Abstract
Aims: Long-term administration of statin therapy has been shown to reduce major coronary events and cardiac mortality within randomised clinical trials. Statins favourably affect platelet adhesion, thrombosis, endothelial function, inflammation, plaque stability, and ventricular arrhythmia, which may potentially improve outcome after percutaneous coronary intervention (PCI) for ST-elevation myocardial infarction (STEMI) and shock. Therefore, we hypothesised that statin therapy has an early beneficial effect among patients undergoing PCI for STEMI complicated by cardiogenic shock.
Methods and results: We retrospectively collected data of consecutive patients undergoing emergency PCI for STEMI complicated by cardiogenic shock between January 2000 and June 2008. Baseline, procedural, and in-hospital data of statin-treated and non-statin-treated patients were compared. Propensity score and multivariate survival analysis were used to adjust for heterogeneity between the two groups. Of 111 patients who comprised the study population, 30/111 (27%) were treated with statin at the time of the procedure. Statin therapy was associated with an in-hospital mortality reduction (46.7% versus 70.4%; odds ratio, 0.32; 95% CI, 0.11-0.89; P=0.029). After adjusting for the propensity to receive statin therapy before the procedure and other confounders, statin therapy remained an independent predictor for in-hospital survival after coronary intervention (odds ratio, 0.35; 95% CI, 0.15 to 0.88; P=0.026).
Conclusions: In this study cohort, statin therapy among patients undergoing emergency PCI for STEMI and cardiogenic shock is associated with a significant mortality advantage at early follow-up.
Introduction
Although prevention trials of statins have shown a 25% to 30% reduction in ischaemic cardiovascular events at long-term follow-up1,2, recent studies suggest that the incidence of early deaths and recurrent ischaemic events are also reduced by statin therapy among acute coronary syndrome (ACS) patients3,4. Among patients with ST-elevation MI referred to the cardiac catheterisation laboratory, those with cardiogenic shock are relatively rare, accounting for less than 10% of all patients. Accordingly, cardiogenic shock represents a common criterion for exclusion in the vast majority of clinical trials. Consequently, little data are available regarding the effect of these drugs on STEMI-patients with shock undergoing emergency percutaneous coronary intervention (PCI).
Beyond lowering lipids, statins have favourable effects on platelet adhesion5, thrombosis6, endothelial function7, plaque stability, inflammation8,9, and ventricular arrhythmia10. Myocardial infarction, cardiogenic shock and stent placement are strongly associated with platelet activation, thrombosis, inflammation within the vessel wall and the distal microvasculature, and malign ventricular arrhythmia. Therefore, we postulated that statin therapy may play a beneficial role early after PCI for STEMI and cardiogenic shock. To assess this, we compared the outcomes of PCI patients who were treated, with those who were not.
Methods
Study population
The study population comprised 111 consecutive patients admitted between January 2000 and June 2008, to the catheterisation laboratory of our centre to undergo immediate PCI for 1) STEMI complicated by 2) cardiogenic shock. Shock was defined as a prolonged (>20 minutes) drop in systolic blood pressure <90 mm Hg despite fluid administration, after excluding right ventricular infarction, and requiring the administration of inotropic drugs along with heart rate >100 bpm. The diagnosis of shock was confirmed by an independent review of source documents in all patients. Similarly, the presence of statin therapy within at least four weeks of PCI was confirmed by an independent review of source documents in all patients. Prompt PCI of the culprit coronary artery was performed using standard balloon and stents techniques, after a loading dose of ticlopidine (2000-2001) or clopidogrel (since 2002). In some cases, ticlopidine or clopidogrel were given after the procedure. Adequate medication was administered, including inotropic drugs as well as anti-thrombotic and anti-ischaemic agents that were given, according to guidelines, when not contra-indicated. We retrospectively collected demographic and procedural data, including medication use, haemodynamic status, equipment use, and final results for each case, that were recorded in an interventional database. Clinical event data during the index hospitalisation were collected by cardiology nurses and research coordinators through patient interview and chart review. The patient population was broken down according to whether statin therapy was currently used within at least four weeks of PCI.
