The ODYSSEY OUTCOMES trial found a significant reduction in death from coronary heart disease, myocardial infarction, ischaemic stroke, and unstable angina requiring hospitalisation in patients who had an acute coronary syndrome (ACS) 1-12 months prior and were treated with the proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor alirocumab versus placebo1. A subsequent analysis showed that there was also lower all-cause mortality in the patients randomised to alirocumab, with evidence that the mortality benefit persisted down to an achieved low-density lipoprotein (LDL) cholesterol level of approximately 30 mg/dL – which is within the range of LDL cholesterol in newborns and what might be considered “normal”23. A limitation of the trial was that the PCSK9 inhibitor was not started prior to hospital discharge. Such an initiation would, at a minimum, lead to an increase in initial appropriate prescription for patients and longer-term adherence to this therapy, as therapies initiated at the time of an acute event tend to make a more profound impression on the patient as to the importance of taking medications to prevent future similar events4. Beyond the adherence issue, a more provocative thought is that very early initiation of a PCSK9 inhibitor might modify the expected clinical trajectory of patients with ACS by modifying plaque vulnerability.
In this issue of EuroIntervention, the EPIC STEMI trial results presented in the article by Mehta et al provide an important step in the evolution of early initiation of potent PCSK9 inhibition5. In this well-designed mechanistic trial, a total of 68 ST-segment elevation myocardial infarction (STEMI) patients undergoing primary percutaneous coronary intervention (PCI) were randomised to alirocumab or a sham placebo (consisting of a simulated injection, though without an actual needle). The primary endpoint was the percent reduction in LDL cholesterol up to 6 weeks. At a median of 45 days, the LDL cholesterol decreased by 72.9% with alirocumab versus 48.1% with sham control, for a mean between-group difference of 22.3% (p<0.001). The trial was not powered for clinical events. There were no safety concerns identified.
Thus, EPIC STEMI has established the feasibility and safety of very early initiation of PCSK9 inhibition in the highest-risk patients – those with STEMI undergoing primary PCI. Significant early reductions in LDL cholesterol with PCSK9 inhibition have now been established. Even within the first 24 hours, the LDL cholesterol decrease, beyond that afforded by high intensity statins, was evident. The data are relevant to all physicians but do underscore the potential importance of interventional cardiologists in initiating this therapy.
The PACMAN-AMI trial had previously demonstrated in 300 acute myocardial infarction patients that alirocumab versus placebo significantly reduced the percent atheroma volume at 52 weeks as assessed by serial intravascular ultrasound imaging, providing true evidence of plaque regression6. The ongoing EVOLVE-MI trial will randomise 4,000 patients with an acute myocardial infarction to evolocumab or to usual care and assess the impact of early PCSK9 inhibitor initiation on myocardial infarction, ischaemic stroke, any arterial revascularisation procedure, and all-cause death in a trial powered for clinical events7.
As mentioned above, in-hospital initiation is one way to improve adherence (Figure 1). Another potential method is to decrease the dosing frequency. This has been accomplished with the PCSK9 inhibitor inclisiran which appears to provide substantial LDL cholesterol reduction with a dosing interval of 6 months, allowing monitored administration during visits with the cardiologist or primary care that probably would occur at least every 6 months in a secondary prevention patient, especially a high-risk one. Cardiovascular outcome trials with this agent are ongoing8.
A major concern to date has been the relatively poor uptake of PCSK9 inhibitors due, in part, to cost concerns9, though in very high-risk patients this therapy is indeed cost-effective. Many patients who could already benefit from PCSK9 inhibition are not receiving this therapy, as demonstrated in the GOULD registry10. Hopefully, trials such as EPIC STEMI and ongoing long-term cardiovascular outcome trials will push this major advance in cardiovascular medicine forward.
