Bioscience) to determine the CYP2C19 (*1,*2,*3,*17) allele status16. Lipid profiles and PCSK9 levels (R & D Systems) were also assessed. A detailed description of laboratory assessments is provided in Supplementary Appendix 2 and Supplementary Appendix 3.
Study outcomes and sample size calculation
The primary outcome was the reduction in platelet reactivity, as defined by VerifyNow PRU, between evolocumab and placebo at 30 days after randomisation in each cohort. The primary hypothesis of our study was that evolocumab would be associated with a reduction of 30 PRU in patients with HPR and NPR. This estimated treatment effect was arbitrarily chosen, given the absence of preliminary data in this setting; however, a reduction of 30 PRU approximates that previously observed with LDL-C lowering using statin therapy4. Statistical assumptions were derived for each cohort of patients based on the distribution of their PRU levels in an internal database analysis of a cohort of subjects on maintenance clopidogrel therapy (HPR: 247±29; NPR: 150±35). Based on these assumptions, a total of 34 and 46 patients were needed in the HPR and NPR cohorts, respectively, to detect a 30 PRU difference with 80% power and α of 0.05. Considering a possible dropout rate of 10%, up to a total of 90 patients were planned to be randomised. Secondary outcomes included comparing PD measures between evolocumab and placebo at each timepoint, assessed by VerifyNow PRU, LTA, TEG, HPR rates, and serum LDL-C levels.
Statistical analysis plan
Categorical variables are expressed as frequencies and percentages. Continuous variables are presented as mean±standard deviation (SD) or median and interquartile range (IQR). A t-test was used to evaluate the primary outcome and all between-group comparisons of platelet reactivity and LDL-C levels. The Fisher’s exact test or Pearson’s chi-square were used for assessing comparisons of HPR and NPR rates between groups. Several exploratory analyses were performed to assess the effect of specific variables on the primary study outcome: 1) to assess the effect of the presence of CYP2C19 loss-of-function (LOF) alleles, we compared the PRU values of the poor or intermediate metaboliser versus others; 2) to assess the effect of baseline LDL-C levels, we compared the PRU values of patients with baseline LDL-C levels above median versus below median; 3) to assess the effect of baseline PCSK9 levels, we compared the PRU values between patients with baseline PCSK9 levels above median versus below median; 4) to assess the correlation between the change (Δ: baseline – follow-up) in PRU and LDL-C, we calculated the PRU and LDL-C at 30-day follow-up and compared them by means of Spearman’s rank correlation coefficient.
A 2-tailed p-value of <0.05 was considered to indicate a statistically significant difference for all the above analyses. Our group performed statistical analyses using SPSS v28.0 software (IBM).
The safety population was composed of all patients who had received at least one dose of the study drug. The PD population included all patients with any PD data on the study drug without a major protocol deviation interfering the PD response.
Results
Patient population
Between October 2017 and October 2020, 259 subjects were identified and consented to participate in the study. Of these, 165 patients were screen failures and 10 patients withdrew. Thus, 84 patients (HPR, n=37; NPR, n=47) were randomised; all randomised patients were exposed to the study drug, representing the safety population; 82 patients (HPR, n=36; NPR, n=46) had valid PD data, representing the PD population. In the HPR cohort, 19 were randomised to evolocumab and 18 to placebo; in the NPR cohort, 22 to evolocumab and 25 to placebo. A consort diagram of the study population is illustrated in Figure 1B.
Baseline characteristics of the study population according to baseline platelet reactivity status (NPR or HPR) are summarised in Table 1 and Supplementary Table 1. There were no significant differences between groups, except for a higher frequency of family history of coronary artery disease (CAD) and oral hypoglycaemic drugs in the placebo group in the HPR cohort and older age in the evolocumab group in the NPR cohort. There were no differences in baseline LDL-C levels between patients allocated to evolocumab versus placebo in the HPR (107.3±29.1 mg/dL vs 110.2±43.1 mg/dL; p=0.808) and NPR (91.2±30.5 mg/dL vs 99.6±31.3 mg/dL; p=0.365) cohorts (Supplementary Table 1).
In the HPR cohort, 17 patients were carriers of CYP2C19 LOF alleles (all heterozygotes), without differences in frequency between the evolocumab group and placebo (57.9% vs 33.3%; p=0.169). In the NPR cohort, 10 patients were carriers of CYP2C19 LOF alleles (9 heterozygotes and 1 homozygote) without differences in frequency between the evolocumab group and placebo (18.2% vs 24.0%; p=0.583) (Supplementary Table 2).
One ischaemic adverse event (non-ST-segment elevation myocardial infarction) occurred in the HPR cohort in a patient allocated to placebo. A complete list of adverse events is shown in Supplementary Table 3.
Table 1. Baseline characteristics of the safety population.
