Abstract
Although technological and procedural advances have resulted in substantial improvements in clinical outcomes following percutaneous coronary interventions (PCI), recurrent coronary events may occur despite achieving optimal procedural results. Beyond myocardial revascularisation failure related to anatomical or stent-related factors, adverse cardiovascular events post PCI often arise from non-culprit lesions not treated during index interventions. While stenting treats a focal manifestation of a systemic, progressive disease, the residual risk following an acute coronary syndrome (ACS) or elective PCI is largely related to the systemic pro-atherogenic effects of suboptimally controlled cardiovascular risk factors. Lowering atherogenic lipid levels, in particular low-density lipoprotein cholesterol (LDL-C), can halt the progression of coronary atherosclerosis and improve cardiovascular outcomes to an extent that is proportional to the magnitude of LDL-C reduction. Early (in-hospital) initiation of intensive statin therapy leads to a very early clinical benefit following ACS, and prolonged adherence to optimised lipid-lowering treatment effectively reduces longer-term cardiovascular events following PCI. Therefore, achieving guideline-recommended treatment goals for LDL-C with statins and, if indicated, with the addition of non-statin lipid-lowering drugs should become a priority for all physicians involved in the treatment of patients with coronary heart disease, including comprehensive strategies initiated during the in-hospital care of patients undergoing coronary interventions. This review article summarises current evidence on the role of LDL-C in the development and progression of coronary atherosclerosis, discusses the clinical benefits of intensive lipid-lowering treatments, and presents current guideline recommendations, with emphasis on patients undergoing PCI.
Introduction
Over the past decades, myocardial revascularisation procedures have evolved to provide safer and more effective treatment for patients with acute and chronic manifestations of coronary artery disease (CAD)1. Advances in stenting technologies and interventional techniques, along with invasive diagnostic tools and adjunctive pharmacotherapies, have resulted in improved survival, prevention of future myocardial infarctions (MI), relief of symptoms and better quality of life following percutaneous coronary interventions (PCI)1. Nonetheless, recurrent ischaemic events are frequent following both acute coronary syndromes (ACS) and elective PCI, resulting either from previously treated lesions, or from progression of native atherosclerotic disease. One in five patients will experience a major cardiovascular (CV) event during the first year following an MI2, indicating considerable residual risk and thus a need for prolonged surveillance and optimal risk factor control. Notably, the prevalence of suboptimally controlled modifiable risk factors remains high3, and the use of guideline-directed secondary prevention medications declines over time following a coronary revascularisation procedure, contributing to worse long-term outcomes4,5,6.
Atherogenic lipoproteins, in particular low-density lipoprotein cholesterol (LDL-C), are key causal factors of atherosclerotic cardiovascular disease (ASCVD)7,8. Intensive LDL-C lowering halts the progression of atherosclerosis and improves clinical outcomes, with a clinical benefit that is proportional to the magnitude of LDL-C reduction9,10,11. Importantly, very early clinical benefit has been demonstrated when intensive statin treatment is initiated in the acute phase of ACS12,13,14. Despite the compelling evidence, lipid-lowering treatment is often suboptimal among patients who have experienced an ACS or undergone PCI15,16. Against this background, there is a need for better awareness and implementation of recommended lipid-lowering strategies across the spectrum of the management of CAD patients, including the time point of hospitalisation for an ACS or elective PCI. A patient-centred approach should not only ensure an optimal procedural result of the interventional treatment, but also focus on optimal post-PCI medical care – including lipid-lowering treatment. The contribution of the interventional cardiologist should not be underestimated, as the overall recommendations provided in the interventional report have a considerable impact on post-PCI management for both patients and referring cardiologists/general practitioners and should not be restricted to antithrombotic treatment.
This review article summarises current evidence on the role of LDL-C in the development and progression of CAD, discusses the clinical benefits of intensive lipid-lowering treatments, and presents current guideline recommendations with emphasis on treatment in the acute phase of ACS or following PCI. We propose pragmatic, guideline-conforming algorithms for lipid management starting from the in-hospital period, involving the critical input of interventional cardiologists.
Role of lipids in atherosclerotic disease
Overwhelming evidence from preclinical research, Mendelian randomisation studies, epidemiological investigations and randomised controlled trials (RCTs) unequivocally shows that LDL-C has a causal and cumulative effect on the risk of ASCVD7,8. Several mutations in genes involved in LDL-C homeostasis leading to elevated plasma LDL-C levels (typically in the context of familial hypercholesterolaemia [FH]) are closely associated with progressive, early-onset CAD17,18; in contrast, naturally occurring mutations that result in lifelong exposure to very low LDL-C levels (e.g., loss-of-function mutations of the PCSK9 enzyme) are linked to a remarkably low incidence of CAD19. In epidemiological observations, high LDL-C levels are consistently associated with a higher risk of clinical ASCVD20. Finally, large-scale RCTs clearly demonstrate a significant reduction in cardiovascular disease (CVD) events with statins as well as non-statin lipid-lowering medications9,10,11. Taken together, the available evidence shows that the higher the LDL-C levels and the longer the exposure to such elevated levels, the higher the risk of developing ASCVD. Conversely, lowering LDL-C reduces the risk of ASCVD proportionally to the absolute reduction in LDL-C levels7. Other lipids are also involved in the pathobiology of atherosclerosis; however, LDL-C is currently recommended as the primary lipid measurement for risk assessment and treatment guidance.
On the basis of this evidence, current European Guidelines21 recommend (class I/A) a reduction of LDL-C levels by at least 50% and the achievement of an LDL-C goal <1.4 mmol/L (<55 mg/dL) for all patients with ASCVD – i.e., including those who present with ACS or undergo PCI. All patients should receive long-term lipid-lowering therapy starting with a statin, and treatment should be intensified in a stepwise approach (uptitration of statin dose or addition of a non-statin drug, if needed) in order to reach the recommended goal21. For patients who experience a second vascular event within two years while taking maximally tolerated statin-based therapy, an even lower LDL-C goal <1.0 mmol/L (<40 mg/dL) may be considered (IIb/B recommendation)21.
Of particular relevance for interventional practice, atherogenic lipids may be involved in the development of in-stent neoatherosclerosis, one of the leading causes of late stent failure including restenosis and stent thrombosis22. An observational study suggested that in-stent neoatherosclerosis is more common among patients with angiographic and clinical evidence of native atherosclerosis progression, suggesting similar pathophysiological mechanisms23. These observations raise the possibility that LDL-C lowering therapies may reduce the risk of late-occurring stent failure. As a proof of concept, a small randomised trial comparing treatment with 10 mg rosuvastatin and eicosapentaenoic acid versus 2.5 mg rosuvastatin in 50 Japanese CAD patients with evidence of established neoatherosclerosis showed a greater decrease of lipid content and macrophage accumulation by means of optical coherence tomography (OCT) in the more intensive treatment group24. A larger ongoing imaging trial on the vascular effects of the PCSK9 inhibitor (PCSK9i) alirocumab, focused primarily on the progression of native atherosclerosis in non-culprit vessels of patients with MI, will also test whether the occurrence of neoatherosclerosis can be reduced among patients receiving very intensive LDL-C lowering treatment (NCT03067844).