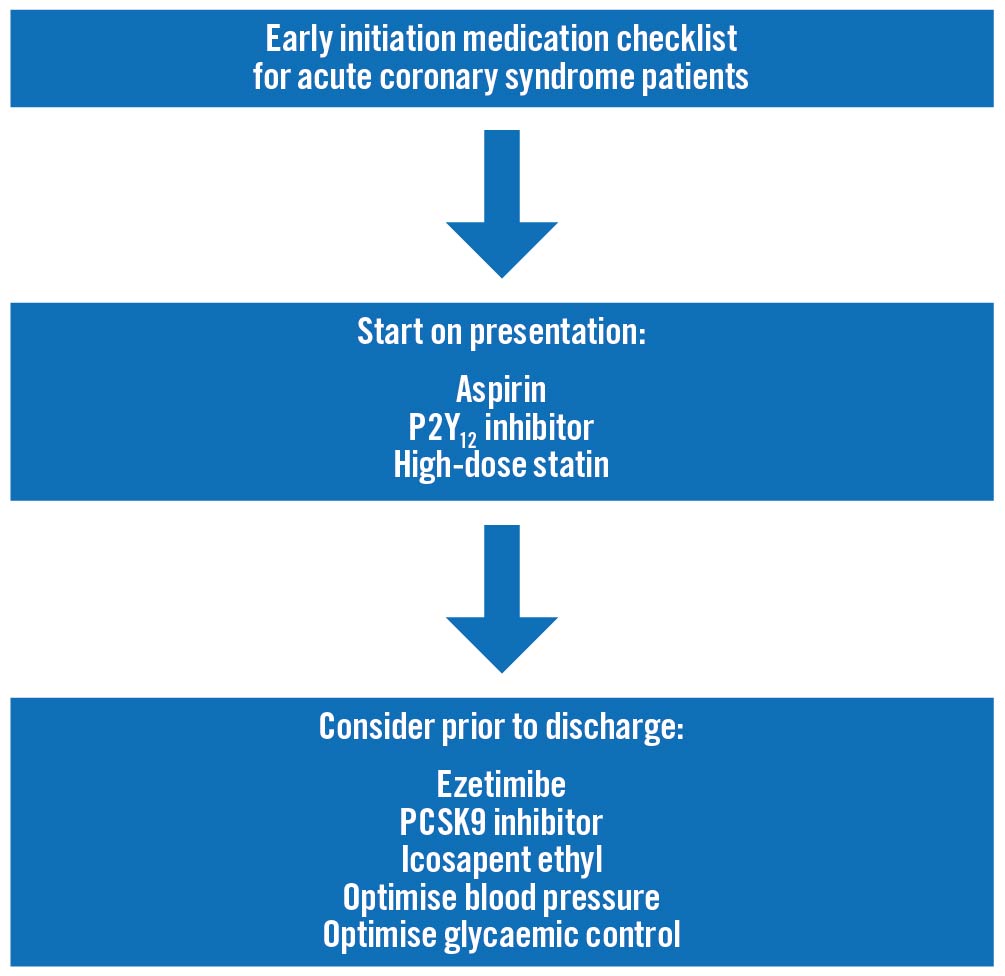
Figure 1. Early initiation medication checklist for acute coronary syndrome patients. Aspirin should be given as soon as an acute coronary syndrome is suspected, with a P2Y12 inhibitor given after the coronary anatomy has been delineated. It would be reasonable to start high intensity statins on presentation as well. While there may be incremental clinical event reduction by starting ezetimibe, PCSK9 inhibitors, and icosapent ethyl at presentation, those strategies need to be tested in adequately powered cardiovascular outcome trials. However, all acute coronary syndrome patients should be assessed for these lipid-modifying therapies, as well as have their blood pressure and, for those with diabetes, glycaemic control, optimised prior to hospital discharge.
Conflict of interest statement
D.L. Bhatt is on the advisory board of AngioWave, Bayer, Boehringer Ingelheim, Cardax, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, High Enroll, Janssen, Level Ex, McKinsey, Medscape Cardiology, Merck, MyoKardia, NirvaMed, Novo Nordisk, PhaseBio, PLx Pharma, Regado Biosciences, and Stasys; is on the board of directors of AngioWave, Boston VA Research Institute, Bristol-Myers Squibb, DRS.LINQ, High Enroll, Society of Cardiovascular Patient Care, and TobeSoft; has stock in Bristol-Myers Squibb and High Enroll; has stock options in AngioWave and DRS.LINQ; is the inaugural Chair of the American Heart Association Quality Oversight Committee; is the Chair of the VA CART Research and Publications Committee and the NCDR-ACTION Registry Steering Committee; is the Deputy Editor for Clinical Cardiology; is a consultant for Broadview Ventures; is on the Data Monitoring Committee of Acesion Pharma, Assistance Publique-Hôpitaux de Paris, Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Boston Scientific (Chair, PEITHO trial), Cleveland Clinic (including for the ExCEED trial, funded by Edwards Lifesciences),Contego Medical (Chair, PERFORMANCE 2), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo; for the ABILITY-DM trial, funded by Concept Medical), Novartis, Population Health Research Institute, Rutgers University (for the NIH-funded MINT Trial); has received honoraria from the American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Chair, ACC Accreditation Oversight Committee), Arnold and Porter law firm (work related to Sanofi/Bristol-Myers Squibb clopidogrel litigation), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor-in-Chief, Harvard Heart Letter), Canadian Medical and Surgical Knowledge Translation Research Group (clinical trial steering committees), Cowen and Company, Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor-in-Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), K2P (Co-Chair, interdisciplinary curriculum), Level Ex, Medtelligence/ReachMD (CME steering committees), MJH Life Sciences, Oakstone CME (Course Director, Comprehensive Review of Interventional Cardiology), Piper Sandler, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co-leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees), Wiley (steering committee); holds a patent for sotagliflozin (named on a patent for sotagliflozin assigned to Brigham and Women's Hospital who assigned to Lexicon; neither he nor Brigham and Women's Hospital receive any income from this patent); has received research funding from Abbott, Acesion Pharma, Afimmune, Aker BioMarine, Amarin, Amgen, AstraZeneca, Bayer, Beren, Boehringer Ingelheim, Boston Scientific, Bristol-Myers Squibb, Cardax, CellProthera, Cereno Scientific, Chiesi, CinCor, CSL Behring, Eisai, Ethicon, Faraday Pharmaceuticals, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Garmin, HLS Therapeutics, Idorsia, Ironwood, Ischemix, Janssen, Javelin, Lexicon, Lilly, Medtronic, Merck, Moderna, MyoKardia, NirvaMed, Novartis, Novo Nordisk, Owkin, Pfizer, PhaseBio, PLx Pharma, Recardio, Regeneron, Reid Hoffman Foundation, Roche, Sanofi, Stasys, Synaptic, The Medicines Company, Youngene, and 89Bio; has received royalties from Elsevier (Editor, Braunwald’s Heart Disease); is a site co-investigator for Abbott, Biotronik, Boston Scientific, CSI, Endotronix, St. Jude Medical (now Abbott), Philips, SpectraWAVE, Svelte, and Vascular Solutions; is a trustee of the American College of Cardiology; and has performed unfunded research for FlowCo and Takeda.