| HPR (n=37) | NPR (n=47) | ||||||
|---|---|---|---|---|---|---|---|
| Evolocumab (n=19) | Placebo (n=18) | p-value | Evolocumab (n=22) | Placebo (n=25) | p-value | ||
| Age, yrs | 63.4±8.1 | 62.5±8.1 | 0.794 | 63.3±8.2 | 58.3±8.1 | 0.040 | |
| Female gender | 8 (42.1) | 12 (66.7) | 0.191 | 10 (45.5) | 8 (32.0) | 0.382 | |
| BMI, kg/m2 | 32.8±8.6 | 32.0±7.0 | 0.747 | 30.3±5.6 | 31.2±6.1 | 0.561 | |
| Active smoking | 6 (31.6) | 5 (27.8) | 0.451 | 8 (36.4) | 7 (28.0) | 0.280 | |
| Hypertension | 17 (89.5) | 17 (94.4) | 0.521 | 20 (90.9) | 23 (92.0) | 0.645 | |
| Diabetes | 7 (36.8) | 11 (61.1) | 0.194 | 13 (59.1) | 14 (56.0) | 0.533 | |
| Hyperlipidaemia | 19 (100.0) | 18 (100.0) | - | 20 (90.9) | 24 (96.0) | 0.593 | |
| Family history of CAD | 8 (42.1) | 14 (77.8) | 0.045 | 10 (45.5) | 8 (32.0) | 0.313 | |
| Peripheral artery disease | 6 (31.6) | 5 (27.8) | 0.543 | 9 (40.9) | 6 (24.0) | 0.347 | |
| Stroke/TIA | 4 (21.1) | 7 (38.9) | 0.295 | 5 (22.7) | 3 (12.0) | 0.446 | |
| Hepatic dysfunction | 0 | 0 | - | 0 | 0 | - | |
| Prior MI | 9 (47.4) | 8 (44.4) | 0.560 | 11 (50.0) | 12 (48.0) | 0.562 | |
| Prior PCI | 12 (63.2) | 12 (66.7) | 0.548 | 14 (63.6) | 15 (60.0) | 0.502 | |
| Prior CABG | 3 (15.8) | 4 (22.2) | 0.490 | 7 (31.8) | 10 (40.0) | 0.762 | |
| CHF | 3 (15.8) | 4 (22.2) | 0.693 | 4 (18.2) | 3 (12.0) | 0.450 | |
| LVEF, % | 55.0±12.2 | 51.9±11.3 | 0.720 | 52.1±9.5 | 50.6±9.3 | 0.603 | |
| Medications | Aspirin | 16 (84.2) | 13 (72.2) | 0.447 | 18 (81.8) | 22 (88.0) | 0.690 |
| β-blocker | 15 (78.9) | 15 (83.3) | 0.553 | 18 (81.8) | 20 (80.0) | 0.586 | |
| ACEi/ARB | 16 (84.2) | 18 (100.0) | 0.230 | 12 (54.5) | 15 (60.0) | 0.549 | |
| High-dose statin | 18 (94.7) | 18 (100.0) | 0.528 | 21 (95.4) | 21 (84.0) | 0.352 | |
| Nitrates | 7 (36.8) | 7 (38.9) | 0.583 | 6 (27.3) | 9 (36.0) | 0.279 | |
| Proton pump inhibitor | 4 (21.1) | 3 (16.7) | 0.532 | 6 (27.3) | 13 (52.0) | 0.136 | |
| Calcium channel blocker | 7 (36.8) | 10 (55.6) | 0.330 | 12 (54.5) | 11 (44.0) | 0.564 | |
| Oral hypoglycaemic | 3 (15.8) | 9 (50.0) | 0.038 | 5 (22.7) | 12 (48.0) | 0.127 | |
| Insulin | 5 (26.3) | 8 (44.4) | 0.336 | 6 (27.3) | 6 (24.0) | 0.963 | |
| Values are mean±SD or n (%). ACEi: angiotensin-converting enzyme inhibitors; ARB: angiotensin receptor blockers; BMI: body mass index; CABG: coronary artery bypass graft; CAD: coronary artery disease; CHF: congestive heart failure; HPR: high platelet reactivity; LVEF: left ventricular ejection fraction; MI: myocardial infarction; NPR: normal platelet reactivity; PCI: percutaneous coronary intervention; SD: standard deviation; TIA: transient ischaemic attack | |||||||