Effect of statins on cardiovascular outcomes in patients with CAD (including ACS and post PCI)
Statins are the cornerstone for lowering lipid levels. There is robust RCT evidence for the safety and efficacy of these medications, and vast real-world experience with hundreds of millions of individuals worldwide currently receiving statin treatment. The most comprehensive meta-analysis of statin trials pooled data from 26 RCTs with 170,000 individuals in primary and secondary prevention and showed that lowering of LDL-C by 1.0 mmol/L reduces the risk of major CV events by 22% and lowers all-cause mortality by 10%. Of note, the mean untreated LDL-C of patients with CAD is in the range of about 3.0-3.5 mmol/L; considering that potent statin regimens can reduce LDL-C levels by about 45-50% (Figure 1), this would translate to an absolute LDL-C reduction of about 1.5 mmol/L on average and thus a statin-mediated reduction in the risk of CV events by approximately one third.
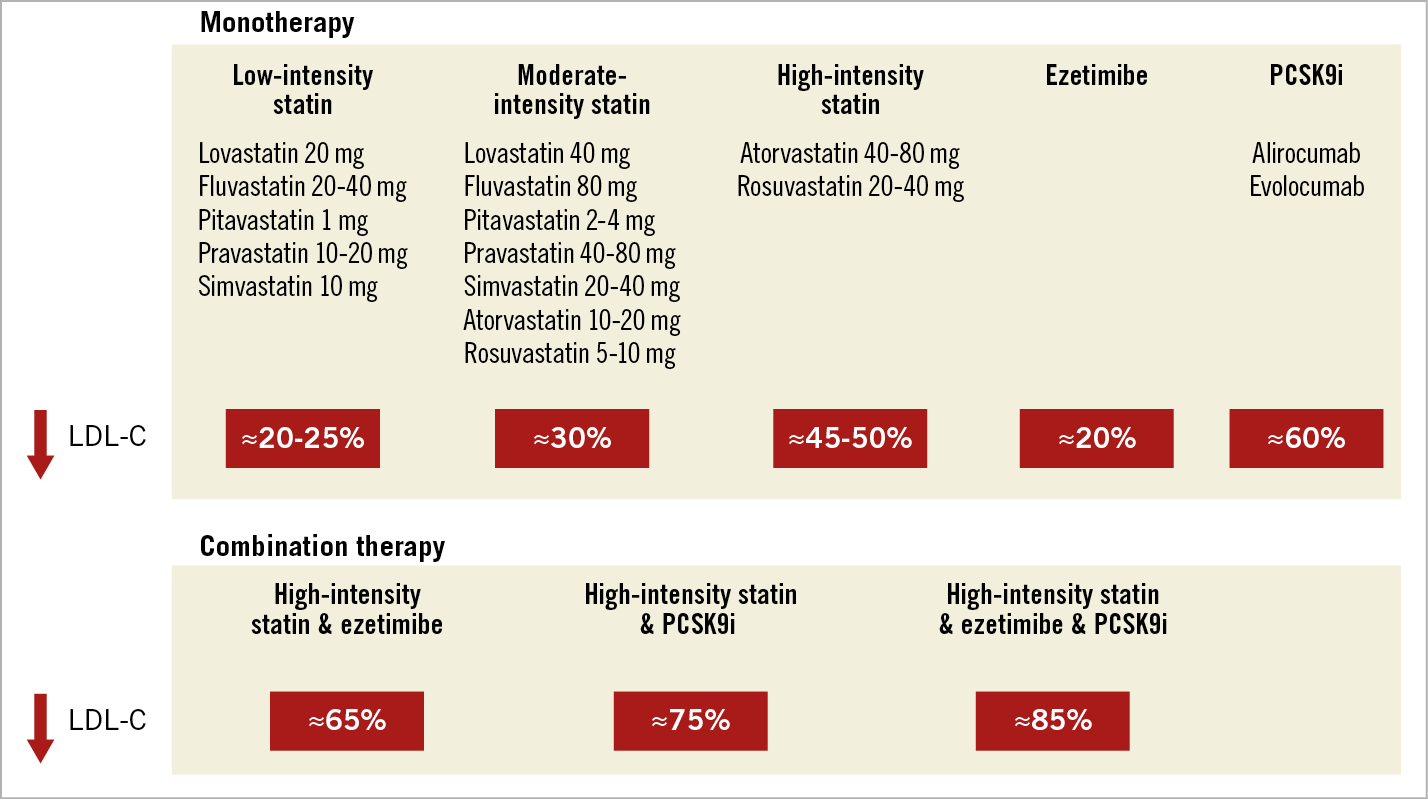
Figure 1. Anticipated average reduction in LDL-C levels with different lipid-lowering monotherapies or combination therapies.
Studies that focused specifically on the acute ACS period were able to show a very early clinical benefit associated with early initiation of intensive statin treatment in this setting. The MIRACL trial compared atorvastatin 80 mg versus placebo, initiated 24-96 hours after admission for an ACS in 3,086 patients and found a significant, 16% reduction in major adverse cardiovascular events (MACE) within only 16 weeks of follow-up (14.8 vs 17.4%; p=0.048)12. The PROVE-IT trial compared atorvastatin 80 mg versus pravastatin 40 mg in 4,162 stabilised ACS patients, enrolled within <10 days of the index event. This study found a 16% reduction in MACE (26.3% vs 22.4%; p=0.005) within a mean follow-up of 24 months13. A post hoc analysis of PROVE-IT found a positive effect as early as 30 days after the ACS event (HR 0.72, CI: 0.52-0.99; p<0.05)14. Based on this evidence, current ESC guidelines recommend that patients who present with an ACS are treated with a high-intensity statin (atorvastatin ≥40 mg or rosuvastatin ≥20 mg daily), initiated as early as possible and irrespective of their baseline LDL-C levels, unless such a treatment is contraindicated or not tolerated (class I/A recommendation) (Figure 2),21.
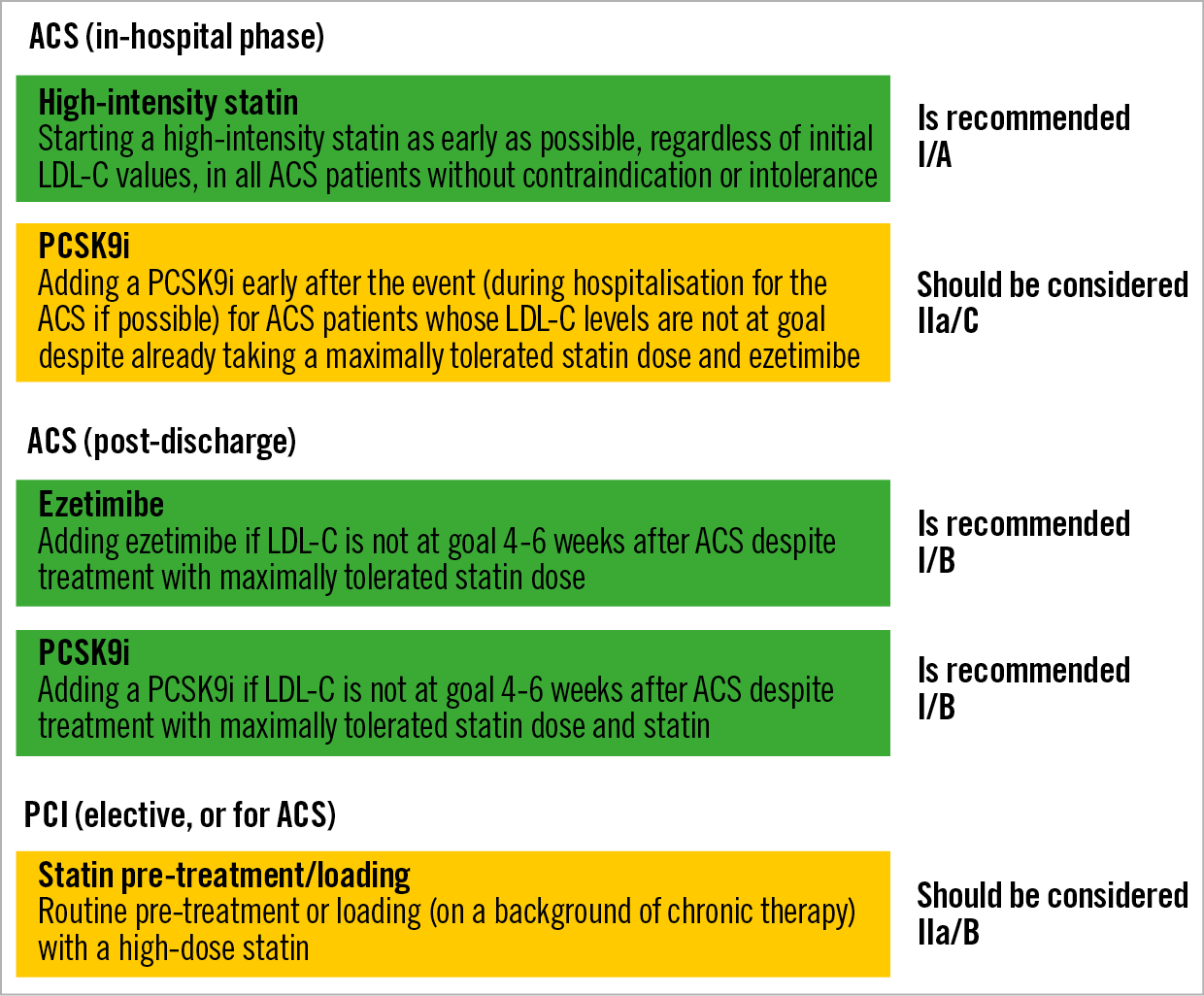
Figure 2. Recommendations of current ESC dyslipidaemia guidelines pertinent to the treatment of patients who present with ACS or undergo PCI.