Statistical analysis
Continuous variables were expressed as mean ±SD, and they were compared by Student’s t test or Mann-Whitney rank sum test. Categorical data were displayed as frequencies and percentages. χ2 test was used for bivariate analysis for categorical data. Kaplan-Meier estimation and Cox proportional hazards modelling were used for unadjusted and adjusted survival analysis, respectively.
To adjust for the bias inherent to the statin therapy decision taking prior PCI, propensity analysis was performed11. Propensity analysis aims to identify patients with similar probability of receiving statin therapy on the basis of observed clinical characteristics. Using a multivariable logistic regression model including the baseline characteristics, the probability of being under statin therapy was determined. The variables that were included in the propensity score model were age, sex, anterior location STEMI, history of STEMI, use of aspirin and use of β-blockers. The population was divided into deciles according to the propensity score. Within each decile, the mean propensity scores of the statin and non-statin groups were compared, as well as their clinical and procedural characteristics. To adjust for the heterogeneity between the two groups, the propensity score was then entered as a continuous variable in the Cox proportional hazards model along with potential covariates. All statistical analyses were performed with the STATA 10.0 program (STATA Corp., College Station, TX, USA).
Results
Baseline characteristics
Data of 111 consecutive patients were entered in the database between 2000 and 2008. All patients had complete in-hospital follow-up and were included in the final analysis. Of these, 30 patients (27%) were taking statin within at least four weeks of the procedure. Table 1 shows the baseline characteristics according to statin treatment.
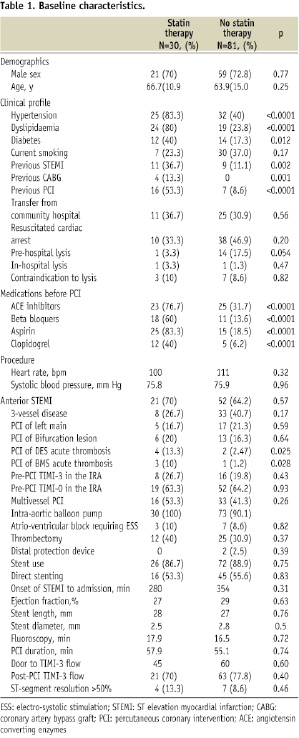
Important differences existed between the two groups. Patients who were on statin therapy before PCI were more likely to have diabetes mellitus, hypertension, hypercholesterolaemia, prior STEMI, previous PCI, previous coronary bypass surgery, and to be taking concomitant ACE inhibitors, β-blockers, aspirin and clopidogrel. During the procedure, statin recipients were more likely to have interventions for drug-eluting or bare metal stent thrombosis. The rates of pre-PCI reperfusion, procedural success, direct stent implantation, and left ventricular ejection fraction as well as the mean time elapsed between the onset of STEMI and admission to the catheterisation laboratory were similar between the two groups.
Unadjusted in-hospital all-cause mortality
As depicted on the Kaplan-Meier curve (Figure 1), administration of a statin at the time of procedure was associated with an in-hospital mortality benefit (46.7% versus 70.4%; log-rank P=0.0275).
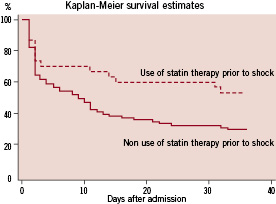
Figure 1. Kaplan Meier Survival estimates in the study population according to the use or non use of statin therapy at the time of PCI for STEMI and shock.
Comparison of the two groups with respect to other major clinical cardiac and procedure-related events showed no significant difference in the incidence of repeat STEMI requiring repeat emergency PCI (6.7% vs. 1.2%, P=0.12), coronary artery bypass surgery (0% vs. 2.5%, P=0.38), and stroke (0% vs. 2.5%, P=0.38). The composite endpoint of death, STEMI, stroke and repeat revascularisation was 56.7% for the statin group and 75.3% for the non-statin group (P=0.056).
Propensity analysis
Within the propensity score analysis, variables that predicted the prescription of a statin before PCI were (in descending order) concomitant prescription of aspirin, anterior STEMI location, male gender, and concomitant prescription of ß-blockers. The goodness of fit of the propensity score was given by c statistic (or area under the receiver operating characteristic curve) being 0.80, indicating the propensity model discriminated well between patients who were prescribed statins before PCI and patients who were not. Within each decile of the study population, the propensity scores and baseline characteristics were similar among the statin-treated and non-statin-treated groups.
