Proprotein convertase subtilisin/kexin type 9 (PCSK9) is a serine protease that is secreted by the liver, binds to the low-density lipoprotein (LDL) receptor, and directs the LDL receptor toward lysosomal degradation, resulting in decreased cellular LDL cholesterol (LDL-C) uptake and higher plasma LDL-C levels. PCSK9 affects not only LDL-C metabolism but also other lipoproteins, inflammation, and thrombosis. Previous in vitro and in vivo studies have suggested that PCSK9 inhibition reduces platelet activation by decreasing platelet LOX-1 expression and generation of oxidised LDL (Figure 1)34. To date, randomised data investigating the in vivo effect of PCSK9 inhibitor on platelet function are limited.
In this issue of EuroIntervention, Franchi et al5 report the results of a single-centre, randomised, double-blind, placebo-controlled study which investigated the effect of evolocumab 420 mg on top of maximally tolerated statin therapy on pharmacodynamic profiles of clopidogrel among 84 ASCVD patients (prior coronary revascularisation: 92%, peripheral artery disease: 31%) treated with clopidogrel. Patients were further stratified according to the level of platelet reactivity into 2 cohorts: high platelet reactivity (HPR, n=37) and normal platelet reactivity (NPR, n=47). The primary endpoint was P2Y12 reaction units (PRU) as assessed by VerifyNow at 30 days. VerifyNow is a broadly available, simple, rapid, whole blood point-of-care test with a broad clinical validation and, therefore, a good choice for the assessment of the primary endpoint. As expected, evolocumab 420 mg s.c. once monthly significantly reduced LDL-C compared with placebo at 14 (36.1±27.0 mg/dL vs 100.9±41.1 mg/dL; p<0.001) and 30 days (45.9±15.9 mg/dL vs 103.8±42.6 mg/dL; p=0.001) in the HPR cohort. At 14 days, PRU levels were significantly lower in the evolocumab group compared with placebo in the HPR (218.2±29.7 vs 246.6±35.2; p=0.017) cohort but not in the NPR cohort (141.2±42.8 vs 148.2±41.7; p=0.578). At 30 days, there were no significant differences in PRU in the HPR (219.3±38.3 vs 240.9±51.8; p=0.161) or NPR (141.5±54.3 vs 158.6±40.8; p=0.229) cohort.
Statins employ multiple pleiotropic effects including antithrombotic action, anti-inflammation, and cardioprotective effects during PCI6. In particular, statins have been shown to reduce platelet aggregation, dense granule release, and platelet-mediated thrombus formation in a rapid and dose-dependent manner7. A previous randomised trial has demonstrated that high-dose (80 mg) atorvastatin significantly reduced PRU at 30 days compared with the control group (188±48 vs 223±53; p<0.01) among 76 patients with HPR who were treated with double-dose clopidogrel (150 mg/day)8. Similar to statins, the antiplatelet effects of PCSK9 inhibitors were expected based on the potential association between PCSK9 and several metabolic pathways beyond LDL receptors. In the current study, a significant difference in PRU was only observed at 14 days (and not at 30 days) in the HPR cohort, i.e., the timepoint at which LDL-C reduction was most intense. This may imply the potential effect of PCSK9 inhibitors on clopidogrel-induced platelet inhibition, via a strong LDL-C reduction rather than an LDL-C-independent pathway. Patients included in this study received evolocumab on top of their maximally tolerated statin dose (i.e., reflecting the most frequent clinical indication) and statin-intolerant patients were not eligible. From a scientific point of view, it would have been interesting to get insights into the potential effects of evolocumab on platelet function in the absence of statins (i.e., statin-naïve patients).