Lipid profile findings
At 14 days, there was a significant reduction of the LDL-C levels with evolocumab compared to placebo in both the HPR (36.1±27.0 mg/dL vs 100.9±41.1 mg/dL; p<0.001) and NPR (18.0±14.6 mg/dL vs 91.2±32.2 mg/dL; p<0.001) cohorts. Reduced LDL-C levels persisted, albeit not as marked, at 30 days (HPR cohort: 45.9±15.9 mg/dL vs 103.8±42.6 mg/dL; p=0.001; NPR cohort: 30.9±12.0 mg/dL vs 95.0±34.3 mg/dL; p=0.001) (Figure 2). Complete lipid profile results are provided in Supplementary Table 4.
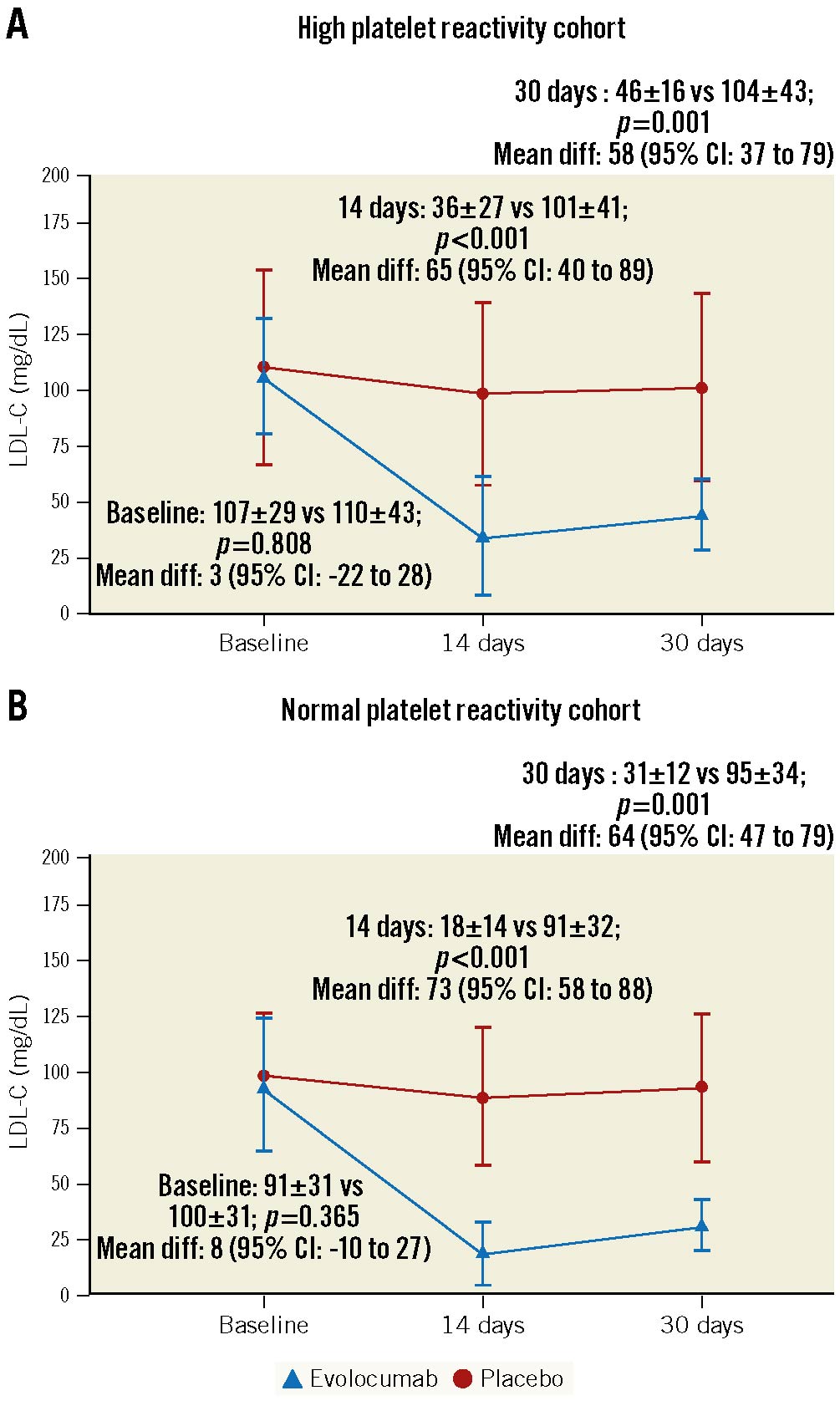
Figure 2. LDL-C levels according to randomised drug and platelet reactivity status. Differences in LDL-C levels between evolocumab and placebo in the high platelet reactivity (HPR, n=36) and normal platelet reactivity cohorts (NPR, n=46) are reported as mean±SD and mean difference with 95% confidence intervals (95% CI). Dashed line represents ≥70 mg/dL. Values are expressed as mean. Error bars indicate SD. LDL-C: low-density lipoprotein cholesterol; SD: standard deviation
Pharmacodynamic findings
There were no differences in baseline PRU levels between patients randomised to evolocumab versus placebo in the HPR (239.5±26.7 vs 248.5±37.5; p=0.419) and NPR (144.9±38.3 vs 148.8±29.7; p=0.704) cohorts. Baseline PD findings are summarised in Supplementary Table 5. PRU levels were significantly reduced in patients randomised to evolocumab compared to placebo at 14 days in the HPR (218.2±29.7 vs 246.6±35.2; p=0.017; mean difference: 28.4 [95% confidence interval {CI}: 5.4 to 51.4]), but not in the NPR (141.2±42.8 vs 148.2±41.7; p=0.578; mean difference: 7.0 [95% CI: −18.1 to 32.1]) cohort (Figure 3, Supplementary Table 6). At 30 days, despite a numerical reduction, there were no significant differences in PRU levels, the primary endpoint of the study, between evolocumab and placebo in the HPR (219.3±38.3 vs 240.9±51.8 PRU; p=0.161; mean difference: 21.6 [95% CI: −9.0 to 52.3]) or NPR (141.5±54.3 vs 158.6±40.8 PRU; p=0.229; mean difference: 17.2 [95% CI: −11.7 to 46.0]) cohorts (Figure 3, Table 2).
No significant differences in PD profiles between evolocumab and placebo were observed using other assays, including LTA with different agonists (AA, ADP, collagen and TRAP) and TEG, with the exception of MPA% with ADP 5 μM (evolocumab: 27.6±14.7 vs placebo: 37.9±18.9; p=0.046) and TEG ADP MA HPR rate (evolocumab: 63.6% vs placebo: 91.7%; p=0.021) in the NPR cohort at 30 days (Figure 4, Table 2, Supplementary Table 6).
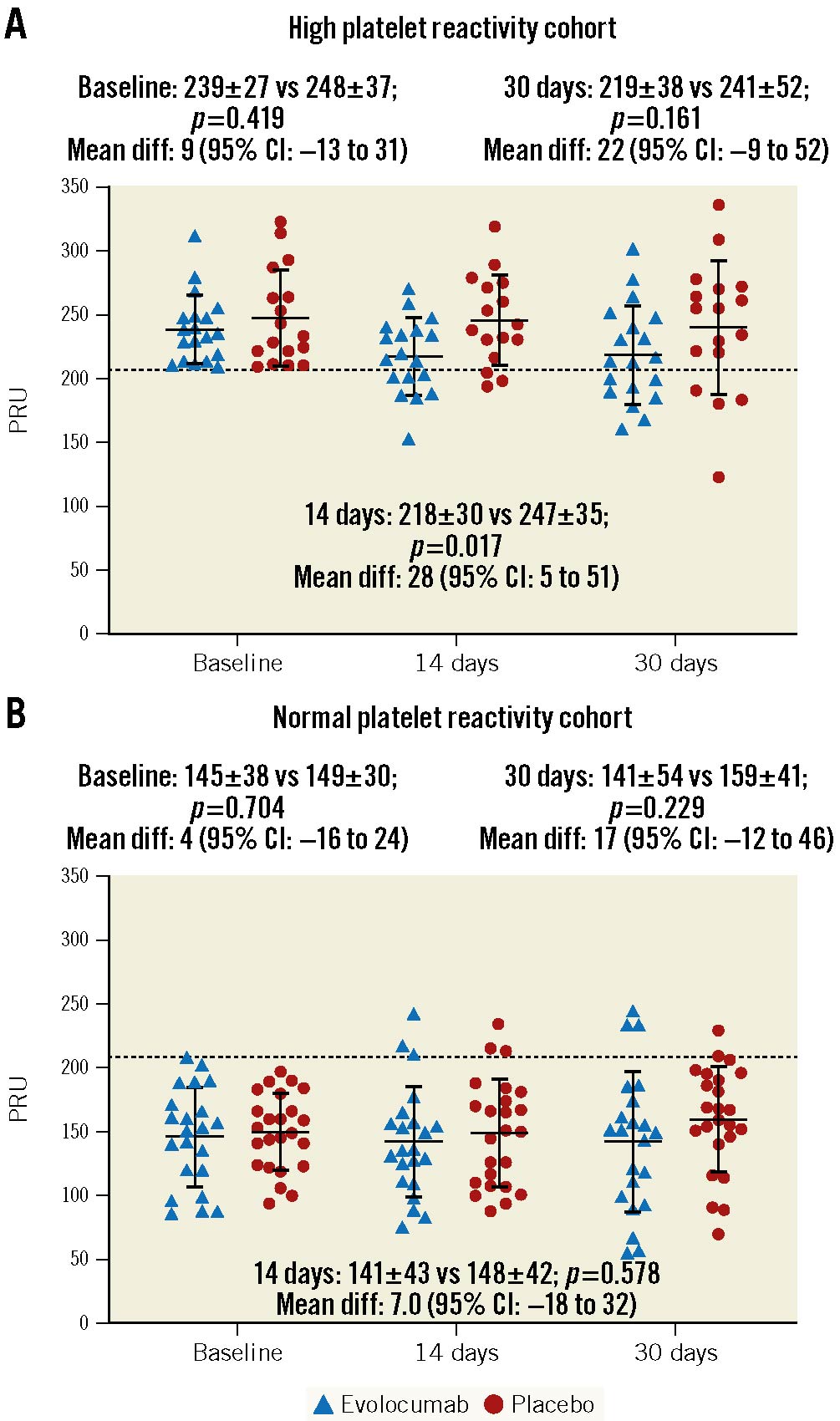