Multivariate analysis of 6-month mortality
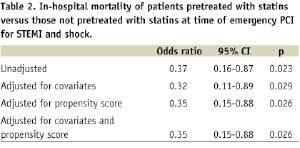
Using Cox proportional hazards model to adjust for all potential variables, statin therapy at the time of emergency PCI for STEMI and cardiogenic shock remained an independent predictor for survival at six months (odds ratio, 0.32; 95% CI, 0.11 to 0.89; P=0.029). When the propensity score was included in the model with all the covariates, the estimation of statin pre-treatment effect did not change significantly (Table 2).
Discussion
The present 8-year registry suggests a reduction of in-hospital mortality in patients treated by statin therapy before emergency PCI for STEMI and cardiogenic shock as compared to those who were not under statin at the time of admission. After adjustment for factors that might be associated with early mortality after PCI for STEMI and shock, statin therapy remained an independent predictor for in-hospital survival benefit. The extent of mortality reduction observed is superior to reductions previously reported in statin-treated patients presenting with acute MI and acute coronary syndromes3,4. However, the present results were obtained in a small sample size population (111 patients with STEMI and shock) and therefore should be considered as potentially resulting from confounding factors in patients’ characteristics in the two groups. Although, this statistical bias should be reduced with the use of regression models and the propensity score, a theoretical risk of patients’ selection still exists.
These findings contrast with the secondary prevention trials with statin therapy (Scandinavian Simvastatin Survival Study, and Cholesterol and Recurrent Events trial1,2, which reported reductions in recurrent infarction and revascularisation rates. However, these trials initiated therapy in the recuperative phase of patients’ index presentation and observed a benefit after 24 months of statin therapy. Indeed, these trials did not examine the effect of early statin therapy in the context of ST-elevation MI. In our study, patients were on statin therapy at the time of coronary re-opening for ST-elevation MI and impaired haemodynamics, and the mortality benefit was seen within days after hospital admission. The present data corroborate the findings of Aronow et al12, who observed an early mortality benefit among ACS-patients on statin treatment at the time of hospital discharge. Similar findings have also been observed in the post-MI patients4. In the MIRACL study3, the primary composite endpoints of death, nonfatal STEMI, cardiac arrest, or recurrent angina were significantly lower among patients treated with atorvastatin therapy compared to those randomised to placebo at 16 weeks, but the mortality reduction was not significant (4.2% for atorvastatin versus 4.4% for placebo, P=0.94). However, this study was not of adequate size to show a mortality difference. Nevertheless, these results raise the possibility that the mechanism of mortality reduction with statins might be independent of its serologic lipid-lowering effect.
Within the context of STEMI, data of large registries have previously focused on the effect of pre-treatment, or early treatment with statins in improving clinical outcomes. The Swedish register of Cardiac Intensive Care (RIKS-HIA) has shown that early initiation of statin therapy in patients with STEMI is associated with reduced 1-year mortality4. The National Registry of Myocardial Infarction 4 Investigators (NRMI-4) reported13 that early statin use was associated with a lower incidence of cardiogenic shock, arrhythmias, cardiac arrest, and cardiac rupture in a large population of >300,000 STEMI patients. Additionally, the Platelet Receptor Inhibition in Ischaemic Syndrome Management (PRISM) investigators have shown that although statin pre-treatment is associated with improved clinical outcome in patients with ACS, discontinuation of statins after onset of symptoms abrogates this beneficial effect14. Recently, Hoffman et al15 have suggested that these results may be related to improved myocardial reperfusion after primary PCI of the infarct-related artery in patients pretreated by statin at the time of STEMI. The beneficial effect of early statin therapy has been reported in survivors of out-of hospital cardiac arrest16, in whom the mortality rate was favourably influenced by the use of an implantable cardioverter-defibrillator (HR 0.32, 95% CI 0.16 to 0.88, p=0.024) and statin therapy (HR 0.68, 95% CI 0.17 to 0.73, p=0.001). Indeed, there has been no clear demonstration so far that pretreatment with statin therapy may be associated with adverse clinical outcome in patients with STEMI and cardiogenic shock. In these patients, Klein et al17 have reported, using data of the American College of Cardiology-National Cardiovascular Data Registry, a 60% rate of in-hospital death despite prompt reopening of the infarct related artery. The patients’ outcome was adversely affected by older age, female gender, no stent use, renal failure and occlusion of the proximal left anterior descending artery. These data were obtained from a large broadcast registry reflecting recent clinical practice but the authors showed no clear benefit of recent therapeutic advances when compared to previous historical reports18.