As it relates to elective PCI, these patients by definition have a very high CV risk and should receive intensive lipid-lowering treatment to achieve the recommended LDL-C goal <1.4 mmol/L21. A practical question, with implications for interventional cardiologists, has been the optimal timing of statin initiation, and whether statin pre-loading prior to elective PCI is beneficial. An individual patient-level meta-analysis of 13 RCTs including 3,341 patients undergoing PCI showed that either pre-treatment with high-dose statin (for statin-naïve patients) or loading of a high-dose statin (for patients already on statin therapy) reduced the risk of periprocedural MI and 30-day adverse CV events by 44% (7.0% vs 11.9%, p<0.0001, and 7.4% vs 12.6%, p=0.05, respectively)25. Moreover, statin pre-treatment appears to be effective in reducing the risk of contrast-induced acute kidney injury, presumably due to the so-called pleiotropic effects of statins26.
Regarding statin pre-loading in the ACS setting, in the Statins Evaluation in Coronary Procedures and Revascularization (SECURE-PCI) trial, patients (n=4,191) with ACS planned for invasive management were randomly assigned to receive loading with 80 mg atorvastatin or placebo before and 24 hours after PCI; all patients were treated with atorvastatin 40 mg 24 hours after the second study drug dose. At 30 days, the rate of the primary outcome (composite of all-cause death, MI, stroke, and unplanned coronary revascularisation) did not differ between groups27. A pre-specified subgroup analysis including only patients who were actually treated with PCI (64% of all patients) showed a significant, 28% relative reduction in 30-day MACE (HR 0.72 [0.54-0.96]; p=0.02), an effect that was more pronounced in patients with ST-elevation myocardial infarction (STEMI)27. Limitations of sub-analyses in the context of an overall neutral study cannot be ignored.
While the evidence for pre-loading remains in part debatable, based on current evidence of potential benefit without evidence of harm, routine pre-treatment or loading (on a background of chronic therapy) with a high-dose statin should be considered in patients undergoing PCI for an ACS or elective PCI according to the current ESC guidelines (class IIa/B recommendation) (Figure 2),21.
Side effects of statins
Although statins are generally very well tolerated, they have some adverse effects28. Muscle-related effects are the most clinically relevant side effect of statins and appear to be dose-dependent. Statin-treated patients frequently report muscle symptoms, and in observational studies their frequency has varied between 10 and 15%29,30. However, these figures are an overestimation of true, statin-induced myopathy, mostly due to the unblinded treatment in observational studies and the non-specific nature of such symptoms31. In sharp contrast, evidence from blinded, placebo-controlled RCTs shows only a slightly increased frequency of muscle symptoms in statin-treated patients32. Importantly, an analysis comparing the incidence of muscle-related symptoms during both the blinded period and the subsequent, open-label extension study of the ASCOTT-LLA trial showed that a “nocebo” effect (caused by negative expectations) may partly explain the higher frequency of reported muscle symptoms in observational studies33. These findings were recently reinforced in the SAMSON study, indicating that side effects are caused mainly by the act of taking tablets rather than the statin per se34. Rhabdomyolysis, the most severe form of statin-induced muscle damage, is extremely rare, with an estimated incidence of 1-3 cases per 100,000 patient-years35. Risk factors for statin-induced myopathy should be considered, particularly older age, history of muscle symptoms, untreated hypothyroidism, excessive alcohol intake, low body mass index, and interaction with concomitant drug therapy. When statin therapy is initiated, safety blood tests including alanine transferase (ALT) and creatine kinase (CK) at baseline are advised to identify the few patients in whom treatment is contraindicated. There is no predictive value of routine repeat CK testing, but CK must be assessed immediately in patients who present with muscle pain and weakness, and statin treatment must be stopped if CK rises to >10 times the upper limit of normal (ULN).
Mild elevations of liver enzymes occur in 0.5-2.0% of statin-treated patients; they do not appear to be associated with true hepatotoxicity and are more frequent with high doses of potent statins36. An increase of three times the ULN on two consecutive occasions is considered clinically relevant. ALT testing is recommended before the start of statin therapy, with the exception of ACS patients where ALT may increase due to myocardial necrosis. Because progression to liver failure is exceedingly rare, routine monitoring of liver enzymes during statin treatment is no longer recommended21; ALT should be tested if deemed clinically indicated.
Statin use can result in a slight increase in the risk of diabetes28. The number needed to cause one case of diabetes is estimated at 255 over four years of statin treatment37; the risk is higher with higher doses of more potent statins38, in the elderly and patients with metabolic syndrome. Overall, the absolute reduction in the risk of CVD in high-risk patients clearly outweighs the small increase in the incidence of diabetes (1 out of 1,000 per year with new-onset diabetes vs 5 CV events prevented)31. In addition, the use of statins is associated with a minimal increase in haemorrhagic stroke9,39; however, the overall benefit on other stroke subtypes greatly outweighs this small hazard9,40.
Effect of non-statin LDL-C lowering medications on cardiovascular outcomes
Despite the potent LDL-C lowering effect of statins (Figure 1), many patients cannot achieve an adequate reduction in LDL-C levels with statins alone (typically due to particularly elevated untreated levels) or cannot tolerate regimens with high doses of potent statins. In these patients, add-on treatment with non-statin medications (ezetimibe or PCSK9 inhibitors [PCSK9i]) is a valuable option.
Ezetimibe reduces the intestinal absorption of dietary and biliary cholesterol. It lowers LDL-C levels by 15-22% when given as monotherapy, and by 21-27% on top of statin therapy41,42. Regarding the impact of CV outcomes, the IMPROVE-IT trial was an RCT including 18,144 ACS patients enrolled <7 days from the index event43. Patients were treated with statin alone (simvastatin 40 mg) versus the combination of statin plus ezetimibe. The study found a modest but significant reduction in MACE within seven years of follow-up (HR 0.936, p=0.016)43. Based on this trial, the addition of ezetimibe is recommended (class I/B indication) for all patients who have not achieved the LDL-C goal despite treatment with maximally tolerated statin therapy (Figure 2),21.