Figure 3. Individual values of PRU according to randomised drug and platelet reactivity status. Differences in P2Y12 reactivity units (PRU) between evolocumab and placebo in the high platelet reactivity (HPR, n=36) and normal platelet reactivity cohorts (NPR, n=46) are reported as mean±SD and mean difference with 95% confidence intervals (95% CI). Dashed line represents the predefined cut-off of HPR, >208 PRU. Solid lines with error bars indicate mean and SD. SD: standard deviation
Table 2. Pharmacodynamic findings at 30-day follow-up.*
| HPR (n=36) | NPR (n=46) | |||||
|---|---|---|---|---|---|---|
| Evolocumab (n=19) | Placebo (n=17) | p-value | Evolocumab (n=22) | Placebo (n=24) | p-value | |
| VerifyNow PRU | 219.3±38.3 | 240.9±51.8 | 0.161 | 141.5±54.3 | 158.6±40.8 | 0.229 |
| HPR status | 11 (57.9) | 13 (76.5) | 0.238 | 3 (13.6) | 1 (4.2) | 0.255 |
| ADP 20 µL | 61.2±13.0 | 61.4±12.2 | 0.963 | 42.7±17.3 | 53.7±20.5 | 0.057 |
| HPR status | 10 (52.6) | 9 (52.9) | 0.985 | 4 (19.0) | 8 (33.3) | 0.280 |
| ADP 5 µL | 43.5±14.3 | 48.4±14.5 | 0.311 | 27.6±14.7 | 37.9±18.9 | 0.046 |
| HPR status | 8 (42.1) | 8 (47.1) | 0.765 | 3 (14.3) | 7 (29.2) | 0.231 |
| AA 1 mM† | 2.0 (1.0-3.5) | 34.0 (1.5-69.5 | 0.260 | 1.0 (0.0-3.0) | 2.0 (0.0-42.0) | 0.308 |
| TRAP 15 µM | 73.9±8.8 | 75.1±10.4 | 0.707 | 66.4±17.6 | 72.1±10.4 | 0.204 |
| Collagen 3 µg/mL† | 53.8±24.2 | 65.3±14.3 | 0.127 | 36.9±29.9 | 44.5±30.5 | 0.446 |
| TEG HKH MA | 66.1±2.7 | 65.8±2.4 | 0.777 | 63.5±4.9 | 63.6±3.7 | 0.949 |
| TEG ADP MA | 63.3±11.3 | 62.8±4.1 | 0.838 | 51.2±15.5 | 56.9±9.4 | 0.145 |
| HPR status | 17 (89.5) | 17 (100.0) | 0.169 | 14 (63.6) | 22 (91.7) | 0.021 |
| Values are mean±SD, median (interquartile range), or n (%). *Calculated in the pharmacodynamic population. †Calculated only in patients on concomitant aspirin treatment. AA: arachidonic acid; ADP: adenosine diphosphate; HKH: kaolin with heparinase; HPR: high platelet reactivity; MA: maximum amplitude; NPR: normal platelet reactivity; PRU: P2Y12 reactivity units; SD: standard deviation; TEG: thromboelastography coagulation analyser; TRAP: thrombin receptor activator peptide | ||||||
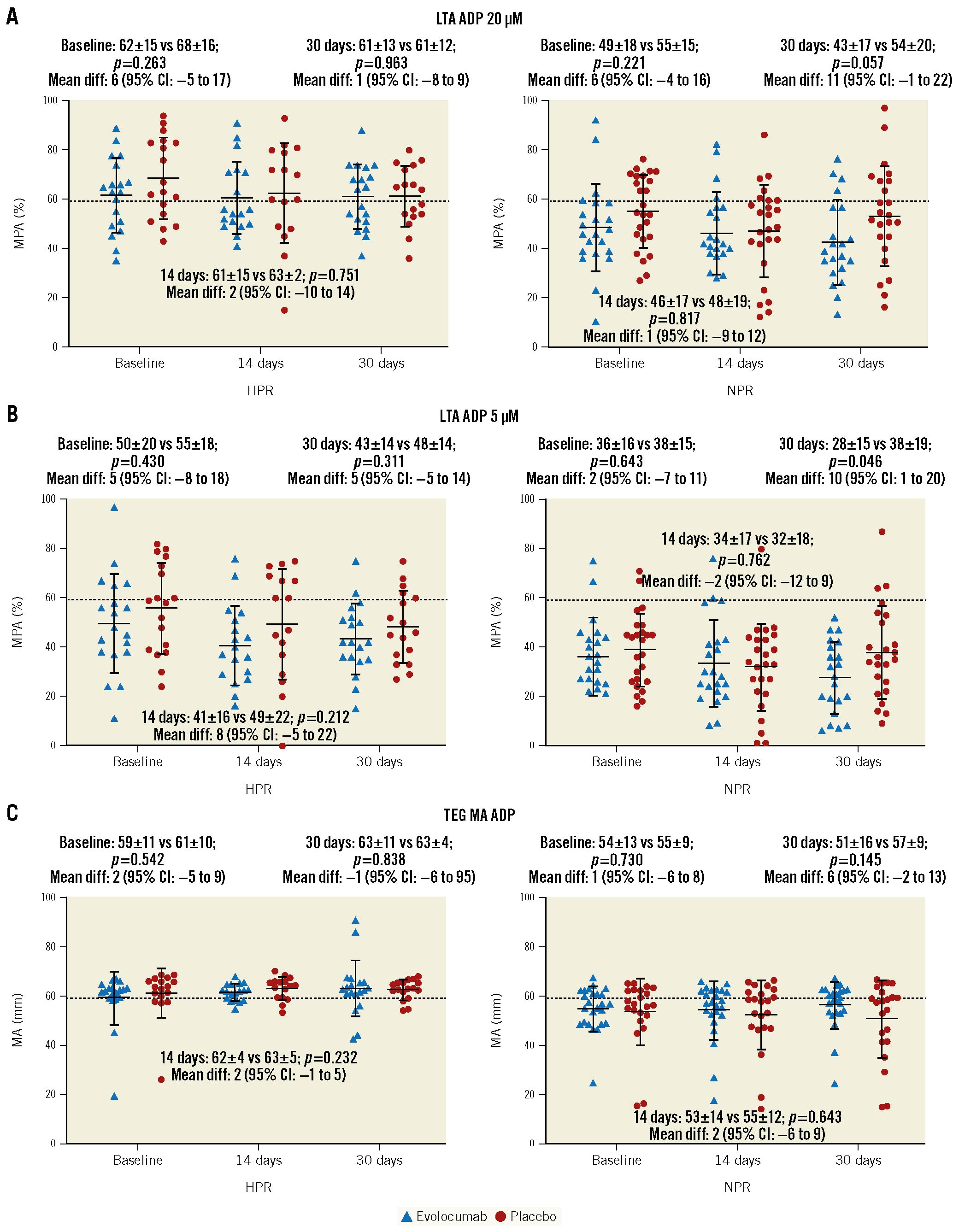
Figure 4. Individual values of platelet reactivity measured by different assays according to randomised drug and platelet reactivity status. Differences in platelet reactivity tests between evolocumab and placebo in the high platelet reactivity (HPR, n=36) and normal platelet reactivity cohorts (NPR, n=46) are reported as mean±SD and mean difference with 95% confidence intervals (95% CI). A) Light transmission