A recent meta-analysis19 of randomised trials including 13,024 ACS-patients have shown that statin therapy is not associated with any reduction in rates of death, STEMI, stroke, and repeat revascularisation procedures at four months. The discrepancy between this meta-analysis and other reports deserves particular attention since it increases the potential patho-physiological mechanisms of these agents. Indeed, data of trials were included in this meta-analysis if statins were initiated within 14 days of ACS and if data of at least 30-day follow-up were available. It could be, therefore, postulated that this negative trial was possibly the result of an inappropriately delayed administration of statins in these patients. Beyond their lipid lowering effect, the potential of statins to reduce thrombogenesis and their anti-inflammatory effect seem to play a very important role in ACS-patients. As suggested in the experimental and clinical settings, mechanistic explanations for thrombogenesis reduction include plaque stabilisation20 (e.g., decreased secretion of matrix metalloproteinases by macrophages, inhibition of endogenous cholesterol synthesis in macrophages, and reduction of number of inflammatory cells in atherosclerotic plaques), improvement of endothelial function7, increased nitric oxide synthesis21, and reduction of platelet activation and adhesion6. The association between systemic inflammation and adverse outcome among ACS-patients is primarily expressed as an excess in mortality. The Pravastatin Inflammation C-Reactive Protein Evaluation (PRINCE) study22 confirms the effect of pravastatin on C-reactive protein lowering, a marker of the anti-inflammatory effect. More recently, the JUPITER Trial23 has strengthened the role played by the anti-inflammatory effects of statins in their beneficial effects observed in large populations. This trial showed that healthy persons with elevated high-sensitivity C-reactive protein levels but without hyperlipidaemia and treated with rosuvastatin had, compared to those under placebo, a significant decrease in MI, revascularisation unstable angina and STEMI +stroke +cardiac death (–54%, –47%, –47%, respectively). Accordingly, Brugts et al24 showed, in a large meta-analysis of 70,388 people without cardiovascular disease but with cardiovascular risk factors that statin use was associated with lower rates of mortality (–12%) and of major coronary (–30%) and cerebrovascular events (–19%). In STEMI patients with shock, thrombogenesis and inflammation are major and sudden, and are obviously associated with myocardial ischemia, refractory cardiac failure, and ventricular arrhythmia, usually causing death in these patients. Therefore, pre-treatment with statins or at least very early administration of these agents is required in order to benefit from their potential positive role in improving patient outcome.
Limitations
Our analysis has several limitations. The treatment with statin therapy was nonrandomised. Despite careful use of a propensity score and regression models to adjust for potential confounders that may affect major cardiac events, unmeasurable factors may still exist, particularly in this small sample size population. Physician or patient’s related bias may also affect the procedure of stenting. However, the statin-treated patients in our cohort carried more high-risk comorbidities at the time of PCI. The compliance with statin therapy before hospital admission was difficult to appreciate. However, any crossover of the comparison groups would only lead to underestimation of the treatment effect and, hence, may strengthen our conclusion about the beneficial effect of statins before PCI. Likewise, the length of statin pre-treatment that would be required to produce the observed mortality reduction remains unknown. In the present study, patients have been selected on the basis of at least 4-week pre-treatment with statins. Previous studies have shown that statin therapy improves endothelial function and lowers serum inflammatory markers as early as six to 12 weeks7-9, the treatment effect may again be underestimated in our study if the duration of statin pre-treatment was shorter than this amount of time in some patients.
Conclusions
In this observational study, pre-treatment with statins is associated with an independent in-hospital mortality reduction in patients with STEMI and cardiogenic shock. Although the mechanism of mortality reduction remains unclear in these patients with STEMI and impaired haemodynamics, additional research is warranted to elucidate this process.