More recently, monoclonal antibodies inhibiting the PCSK9 enzyme have emerged as a promising treatment option to lower LDL-C levels further44. The currently approved antibodies evolocumab and alirocumab are administered subcutaneously every 2-4 weeks and lower LDL-C levels by approximately 60% on top of statin, while maintaining a favourable safety profile45,46. Large outcomes trials have shown a significant clinical benefit in the context of secondary CV prevention. In the FOURIER trial, 27,564 patients with a history of MI (81.1% of patients) and/or non-coronary ASCVD manifestations were treated with optimised statin alone versus optimised statin plus evolocumab. The study found a 15% relative risk reduction for the primary endpoint (a composite of CV death, MI, stroke, hospitalisation for unstable angina, or coronary revascularisation), and a 20% relative risk reduction for the “harder” triple endpoint (CV death, MI or stroke) within a median follow-up of 2.2 years47. Very similarly, in the ODYSSEY OUTCOMES trial involving 18,924 patients with a history of ACS (1-12 months prior to enrolment), treatment with statin plus alirocumab versus statin alone resulted in a 15% reduction in MACE and was associated with a significant nominal p-value for reduction in all-cause mortality within a median 2.8-year follow-up48. With the exception of injection-site reactions, adverse events and laboratory abnormalities were similar in the alirocumab and placebo groups48. Both trials included patients with a history of ACS/MI (on average 2.6 months post ACS for ODYSSEY OUTCOMES vs a longer interval [more than one year in 74% of patients] after the qualifying MI for FOURIER). More recently, the EVOPACS trial showed the feasibility, safety, and potent LDL-C lowering efficacy of evolocumab initiated on top of high-intensity statin in the acute phase of ACS (within 24-72 hours of symptom onset)49. More than 90% of patients treated with atorvastatin 40 mg plus evolocumab, versus 10.7% of those treated with atorvastatin alone, achieved the recommended LDL-C target (<1.4 mmol/L) eight weeks after the index ACS event, without raising safety concerns49. Thereby, this proof-of-concept study proposed a new paradigm for very early, very intensive LDL-C reduction for selected ACS patients who are not anticipated to reach the recommended LDL-C target with intensive statin therapy alone. The ESC guidelines recommend PCSK9i (class I/A) for all patients with ASCVD and LDL-C levels >1.4 mmol/L despite treatment with maximally tolerated statin plus ezetimibe21. The addition of a PCSK9i early after an ACS event (during hospitalisation, if possible) should be considered in patients who present with an ACS and whose LDL-C is not at goal level, despite already being on a maximally tolerated statin dose and ezetimibe (IIa/C recommendation) (Figure 2),21.
Beyond LDL-lowering drugs, therapies aimed primarily at lowering triglyceride levels (polyunsaturated fatty acids) have resulted in a reduction of MACE in patients with ASCVD or diabetes with additional risk factors50, although these effects were not confirmed in a recent outcomes trial.
Factors affecting the clinical benefit of lipid-lowering therapies
The expected clinical benefit of LDL-C-lowering treatment depends on the baseline (untreated) LDL-C levels, the intensity of lipid-lowering therapy, and the baseline estimated absolute CV risk9. The anticipated relative (%) reduction of LDL-C levels with each monotherapy or combination therapy is summarised in Figure 1; it should be noted, however, that there is marked inter-individual variability in treatment response. For any given baseline LDL-C level, the relative reduction dictates the magnitude of absolute LDL-C reduction. Based on the pooled evidence from statin trials discussed above, an approximately 20% relative reduction in the risk of CV events is anticipated per 1.0 mmol/L (38 mg/dL) of lowering LDL-C9. Thereby, larger absolute reductions in LDL-C levels lead to larger proportional (%) reductions in risk. Finally, the absolute risk reduction can be calculated by multiplying the absolute baseline risk by the anticipated relative (proportional) risk reduction: the greater the baseline risk, the greater the absolute benefit in terms of lowering the risk of CV events for any given relative risk reduction21 (Figure 3). By definition, CV risk is considered “very high” for all patients with ASCVD, i.e., including those with ACS or undergoing PCI. However, there is substantial heterogeneity of risk within this broad group of secondary prevention patients: the likelihood of recurrent ischaemic events is higher among patients with progressive disease (i.e., history of multiple previous events), anatomically more complex disease (particularly residual disease following revascularisation), or multiple, uncontrolled risk factors. Along these lines, outcomes trials of PCSK9i showed that the risk of recurrent events among patients with clinically manifest CAD was higher for patients with multiple prior MIs versus only one MI, with a recent MI versus a more remote MI, residual multivessel CAD, and polyvascular disease (i.e., including other vascular beds besides the coronary arteries)49,50. Importantly, and consistent with the concept summarised in Figure 3, these patients experienced greater risk reductions with intensive LDL-C lowering with evolocumab or alirocumab compared to patients without these additional very high-risk characteristics52,53.