aggregometry (LTA) adenosine diphosphate (ADP) 20 µM is reported as maximum platelet aggregation percentage (MPA%). B) LTA ADP 5µM is reported as maximum platelet aggregation percentage (MPA%). C) Thromboelastography coagulation analyser (TEG) thrombin reported as maximum amplitude (MA) in mm. Dashed lines represent the predefined cut-off of HPR in the respective test. A and B: >59 MPA%; C: >47 mm. Solid lines with error bars indicate mean and SD. SD: standard deviation
Correlation and exploratory analyses at 30-day follow-up
At baseline, in the HPR and NPR cohorts, there were moderate to high correlations between PRU and baseline levels of LDL-C (rs=0.641; p=0.001 and rs=0.410; p=0.005, respectively); there was no correlation with circulating PCSK9 levels in any cohort. At 14 and 30 days, there was a moderate correlation between PRU and LDL-C in the HPR cohort (rs=0.453; p=0.008 and rs=0.356; p=0.033, respectively), without a correlation in the NPR cohort (rs=0.175; p=0.245 and rs=0.245; p=0.101, respectively). In the HPR cohort, at 14 days, there was a moderate correlation between ΔLDL-C and ΔPRU (rs=0.361; p=0.039), but not at 30 days (rs=0.104; p=0.545). In the NPR cohort, there were no correlations between ΔLDL-C and ΔPRU, neither at 14 days (rs= −0.111; p=0.463) nor 30 days (rs= −0.017; p=0.908).
At 30 days, among patients with baseline circulating levels of PCSK9 above the median, in the HPR cohort, there was a significant difference in PRU between evolocumab and placebo (209.7±33.3 vs 253.9±42.0; p=0.020), without a difference in the NPR cohort (162.3±55.1 vs 165.6±39.7; p=0.880). Among patients with baseline circulating levels of PCSK9 below the median, there were no differences in PRU between evolocumab and placebo in the HPR (227.8±44.8 vs 226.4±60.6; p=0.960) and NPR (130.5±46.2 vs 150.5±44.1; p=0.300) cohorts (Figure 5, Supplementary Table 7, Supplementary Table 8).
At 30 days, among carriers of a CYP2C19 LOF allele in the HPR cohort, there were significant differences in PRU levels between evolocumab compared to placebo (214.5±30.4 vs 261.8±45.8; p=0.027); there were no differences in the NPR cohort (176.8±51.5 vs 173.8±25.5; p=0.907). In non-carriers of a CYP2C19 LOF allele, there were no significant differences in PRU levels between evolocumab and placebo in the HPR cohort (225.3±52.4 vs 240.8±45.1; p=0.523) or NPR (127.1±51.5 vs 161.5±42.3; p=0.059) cohort (Figure 5, Supplementary Table 9, Supplementary Table 10). Full results of the exploratory analyses are reported in Figure 5 and Supplementary Table 7-Supplementary Table 13.
