Periprocedural cardiac myonecrosis following percutaneous coronary intervention (PCI) is frequently observed in daily practice and related to distal embolisation, side branch occlusion, dissection, or vasospasm. Several therapeutic strategies, including statins, angiotensin-converting enzyme inhibitors, β-blockers, nicorandil, and antiplatelet agents, were previously evaluated for the prevention of periprocedural myocardial injury1.
Among those, statins are the most established treatment for reducing the risk of periprocedural myocardial injury2, and, accordingly, routine pretreatment or loading with a high-dose statin prior to PCI is endorsed by the current European guidelines (class IIa, level of evidence B)3. Pleiotropic effects have been considered the primary mechanism of cardioprotective action as benefits have been observed following a short-term pretreatment (12 hours to 2 weeks prior to PCI) with statins, i.e., a time range that is too short to result in significant low-density lipoprotein cholesterol (LDL-C) lowering2.
Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors rapidly and effectively lower LDL-C levels and reduce major cardiovascular events. Although previous investigations have suggested a potential association between PCSK9 levels and lipoprotein metabolism, inflammation, thrombosis, and immune function4, little is known regarding the pleiotropic effects of PCSK9 antibodies.
The EVOCATION investigators previously conducted a similarly designed randomised trial and found a benefit in pretreatment with pravastatin 20 mg/day initiated 4 weeks before PCI for the prevention of post-PCI microvascular dysfunction as assessed by the index of microcirculatory resistance (IMR)5. IMR is a thermodilution-derived measure, which can specifically assess microvascular dysfunction caused primarily by distal embolisation during PCI. In this issue of EuroIntervention, Ishihara et al report the results of the EVOCATION trial, a multicentre, randomised, open-label study including 100 Japanese patients with chronic coronary syndrome (CCS), which investigated the effect of biweekly evolocumab (140 mg) pretreatment on top of statin therapy as verified by post-PCI IMR6. Evolocumab was initiated 21.0±8.6 days before PCI. Despite the substantial decrease in LDL-C in the evolocumab group (25.6 mg/dl, 95% confidence interval [CI]: 21.9-30.0) compared with the statin alone group (79.8 mg/dl, 95% CI: 73.9-86.3), there was no significant difference between treatment groups with respect to the primary endpoint post-PCI IMR (20.6, 95% CI: 17.2-24.6 vs 20.6, 95% CI: 17.0-25.0; p=0.98) or the secondary endpoint post-PCI troponin T (20.6 ng/ml, 95% CI: 17.2-24.6 vs 20.6 ng/ml, 95% CI: 17.0-25.0; p=0.98).
The pleiotropic effects of statins mitigating the risk of myocardial damage during PCI include antithrombotic action, anti-inflammation, improvement of coronary flow velocity reserve by vasodilation of coronary microvessels, and rapid improvement of endothelial function2. Similar to statins, PCSK9 inhibitors were expected to reveal pleiotropic effects based on the potential association between PCSK9 and several metabolic pathways beyond LDL receptors. Despite substantial LDL-C lowering, the current study showed no cardioprotective effects of evolocumab during PCI. Given the lack of randomised data in this field, the EVOCATION trial has provided important insights on the absence of pleiotropic effects of PCSK9 inhibitors.
These findings are consistent with the lack of high-sensitivity C-reactive protein (CRP) reduction following treatment with PCSK9 inhibitors789. During the 2022 European Society of Cardiology Congress in Barcelona, more data on potential pleiotropic effects of PCSK9 inhibitors were presented (not yet published). Two studies investigating the effect of either alirocumab (Rexhaj et al) or evolocumab (Kannankeril et al) on endothelial function, as assessed by flow-mediated vasodilatation, did not show an improvement of endothelial function in stabilised patients with acute coronary syndrome or CCS (only unvalidated endpoints differed in the evolocumab study). Clinically relevant inhibition of platelet function as assessed by VerifyNow (Accumetrics) was absent in a PACMAN-AMI substudy following treatment with alirocumab (Räber et al).
The study by Ishihara et al does have some limitations that merit consideration when interpreting the robustness of the findings. First, this was an open-label, non-placebo-controlled study. Second, pre-PCI IMR was not measured in the current study. Previous studies have demonstrated that pre-PCI IMR was an independent predictor for post-PCI changes in IMR10 and that a high pre-PCI IMR value was significantly associated with an increased risk of post-PCI myocardial injury. Third, relatively low-risk patients/lesions were included in the current study (mean age of 68 years, two-thirds with single stent implantation, mean stent length <30 mm) reflecting a population in which periprocedural MI prevention using a costly drug may not be the first priority. Finally, it would have been of scientific interest to add a third treatment arm receiving only PCSK9 inhibitors to exclude any impact by the statin background therapy.
Collectively, the current study importantly adds to previous evidence on the limited or even absent clinically relevant pleiotropic effects of PCSK9 inhibitors in the presence of statin treatment (Figure 1). As a broader clinical consequence, patients in whom PCSK9 inhibitor therapy has been initiated to achieve guideline-recommended LDL-C goals should be motivated to continue their statin therapy not only to combine the LDL-C lowering effects but also to benefit from the additional pleiotropic effects of statins.
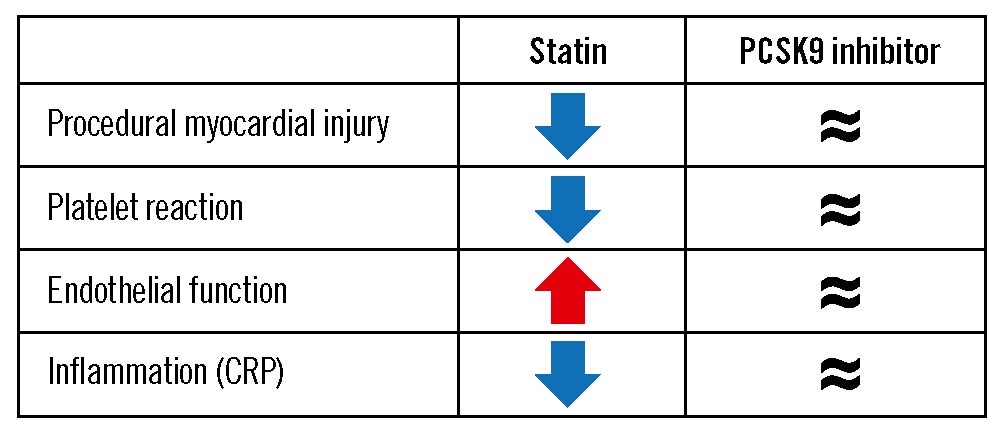
Figure 1. Clinically relevant pleiotropic effects of statins and PCSK9 inhibitors in the PCI setting. CRP: C-reactive protein; PCI: percutaneous coronary intervention; PCSK9: proprotein convertase subtilisin/kexin type 9
Conflict of interest statement
L. Räber received research grants to the institution from Abbott Vascular, Biotronik, Boston Scientific, HeartFlow, Sanofi, and Regeneron; and speaker or consultation fees from Abbott Vascular, Amgen, AstraZeneca, CSL Behring, Novo Nordisc, Occlutech, Sanofi, and Vifor. Y. Ueki reports travel grants from Infraredx.
Supplementary data
To read the full content of this article, please download the PDF.