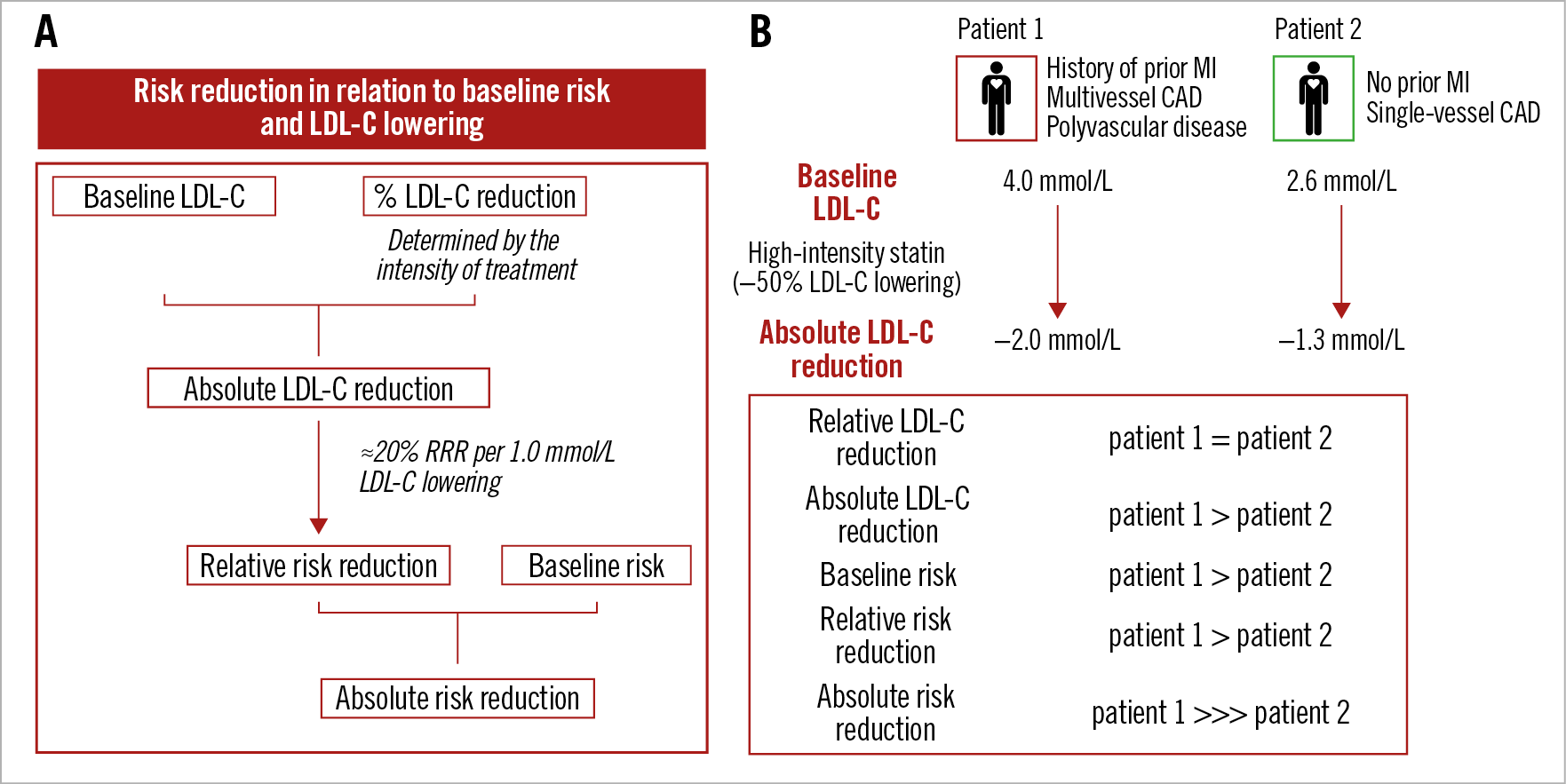
Figure 3. Expected clinical benefit of LDL-C lowering. A) Baseline LDL-C levels and the anticipated relative LDL-C reduction with a lipid-lowering medication both determine the expected magnitude of absolute achieved LDL-C lowering. Each 1.0 mmol/L absolute reduction in LDL-C levels is associated with an approximately 20% relative reduction in the risk of cardiovascular events, such that larger absolute LDL-C reductions result in larger proportional reductions in risk. The absolute risk reduction is driven by both the relative risk reduction and absolute baseline risk. B) Case example of two patients presenting with ACS but with different baseline (untreated) LDL-C levels and risk profiles. In both patients, guideline-recommended treatment with a high-intensity statin is expected to bring a similar (approximately 45-50%) reduction in LDL-C levels; a larger absolute LDL-C reduction, and thereby a 1.5-fold larger proportional reduction in CV risk (about 20% per 1.0 mmol/L LDL-C lowering), is estimated for patient 1 due to the higher baseline LDL-C levels. Coupled with a higher baseline risk in patient 1 (due to a history of previous MI, multivessel CAD and polyvascular disease), and although a significant risk reduction is estimated for both patients, the absolute risk reduction is expected to be substantially higher for patient 1.
Specific considerations with implications for interventional cardiologists
PATIENTS WITH ACUTE CORONARY SYNDROME
Patients presenting with ACS carry the highest risk for recurrent events54, as the systemic inflammatory reactions in this setting may trigger plaque growth and destabilisation at non-culprit lesion sites55. ACS patients harbour more vulnerable plaques in their non-infarct-related arteries compared with patients with chronic coronary syndromes (CCS)56. Optimal secondary prevention therapies are therefore of particular relevance following ACS in order to improve early and long-term prognosis. Early (i.e., during index hospitalisation) initiation of high-intensity statin therapy (atorvastatin ≥40 mg or rosuvastatin ≥20 mg) is a cornerstone of ACS therapy, independent of the presence of CV risk factors or LDL-C levels21. Treatment should be initiated as early as possible to increase patient adherence after discharge, unless contraindicated or not tolerated, or unless the origin of the ACS is non-atherosclerotic (e.g., spontaneous dissection or embolism).
LDL-C should be measured during the index hospitalisation in all ACS patients, either prior to or after the intervention. The difference between fasting and non-fasting LDL-C measurement is minimal in most cases, so that non-fasting samples are generally preferred for practical reasons. Although LDL-C levels decrease during the first days following ACS admission (in part related to the acute phase response resulting in upregulation of LDL-receptor activity) and subsequently increase again, these changes are in the range of 2-5% and thus have no clinical relevance57.
For patients who present with an ACS and are statin-naïve or have been on a low- or moderate-intensity statin regimen, the first step during hospitalisation should be initiation of, or uptitration to, high-intensity statin. If patients are on a high-intensity statin, but not at the highest approved dose (i.e., on atorvastatin 40 mg or rosuvastatin 20 mg), increasing the dose further (i.e., to atorvastatin 80 mg or rosuvastatin 40 mg) is reasonable, but one should consider the minimal (≈6%) incremental LDL-C reduction with this uptitration as well as the dose-dependent nature of side effects of statins for patients at higher risk of such effects. In a subsequent, ambulatory control 4-6 weeks later, LDL-C levels should be measured, and ezetimibe should be added if LDL-C is still >1.4 mmol/L (Figure 4),21. The recommendation for subsequent measurement (and ezetimibe addition, if needed) should be explicitly stated in the discharge letter. However, if a patient presents already on optimised (high-intensity, or maximally tolerated) statin therapy and has an LDL-C >1.4 mmol/L, or is not on treatment and presents with an untreated LDL-C of >3.5 mmol/L (which is unlikely to be lowered to <1.4 mmol/L given the approximately 50% LDL-C-lowering effect of high-intensity statin alone), then it may be reasonable to add ezetimibe during the hospitalisation for an ACS event rather than delay the treatment intensification to a later point in time. The latter approach is not specifically recommended by current guidelines but is a pragmatic approach that is consistent with evidence showing “the lower, the better” and “the sooner, the better” for LDL-C lowering in the ACS context, and is supported by the overall very good tolerability of ezetimibe.
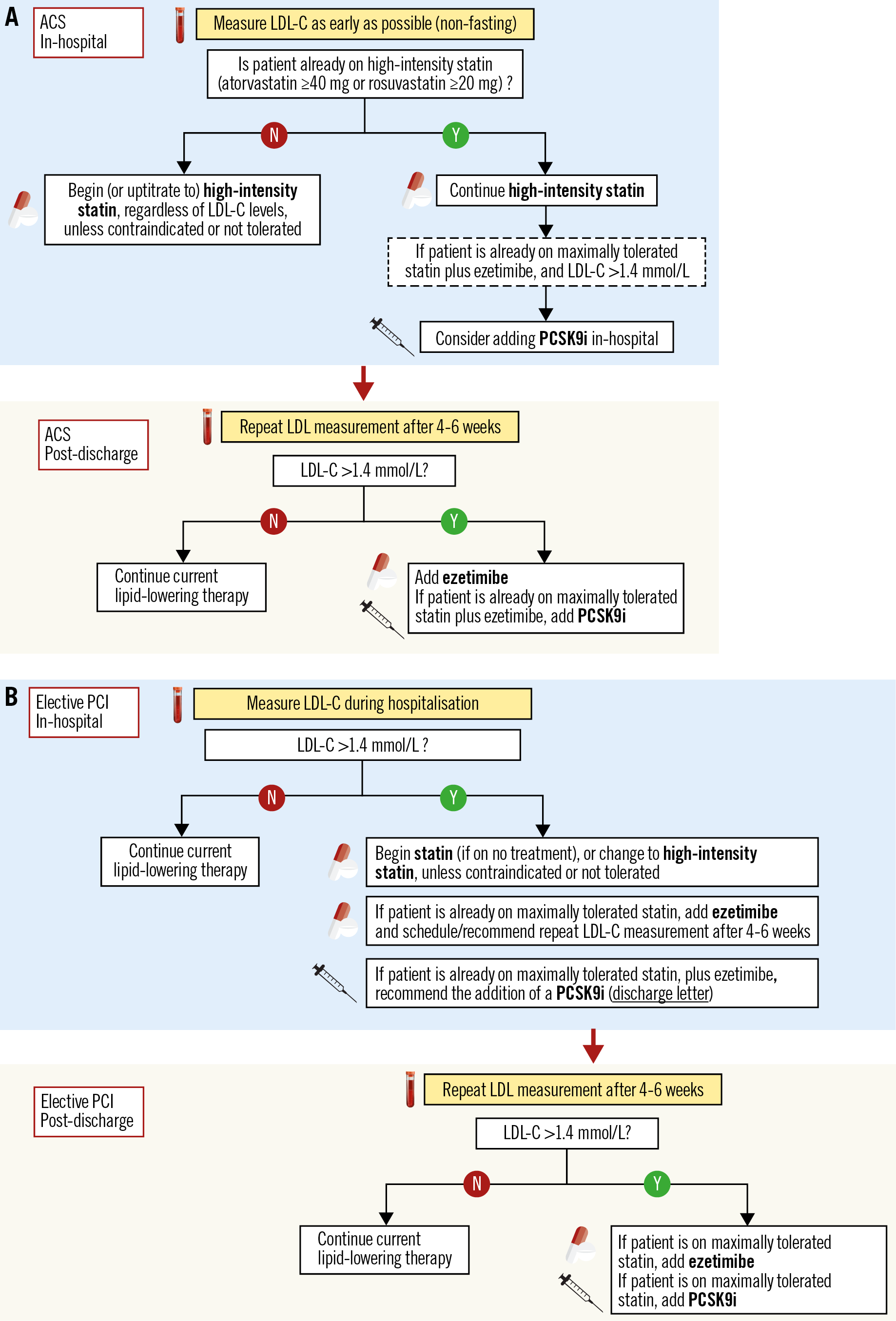
Figure 4. Proposed algorithm for in-hospital and post-discharge lipid management of patients presenting with ACS (A) or undergoing elective PCI (B).