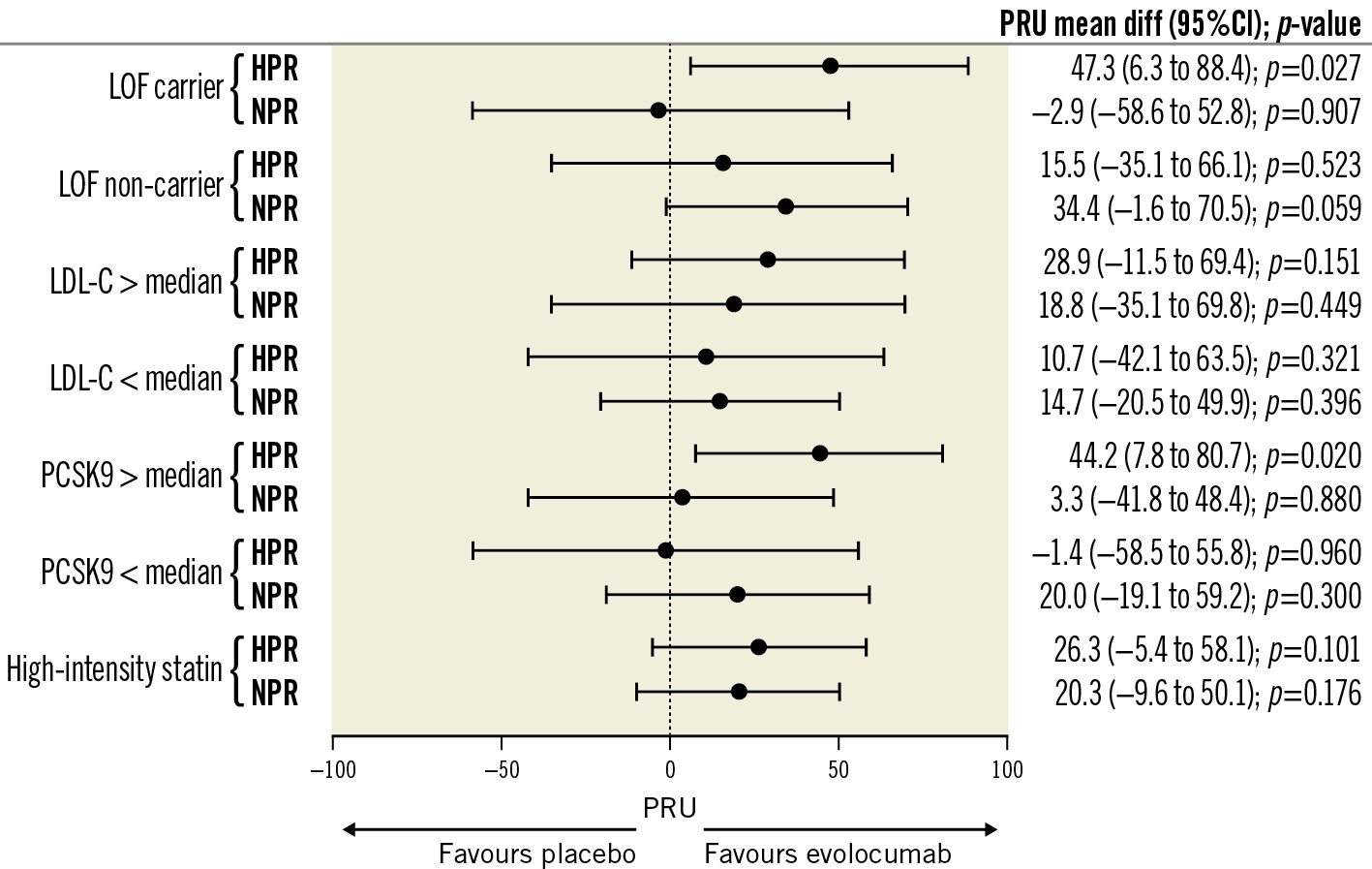
Figure 5. Mean difference in PRU values according to HRP and NPR cohort and randomised drug in different exploratory analyses. Differences in PRU values between evolocumab and placebo are reported as mean±SD and mean difference with 95% confidence intervals (95% CI). >median denotes above the median of plasma levels and
Discussion
The present investigation aimed to assess the PD effects of evolocumab versus placebo on profiles of platelet reactivity in ASCVD patients treated with clopidogrel and a maximally tolerated dose of a statin. The key findings of this study can be summarised as follows: 1) compared to placebo, evolocumab was associated with a significant reduction in LDL-C levels at 14- and 30-day follow-up; 2) in patients with HPR, although at 14 days evolocumab was associated with a significant reduction in PRU, this was not confirmed at 30 days where the reduction did not reach statistical significance (primary endpoint); 3) in patients with NPR, evolocumab compared to placebo was not associated with a significant reduction in PRU at 14 and 30 days; 4) our overall PD findings were corroborated by a number of assays; and 5) exploratory analyses suggest that patients with HPR and elevated PCSK9 levels or who are carriers of CYP2C19 LOF alleles may experience an enhanced degree of reduction in platelet reactivity with evolocumab (Central illustration).
Although clopidogrel therapy has been shown to be efficacious in different high-risk settings of patients with AVSCD, rates of ischaemic recurrences remain elevated despite this treatment regimen23. This can be, in part, attributed to the high interindividual variability in responses to clopidogrel23. Intuitively, the use of the more potent P2Y12 receptor inhibitors, prasugrel and ticagrelor, represents a potential treatment option17. However, the risk of bleeding complications with their prolonged use has limited their broad uptake in clinical practice for long-term secondary prevention. The pleiotropic effects on thrombosis associated with lipid-lowering therapies, in particular statins, have been the subject of extensive research8. In statin-naïve patients with stable CAD and HPR, treatment with high-dose atorvastatin in addition to double-dose clopidogrel reduced platelet reactivity significantly more than double-dose clopidogrel alone4. To date, the exact biological mechanisms involved in statin-mediated modulation of platelet function are not fully understood, although likely attributed to both their lipid-lowering and non-lipid-related effects18. In fact, there is increasing evidence that circulating lipoproteins affect platelet function. In particular, LDL-C, VLDL, and especially oxidised LDL-C are atherogenic lipoproteins and increase platelet activation9. Also, familial hypercholesterolaemia is associated with increased platelet activity, such as hyperaggregability, and the application of a lipid-lowering drug resulted in a reduction of platelet reactivity19. It may therefore be argued that achieving intensified reduction of circulating lipoproteins may have an even greater impact on modulating platelet function than that observed with statins alone20.
This is the first randomised controlled study assessing the role of intense LDL-C reduction with evolocumab on platelet function in ASCVD patients on clopidogrel treatment. In the HPR cohort, there was a significant reduction in PRU at 14 days which, however, was not sustained at 30 days, despite the numerical reduction. It may be argued that such findings could be attributed to the different magnitude of the LDL-C lowering effect over time. In fact, in our study evolocumab was associated with intense LDL-C reduction compared to placebo at 14- and 30-day follow-up, but this reduction was more pronounced at 14 than at 30 days. Consistent with our study, dose-finding studies of evolocumab also showed that 420 mg monthly was associated with an intense LDL-C level reduction (~70% reduction from baseline) at 2 weeks with a slight increase over 4 to 8 weeks. Instead, a dosing regimen of 140 mg every 2 weeks was associated with a less intense initial LDL-C level reduction (~55% reduction from baseline) but a more steady effect at 4 to 8 weeks21. These patterns have also been found in PD simulation models comparing different dosing regimens22. Our assumption is supported by findings from an in vitro study showing that atorvastatin dose-dependently inhibited platelet activation2223. In addition, in the HPR cohort, at 14-day follow-up, there was a moderate correlation between ΔLDL-C and ΔPRU, but not at 30 days. Conversely, the reduction in PRU in the NPR cohort was very modest and non-significant. Overall, although our study suggests the potential for a PCSK9 inhibitor to modulate profiles of clopidogrel-induced platelet inhibition with an effect that depends on the magnitude of further LDL-C reduction, this was not fully supported by our investigation. Indeed, a treatment effect is more likely to occur among patients with HPR which is no longer observed or may be negligible if platelets are already adequately inhibited by clopidogrel.