Currently, there is only one clinical study that has investigated the effects of PCSK9 inhibition on platelets. Results of the PACMAN-AMI9 substudy, which investigated the effect of alirocumab added to high-intensity statin therapy on PRU at 4 weeks among 139 patients with acute myocardial infarction undergoing PCI and receiving dual antiplatelet therapy with a potent P2Y12 inhibitor, were presented at the ESC Congress 2022 (Räber et al. Impact of alirocumab added to high-intensity statin therapy on platelet function in AMI patients: a pre-specified substudy of the randomized, placebo-controlled PACMAN-AMI trial. ESC Congress 2022. Barcelona, Spain). Despite the substantial decrease in LDL-C in the alirocumab group compared with the placebo group, there were no significant differences in PRU at 4 weeks (alirocumab: 12.5 [interquartile range 27.0] vs placebo: 19.0 [interquartile range 30.0]; p=0.26). The reduction in PRU by a PCSK9 inhibitor was numerically greater in the study by Franchi et al (mean difference 22 at 30 days in the HPR group) compared with the PACMAN-AMI substudy (median difference 7 at 4 weeks). The difference in the magnitude of PRU reduction appears mainly attributable to the type of P2Y12 inhibitor used in those studies (i.e., clopidogrel vs potent P2Y12 inhibitors), resulting in the much lower PRU value induced by potent P2Y12 inhibitors in the PACMAN-AMI substudy.
In view of the totality of the aforementioned data, the effect of PCSK9 inhibition on platelet function may be more relevant especially in HPR patients with stable ASCVD treated with clopidogrel. Compared with ticagrelor and prasugrel, clopidogrel has a less potent antiplatelet effect with significant interindividual variability mainly due to genetic polymorphisms. Although the continuous relationship between PRU values and ischaemic risks (a 4% increase for every 10-unit increase in PRU) has been demonstrated in the previous meta-analysis1, the clinical relevance of such a small difference (mean difference 22) remains uncertain. Further research is required to investigate the clinical impact of this modest antiplatelet effect on ischaemic outcomes beyond LDL-C lowering as well as bleeding events in this specific population.