If an ACS patient presents with an LDL-C >1.4 mmol/L despite already being on maximal statin plus ezetimibe therapy, then starting a PCSK9i early on, in-hospital if possible, should be considered according to current guidelines (Figure 4),21. One should keep in mind that: (i) in real-world practice, it is very infrequent for a patient with ACS (either first, or recurrent event) to present on a combination therapy of maximal statin plus ezetimibe; and (ii) regulatory restrictions make an approach of very early, in-hospital initiation of a PCSK9i very challenging, and national reimbursement policies are considerably stricter than guideline recommendations. In any case, in order to increase subsequent treatment adherence, potential eligibility for PCSK9i treatment should be evaluated as early as possible, i.e., in-hospital, based on the first LDL-C measurement, and afterwards at the repeat LDL-C measurement 4-6 weeks after the ACS event. Pragmatically, patients who present with an untreated LDL-C >4.0 mmol/L are not likely to reach the <1.4 mmol/L goal under combination treatment with high-intensity statin and ezetimibe (based on the average 65% LDL-C reduction anticipated with this combination) (Figure 1) and are therefore likely to qualify for a PCSK9i treatment21. According to a recent Swiss study, 51% of patients would be eligible for a PCSK9i treatment according to ESC guideline criteria one year following ACS58.
PATIENTS UNDERGOING ELECTIVE PCI
Similar to the ACS scenario, patients receiving PCI for CCS belong to the very high-risk category, and thus similar treatment goals apply (LDL-C goal of <1.4 mmol/L and a reduction >50% from baseline)21.
In statin-naïve patients, routine initiation of statin therapy is indicated unless safety concerns exist, at an intensity that should be tailored to enable reaching the LDL-C goal (based on the untreated LDL-C levels and the anticipated reduction with different statin regimens) (Figure 1). Specifically regarding patient age, based on recent evidence59, the current ESC guidelines recommend that older people with ASCVD receive statin treatment in the same way as younger patients (class I/A recommendation)21. However, it is recommended that the statin is started at a low dose if there is significant renal impairment and/or the potential for drug interactions, and then uptitrated as needed to achieve LDL-C goals. A repeat LDL-C measurement after 4-6 weeks should be scheduled if high-intensity statin therapy is not anticipated to result in an LDL-C of <1.4 mmol/L (i.e., if baseline untreated LDL-C is >2.8 mmol/L), and ezetimibe should then be added in case the treatment goal is not achieved (Figure 4). During a second follow-up, the LDL-C levels should be measured under optimised oral treatment (statin plus ezetimibe), and eligibility for PCSK9i should be assessed. In order to increase the compliance with follow-up visits and coordinate follow-up LDL-C measurements in CCS patients, it is reasonable that the first follow-up (4-6 weeks after elective PCI) is scheduled by the treating interventional cardiologist and conducted by the referring cardiologist, who should perform the guideline-endorsed routine assessment of ischaemia 1-3 months post revascularisation60. The second LDL-C assessment could be conducted at six months latest, i.e., the time point at which decision making on dual antiplatelet therapy (DAPT) is required60, unless it is performed at an earlier time point by the treating general practitioner. The cardiologist should be equipped to assess LDL-C ad hoc, in case it is not measured by the general practitioner.
CCS patients are frequently taking a statin at the time point of presentation for an elective revascularisation procedure; in case LDL-C is >1.4 mmol/L, the statin dose should be increased, the type of statin changed to a more potent statin (atorvastatin or rosuvastatin), or ezetimibe added as needed. The intensification of treatment should be specifically described in the discharge letter, along with recommendations for subsequent LDL-C controls and treatment adjustments if needed. If LDL-C is >1.4 mmol/L despite a maximal dose of either atorvastatin or rosuvastatin (or a maximally tolerated statin treatment) in combination with ezetimibe, PCSK9i are recommended as the next step21. While guidelines recommend PCSK9i in all patients not achieving treatment goals, national health authorities individually define the reimbursement criteria and, de facto, whether a patient can be treated or not. In some healthcare systems, the LDL-C thresholds defining reimbursement of a PCSK9i therapy are lower in case of recurrent CV events. Currently, there are no options for additional treatment if LDL-C is above the guideline-recommended goal but below the local reimbursement restrictions for PCSK9i despite maximal statin plus ezetimibe therapy. Non-pharmacologic means (mainly dietary changes) should be encouraged, as with all patients, and emerging therapies may be considered in the future in such cases.
Heterozygous FH (HeFH) is a common genetic disorder (prevalence 1/200-250 in the general population) resulting in considerably high LDL-C levels61, although only a small minority of these cases are identified and properly treated. The risk of CAD among individuals with definite or probable HeFH is estimated to be increased at least 10-fold18. Early diagnosis and appropriate treatment can substantially reduce this risk of CAD, hence physicians should be sensitised to detect and properly treat this disorder. The diagnosis of FH is usually based on clinical and laboratory findings (including patient history and family history of premature ASCVD, family history of severe hypercholesterolaemia, untreated LDL-C levels, and clinical signs of severe hypercholesterolaemia), and can be verified by DNA analysis. The probability of FH can be assessed using algorithms such as the Dutch Lipid Clinic Network (DLCN) score; probable or definite FH is defined by a score >6 points. Typically, individuals with an untreated LDL-C >5 mmol/l have at least a possible FH. Family cascade screening should be performed if a patient is identified.