In line with prior investigations, we found a moderate correlation between LDL-C levels and PRU at baseline8. However, we did not find a correlation between PCSK9 levels and PRU. This could be related to the use of aspirin in most of our patients, which may modulate PCSK9 levels24. In fact, preclinical models have shown that circulating PCSK9 directly enhances platelet activation and in vivo thrombosis by binding to platelet CD36 and that this effect can be mitigated by the use of aspirin or PCSK9 inhibitors2425. In a prospective observational study conducted in ACS patients undergoing percutaneous coronary intervention treated with more potent P2Y12 inhibitors (i.e., prasugrel or ticagrelor), which are known to be associated with low platelet reactivity, elevated PCSK9 levels have been suggested to be associated with increased platelet reactivity and higher rates of ischaemic events8. However, in our study we enrolled patients with stable ACSVD while on clopidogrel, and we excluded those with low platelet reactivity. Overall, our data, rather than excluding the role of circulating PCSK9 in platelet reactivity in ASCVD, may underscore the gaps in knowledge of these complex biological pathways.
Exploratory analyses showed no differences in most PD outcomes assessed according to baseline LDL-C, baseline circulating PCSK9 levels, or concomitant treatment with high-dose statins. However, we found significant differences in PRU values in favour of evolocumab over placebo in the HPR cohort among carriers of CYP2C19 LOF alleles and patients with baseline levels of PCSK9 above the median. The reasons for these findings are unknown and may be attributed to play of chance. Nevertheless, these findings may be considered as hypothesis-generating for future research.
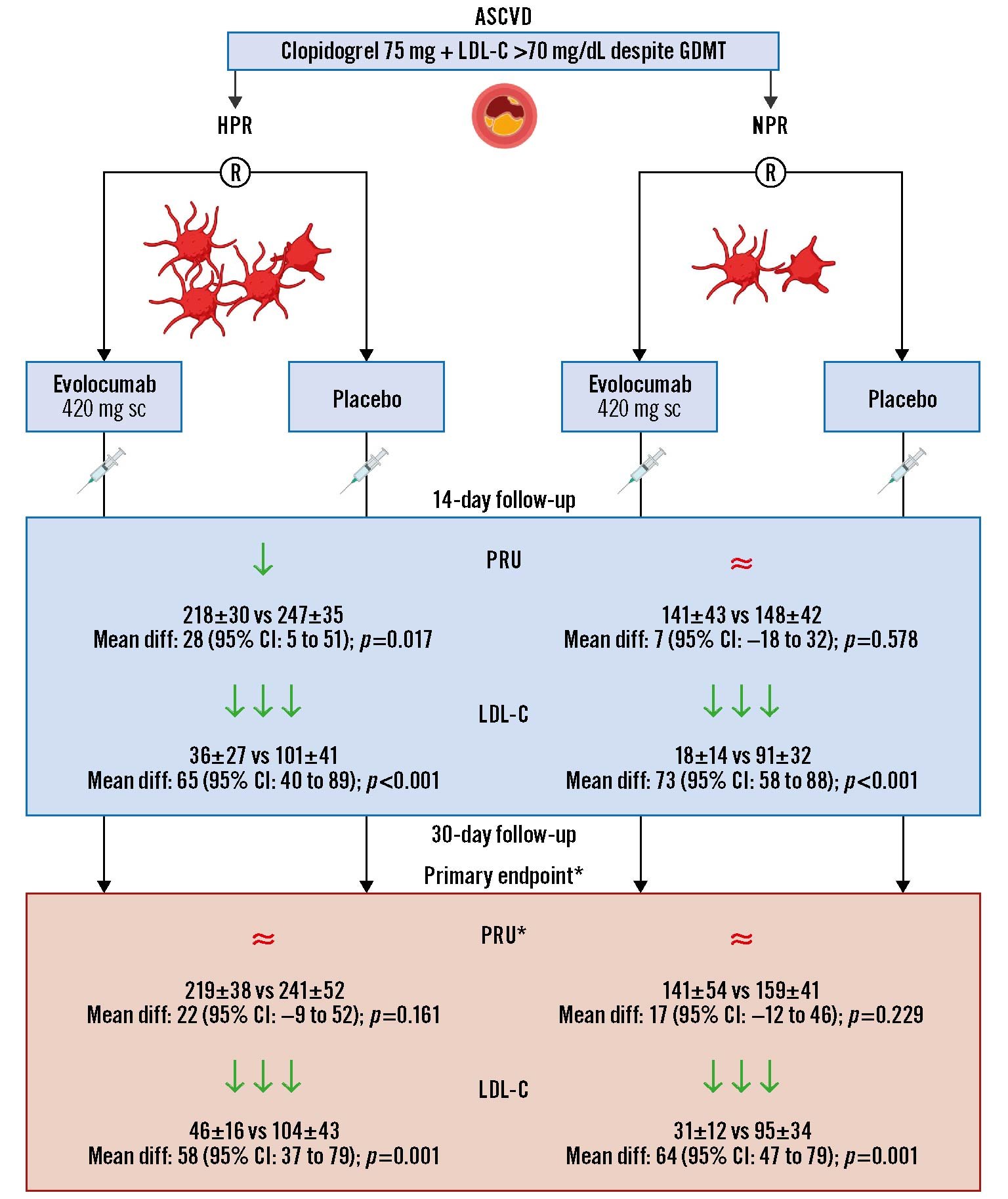
Central illustration. Impact of evolocumab on the pharmacodynamic profiles of clopidogrel in patients with atherosclerotic cardiovascular disease. The green arrow (↓) denotes a statistically significant reduction, and the red approximation symbol (≈) denotes no statistical difference. Values are reported as mean±SD and mean difference (95% confidence interval). ClinicalTrials.gov: NCT03096288. *The primary endpoint was the difference in PRU at 30 days. ASCVD: atherosclerotic cardiovascular disease; CI: confidence interval; LDL-C: low-density lipoprotein cholesterol; GDMT: guideline-directed medical therapy; PRU: P2Y12 reactivity units; R: randomisation; sc: subcutaneous; SD: standard deviation
Limitations
Some limitations should be acknowledged. First, the PD nature of this study does not allow us to make any definitive conclusions on the clinical impact of our findings. Second, only one dosing regimen of evolocumab (420 mg subcutaneous monthly) was studied with a follow-up at 30 days. Third, our study excluded patients with low platelet reactivity and arbitrarily assumed a similar treatment effect in both the NPR and HPR cohorts. The rationale for this was that it would be unlikely for a treatment effect to be observed in these subjects, which is supported by our study findings that only the patients with HPR were those with a PD efficacy signal. Furthermore, at the time of the trial design, there were no specific data on the treatment effects of lipid-lowering therapies in NPR patients with different profiles of clopidogrel response. Fourth, a type II error due to sample size cannot be ruled out. Whether a larger sample size would have resulted in significant differences in platelet reactivity between evolocumab and placebo is unknown and would warrant further investigation. Of note, in our study we found a mean difference in PRU of 28 in the HPR cohort and 10 in the NPR at 14 days, and one of 22 in the HPR and 17 in NPR at 30 days, respectively. Even though our study was powered to detect a difference of 30 PRU, an individual patient data meta-analysis involving over 3,000 patients reported that for every 10-unit increase in PRU value, there is a 4% increase in serious adverse cardiac events26. Moreover, our study was conducted among patients on a maximally tolerated dose of statins, which have previously been shown to enhance clopidogrel-induced platelet inhibition4. Thus, it may be argued that evolocumab could have led to a greater magnitude of clopidogrel-induced