The study by Franchi et al has some limitations that merit consideration when interpreting the study findings. First, the single-centre design of this study may limit the generalisability. Second, the lack of statistical significance may partly be attributable to the relative lack of power due to a small sample size (type II error). In the power calculation of this study, investigators arbitrarily assumed similar effects of evolocumab in patients with HPR and NPR. Based on the observed magnitude of the effect, the study would probably have reached statistical significance with a larger sample size. Third, the current study included only patients with stable ASCVD receiving clopidogrel.
Collectively, the current pioneering study is an important addition to the body of evidence investigating the pleiotropic effects of PCSK9 inhibitors and suggests, although suffering from a negative primary endpoint, small LDL-C-depending effects of PCSK9 inhibitors on platelet inhibition in patients with high platelet reactivity (Figure 1).
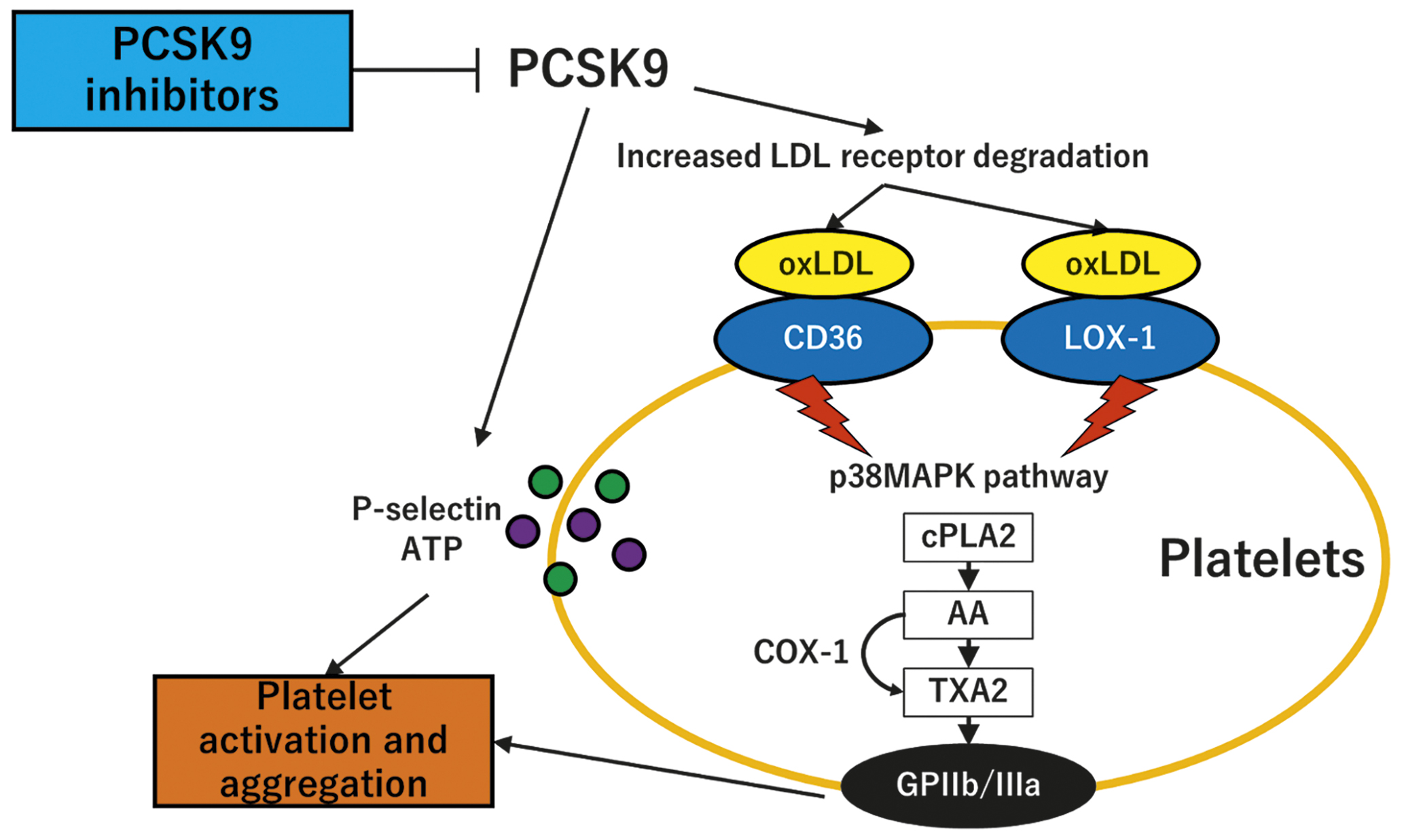
Figure 1. Potential platelet effects of PCSK9 inhibitors. PCSK9 increases LDL receptor degradation and thereby increases the LDL cholesterol level. oxLDL binds to CD36 and LOX-1 and activates the p38MAPK pathway. cPLA2 releases AA that is subsequently converted to TXA2 by the COX-1 enzyme. TXA2 acts with downstream signalling to respective receptors and activates the GPIIb/IIIa receptors. PCSK9 also directly induces platelet release of P-selectin and ATP from granules. The inhibition of PCSK9 suppresses these pathways and potentially decreases the platelet activation and aggregation. AA: arachidonic acid; ATP: adenosine trisphosphate; cPLA2: cytosolic phospholipase; GPIIb/IIIa: glycoprotein IIb/IIIa; LDL: low-density lipoprotein; oxLDL: oxidised low-density lipoprotein; PCSK9: proprotein convertase subtilisin/kexin type 9; p38MAPK: p38mitogen-activated protein kinase; TXA2: thromboxane A2
Conflict of interest statement
L. Räber has received research grants to the institution from Abbott Vascular, Biotronik, Boston Scientific, Canon, HeartFlow, Intrafredx, Medtronic, Sanofi, and Regeneron; and speaker or consultation fees from Abbott Vascular, Amgen, AstraZeneca, CSL Behring, Novo Nordisk, Occlutech, Sanofi, and Vifor. Y. Ueki reports travel grants from Infraredx.