NON-OBSTRUCTIVE AND NON-CULPRIT CORONARY PLAQUES
In patients with suspected CAD referred to coronary angiography, up to 50% show normal coronary arteries or mild, non-ischaemia-inducing lesions. In these patients, the cardiovascular risk category is not necessarily “very high risk” but depends on other characteristics such as presence of diabetes, chronic kidney disease, presence of FH, and calculated 10-year risk for fatal CVD. The extent of non-flow-limiting coronary stenosis should be well documented, as current guidelines categorise CAD patients as “very high risk” in the presence of “characteristics known to be predictive of clinical events”21. This is defined as the presence of two major epicardial arteries with >50% stenosis – a consensus-based rather than an evidence-based approach.
In addition to patients without angiographic evidence of obstructive CAD, non-stenotic atherosclerotic plaques may coexist with stenotic, culprit lesions that are treated by means of PCI in patients with CAD. Natural history studies using intracoronary imaging in patients with CAD demonstrated that certain imaging-defined characteristics of angiographically non-stenotic non-culprit plaques are independently associated with future CV events. These include plaque burden >70% and VH-based thin-cap fibroatheroma (TCFA) phenotype in PROSPECT62, OCT-defined lipid-rich plaques with inflammatory cell infiltration in CLIMA63, and near-infrared spectroscopy (NIRS)-defined lipid-rich plaques in the Lipid-Rich Plaque64 and PROSPECT-II studies (Erlinge et al. PROSPECT II: A Prospective Natural History Study Using NIRS-IVUS Imaging in Patients with Acute Myocardial Infarction. Presented at TCT Connect 2020). In a different setting, i.e., patients presenting with stable chest pain (of whom 10% had known previous CAD and obstructive CAD was identified in 26%), the CT substudy of SCOT-HEART showed that high-attenuation (i.e., lipid-rich) plaques were independently associated with spontaneous myocardial infarctions throughout five years of follow-up, irrespective of coronary artery stenosis65. Collectively, these studies confirm that not only the degree of angiographic stenosis, but also plaque burden (not measurable by angiography alone) and plaque composition should be considered for risk stratification and thus for defining the LDL-C goal for a given patient. Although so far not tested in a dedicated RCT, it may become reasonable to consider such a strategy in routine practice.
Effect of LDL-C lowering therapies on coronary plaques
In addition to the evidence from clinical outcomes trials, mechanistic studies using intracoronary imaging were able to demonstrate beneficial effects of lipid-lowering medications on coronary atherosclerotic lesions (reviewed in detail elsewhere)66 (Figure 5). Intravascular ultrasound (IVUS) can depict the atherosclerotic vessel wall beyond the “lumenography” provided by coronary angiography and is capable of quantifying atheroma volume. Studies applying serial IVUS showed that intensive statin treatment slows the progression of coronary atherosclerosis and achieves modest plaque regression with the highest doses of potent statins. In the REVERSAL study including 654 CCS patients, treatment with atorvastatin 80 mg led to prevention of plaque progression (no significant change in percent atheroma volume [PAV] by −0.4%) during an 18-month follow-up, whereas PAV increased (+2.7%) in the pravastatin arm (p=0.02)67. ASTEROID was the first large-scale serial IVUS study to document coronary plaque regression. In 507 patients treated with the highest dose of rosuvastatin (40 mg) over 24 months, reduction of LDL-C to a mean of 60.8±20 mg/dL was associated with a median PAV reduction of 0.79%68. The SATURN study subsequently compared rosuvastatin 40 mg versus atorvastatin 80 mg and showed a similar magnitude of plaque regression with the two regimens (1.22% [1.52 to 0.90] vs 0.99% [1.19 to 0.63]; p=0.17)69. In addition to data from CCS patients, the IBIS-4 study showed a comparable mean PAV regression of −0.9% (p=0.007) during a 13-month follow-up in the non-infarct-related arteries of acute STEMI patients treated with rosuvastatin 40 mg70. Regarding non-statin drugs, the PRECISE IVUS study compared atorvastatin (uptitrated to reach an LDL-C of <70 mg/dL) plus ezetimibe versus atorvastatin monotherapy in 202 Japanese patients undergoing PCI. The study showed greater coronary plaque regression with the combination of statin plus ezetimibe versus statin alone after 9-12 months (change in PAV: −1.4% [−3.4% to −0.1%] vs −0.3% [−1.9% to 0.9%], respectively; p<0.001)71. Finally, in the GLAGOV trial, 968 patients with angiographic coronary disease were randomly allocated to receive the PCSK9i evolocumab or placebo in addition to statin for 18 months. The study showed greater PAV regression with evolocumab versus placebo (difference: −1.0% [−1.8% to −0.64%]; p<0.001) and a greater proportion of patients with PAV regression over time (64.3% vs 47.3%, respectively; p<0.001)72.
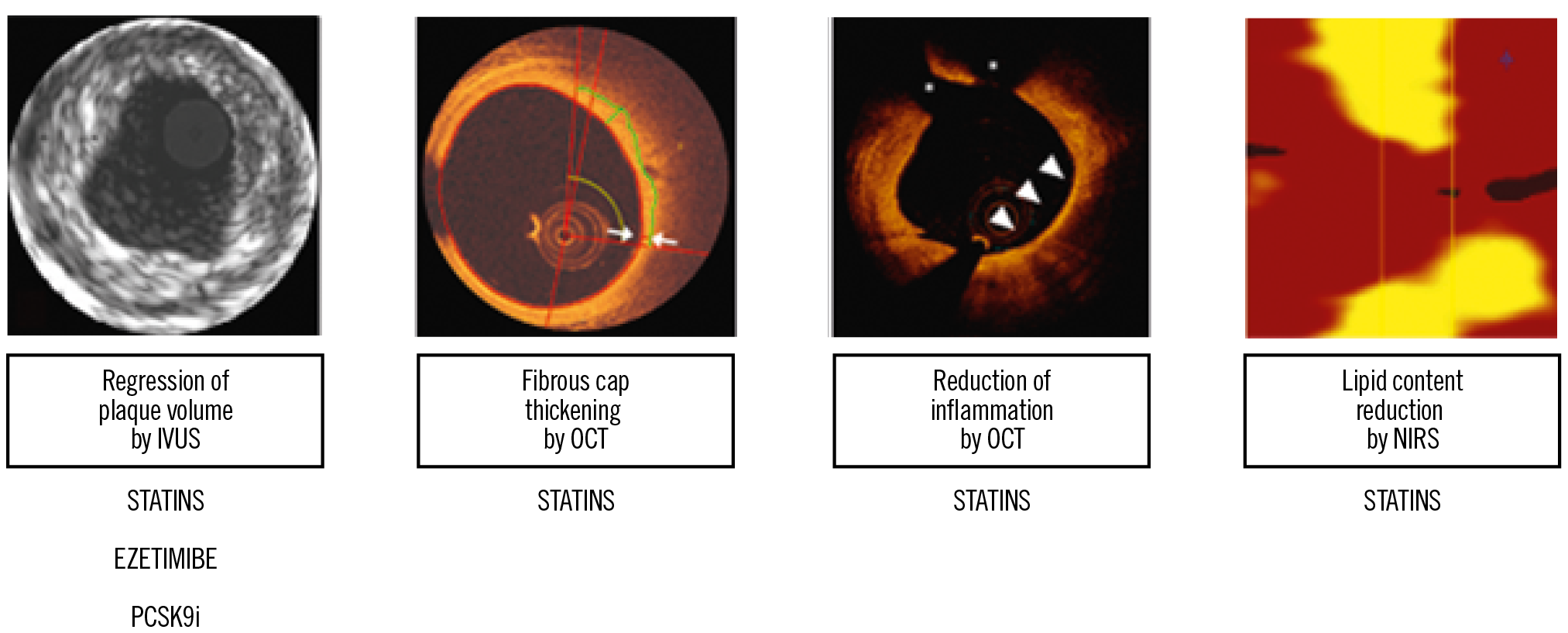
Figure 5. Summary of evidence on the effect of lipid-lowering medications on coronary atherosclerosis defined by intracoronary imaging.