platelet inhibition in stain-naïve patients. However, the effects of evolocumab on profiles of platelet reactivity among statin-naïve patients treated with clopidogrel remain unknown. Fifth, this trial was designed to assess the PD effects of evolocumab in stable ACSVD patients treated with clopidogrel. Therefore, the results cannot be extended to ACS patients or patients treated with other oral P2Y12 inhibitors. However, ongoing randomised controlled trials are evaluating the role of PCSK9 inhibition in ACS patients2728. Ultimately, despite randomisation, there were minor imbalances between the evolocumab and placebo groups in the HPR cohort (family history of CAD and oral hypoglycaemic drugs). However, as these imbalances were observed post hoc and are not correlated with the primary endpoint, no adjustments to the primary analysis were made29.
Conclusions
In patients with ASCVD on clopidogrel treatment and with LDL-C levels above target despite a maximally tolerated statin dose, evolocumab was not associated with a significant reduction in platelet reactivity compared with placebo. Among patients with HPR, at 14 days, evolocumab was associated with a significant reduction in platelet reactivity, but this was not sustained at 30 days. Patients with NPR had non-significant reductions in platelet reactivity throughout the study. In both cohorts, evolocumab was associated with an intense reduction in LDL-C levels at 14 and 30 days.
Impact on daily practice
High on-treatment platelet reactivity while on clopidogrel is a marker of thrombotic risk. Optimising clopidogrel-induced antiplatelet effects is therefore an unmet clinical need. While potent P2Y12 inhibitors can overcome HPR, the increased risk of bleeding limits their long-term use. LDL-C reduction using statins enhances clopidogrel-induced platelet inhibition. However, in this study, intense LDL-C reduction with PCSK9 inhibition with evolocumab did not significantly decrease platelet reactivity compared to a placebo among clopidogrel-treated patients on statin therapy. Nevertheless, among HPR patients, when LDL-C levels were the lowest and certain individual profiles were present, results suggested a potential treatment effect on platelet function with evolocumab. These observations support potential avenues of clinical investigation aimed at identifying patient cohorts in whom pleiotropic effects associated with evolocumab use may be observed.
Guest Editor
This paper was guest edited by Franz-Josef Neumann, MD; Department of Cardiology and Angiology II, University Heart Center Freiburg – Bad Krozingen, Bad Krozingen, Germany.
Acknowledgements
Dr. Ortega-Paz is supported by a grant from the Spanish Society of Cardiology (SEC/MHE-MOV-INT 20/001).
Funding
The present study was funded by an investigator-initiated grant from Amgen. Amgen had no role in the study design conception, conduct of the study, or decision to publish these results.
Conflict of interest statement
F. Franchi declares that he has received payment as an individual for consulting fees or honoraria from AstraZeneca, Bayer and Sanofi; and institutional payments for grants from PLx Pharma and The Scott R. MacKenzie Foundation. D.J. Angiolillo declares that he has received consulting fees or honoraria from Abbott, Amgen, AstraZeneca, Bayer, Biosensors, Boehringer Ingelheim, Bristol-Myers Squibb, Chiesi, Daiichi Sankyo, Eli Lilly, Haemonetics, Janssen, Merck, Novartis, PhaseBio, PLx Pharma, Pfizer, and Sanofi; he also declares that his institution has received research grants from Amgen, AstraZeneca, Bayer, Biosensors, CeloNova, CSL Behring, Daiichi Sankyo, Eisai, Eli Lilly, Gilead, Idorsia, Janssen, Matsutani Chemical Industry Co., Merck, Novartis, and the Scott R. MacKenzie Foundation. The other authors have no conflicts of interest to declare. The Guest Editor reports lecture fees paid to his institution from Amgen, Bayer Healthcare, Biotronik, Boehringer Ingelheim, Boston Scientific, Daiichi Sankyo, Edwards Lifesciences, Ferrer, Pfizer, and Novartis; consultancy fees paid to his institution from Boehringer Ingelheim; and grant support from Bayer Healthcare, Boston Scientific, Biotronik, Edwards Lifesciences, GlaxoSmithKline, Medtronic, and Pfizer.
Supplementary data
To read the full content of this article, please download the PDF.