Other intracoronary imaging modalities can assess indices of plaque morphology and composition in vivo beyond atheroma volume. In a serial OCT trial, higher-dose compared with lower-dose atorvastatin (20 mg vs 5 mg) resulted in greater fibrous cap thickening and reduction of macrophage accumulation73. In another study assessing changes in plaque lipid content by means of serial NIRS, intensive statin treatment (rosuvastatin 40 mg) resulted in greater reduction in the maximal lipid core burden index of obstructive coronary lesions compared with standard-of-care lipid-lowering therapy within a short-term (7-week) follow-up74. Consistent with these RCT data, observational evidence showed an increase in fibrous cap thickness, reduction in inflammation, and regression of high-risk TCFA to presumably more stable lesion phenotypes in non-culprit lesions of patients with STEMI treated with high-dose rosuvastatin75. The effect of the PCSK9i alirocumab on IVUS-, OCT-, and NIRS-defined plaque burden and composition is currently being investigated in the PACMAN-AMI trial (NCT03067844).
With respect to non-invasive imaging of the coronary arteries, CTA studies have assessed changes in coronary atherosclerosis in relation to statin treatment. In the PARADIGM study including 1,255 patients without a history of CAD, use of statins (in 62% of patients) was associated with slower progression of plaque volume, increase in plaque calcification, and reduction of high-risk plaque features (low-attenuation plaque, positive arterial remodelling, or spotty calcifications) at an interscan interval of ≥2 years76. In another observational study including 202 patients who underwent coronary CTA for suspected CAD and were prospectively included to undergo a follow-up scan with a mean interscan period of 6.2±1.4 years, statin use was associated with an increased progression of calcified coronary plaque and a reduced progression of non-calcified coronary plaque77. These data need to be interpreted in view of inherent limitations of non-randomised investigations but provide mechanistic evidence that is complementary to the data derived from catheter-based intracoronary imaging, overall pointing to a stabilising effect of statin treatment on coronary atheroma burden and composition. Regarding triglyceride-lowering medications, the EVAPORATE study involving 80 patients with coronary atherosclerosis (≥20% narrowing) and elevated triglyceride levels despite statin therapy showed significant regression of low-attenuation plaque volume with icosapent ethyl (a highly purified eicosapentaenoic acid ethyl ester) versus placebo over 18 months78.
In summary, there is evidence that lipid-lowering therapies halt the progression of coronary atherosclerosis; statins in particular induce phenotypic changes towards more stable plaque types. As both large plaque volume and certain high-risk plaque characteristics by IVUS-VH, OCT and NIRS have been associated with impaired clinical prognosis62,63,64, it may be reasonable to consider the use of such imaging-based characteristics in order to identify patients who are more likely to derive clinical benefit from very intensive lipid-lowering treatments. Such imaging-guided, risk-tailored approaches might be most reasonable for costly treatments (e.g., clinically available PCSK9 antibodies, or other treatments that are currently under investigation), particularly in view of restraints imposed by the limited resources of national health systems across Europe. Identifying certain high-risk plaque characteristics as “gate-keepers” to potentially maximise the clinical benefit of very intensive LDL-C-lowering treatments would conceptually be in line with evidence indicating greatest risk reduction with PCSK9 antibodies in the presence of certain clinical characteristics of excessive risk (e.g., progressive CAD or polyvascular disease)52,53. However, the clinical value and cost-effectiveness of such imaging-based approaches remain speculative and require investigation in properly designed studies.
Future outlook on lipid-lowering therapies
Statins are the undisputed first-line therapy, and ezetimibe and PCSK9i are currently recommended add-on treatments to lower LDL-C in patients with CAD. Several emerging therapies are under investigation, targeting either LDL-C or lipoprotein(a) [Lp(a)] levels.
Bempedoic acid is an oral small molecule that inhibits cholesterol synthesis in the liver by inhibiting an enzyme upstream of the target enzyme of statins79. It reduces LDL-C levels by approximately 20% in monotherapy, and by about 40% in combination with ezetimibe according to phase III trials in patients with ASCVD, at very high CV risk, or with statin intolerance80,81. A clinical outcomes trial is ongoing.
An alternative approach to monoclonal antibodies inhibiting the PCSK9 enzyme involves RNA interference. In phase II and III trials, the small interfering RNA (siRNA) molecule inclisiran reduced LDL-C by up to 50% in a dose-dependent manner82,83. No specific serious adverse events were observed. The ORION 4 trial, with a planned mean duration of five years, is currently comparing CV outcomes with inclisiran versus placebo in 15,000 patients with prior MI or stroke (NCT03705234).
Lp(a) is an LDL particle that can cross the endothelial barrier and become retained within the arterial wall. Pro-atherogenic as well as prothrombotic effects have been attributed to increased levels of Lp(a)84. According to Mendelian randomisation studies, Lp(a) has a causal effect on the risk of ASCVD that is proportional to the absolute change in plasma levels, and individuals with a genetic disorder twice as frequent as HeFH causing extremely elevated Lp(a) levels >180 mg/dL have an increased lifetime risk of ASCVD, similar to that of patients with HeFH85. RNA-based therapies for selective reduction of Lp(a) concentrations are currently under investigation, and studies of an antisense oligonucleotide have shown a >90% reduction in plasma Lp(a) levels86. A clinical outcomes trial is currently underway (NCT04023552).
Conclusion
Coronary atherosclerotic disease is a chronic, progressive disease; the risk for progression or sudden destabilisation by acute thrombotic events can be lowered with optimally controlled risk factors, lifestyle changes, and adequate therapy for secondary prevention. Myocardial revascularisation provides major benefits to properly selected patients, but suboptimal adherence to guideline-directed medical therapies adversely affects long-term clinical outcomes following an ACS or elective coronary revascularisation. Among modifiable risk factors of ASCVD, lowering of atherogenic lipids can halt the progression of coronary atherosclerosis and substantially reduce the risk of recurrent ischaemic events in patients with manifest CAD. The time point of hospitalisation for interventional treatment, either for ACS or for CCS, provides an excellent opportunity to initiate or intensify lipid-modifying therapies and establish a concrete follow-up scheme with recommended further steps. This approach should be similar to the in-hospital initiation of antiplatelet treatment following PCI, with the guideline-endorsed recommendation57 to reassess this treatment and adjust as needed at certain time points following the acute intervention (e.g., decide on prolonged DAPT with low-dose ticagrelor, or second-line antithrombotic therapy with a low-dose NOAC). Physicians involved in the in-hospital care of patients undergoing PCI (either elective, or for an ACS), clearly including the treating interventional cardiologists, should be sensitised to measuring lipid levels, starting or modifying treatment in accordance with current guideline recommendations, and to the inclusion of concrete suggestions for subsequent controls and treatment adjustments in the interventional report and/or discharge letters (Figure 4). Starting intensive lipid-lowering treatment in the acute, in-hospital setting has major advantages for patients, as it is linked to an early clinical benefit (reduction of recurrent events in the very high-risk, early post-ACS period) and is more likely to increase subsequent, long-term adherence to treatment.
Conflict of interest statement
K.C. Koskinas reports speaker/consultant fees from Amgen, Sanofi, and Daiichi Sankyo. L. Räber receives research grants to the institution from Abbott, Biotronik, Boston Scientific, Medis, Sanofi, and Regeneron, and speaker or consultant honoraria from Abbott, Amgen, AstraZeneca, Canon, Occlutech, Sanofi, and Vifor. The other author has no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

