
Abstract
Aims: The aim of this study was to evaluate clinical outcome for different indications for PCI in an unselected, nationwide PCI population at short- and long-term follow-up.
Methods and results: We evaluated clinical outcome up to six years after PCI in all patients undergoing a PCI procedure for different indications in Sweden between 2006 and 2010. A total of 70,479 patients were treated for stable coronary artery disease (CAD) (21.0%), unstable angina (11.0%), non-ST-elevation myocardial infarction (NSTEMI) (36.6%) and ST-elevation myocardial infarction (STEMI) (31.4%). Mortality was higher in STEMI patients at one year after PCI (9.6%) compared to NSTEMI (4.7%), unstable angina (2.2%) and stable CAD (2.0%). At one year after PCI until the end of follow-up, the adjusted mortality risk (one to six years after PCI) and the risk of myocardial infarction were comparable between NSTEMI and STEMI patients and lower in patients with unstable angina and stable CAD. The adjusted risk of stent thrombosis and heart failure was highest in STEMI patients.
Conclusions: The risk of short-term mortality, heart failure and stent thrombosis is highest for STEMI patients after PCI. Therapies to reduce stent thrombosis and heart failure appear to be most important in decreasing mortality in patients with STEMI or NSTEMI undergoing PCI.
Introduction
Treatment with percutaneous coronary intervention (PCI) improves outcome in patients with ST-elevation myocardial infarction (STEMI) and in patients presenting in the acute phase of non-ST-elevation myocardial infarction (NSTEMI)1-3. In patients with stable coronary artery disease (CAD), PCI has not been proven to result in an improved prognosis compared to optimal medical therapy, and is mainly performed to reduce symptoms4.
In the past decade, differences in mortality have been widely investigated in patients with different presentations of CAD, especially NSTEMI and STEMI. However, the results are inconsistent in the short and long term. In the first year after myocardial infarction, studies have reported a higher5-7, comparable8-10 and lower11 mortality in STEMI patients compared to NSTEMI patients. In relation to the long term, some studies have reported a comparable mortality risk in NSTEMI and STEMI patients6,7,12, while others observed a higher mortality risk among NSTEMI patients11,13. In these studies, various proportions of patients have been treated with PCI as reperfusion and revascularisation strategy and selective groups of patients were included. Therefore, it may be questionable whether the previous studies reflect current real-world clinical practice.
The aim of this study was to evaluate clinical outcome after PCI over the whole spectrum of CAD in a nationwide PCI population. We investigated early and late mortality after PCI, and other clinical events that could contribute to our understanding of differences in mortality at short- and long-term follow-up.
Methods
SELECTION OF PATIENTS
In the present study, we included all patients with CAD undergoing a PCI procedure in Sweden between January 2006 and December 2010 (Figure 1). From 2006 onwards, there were almost no missing data and the proportions of indications for PCI were relatively stable over time. All patients were included only once in the analyses. Patients without a Swedish personal identification number, necessary for merging with other registries, were excluded. In addition, patients with non-ST-elevation acute coronary syndromes (NSTE-ACS) were excluded when data about myocardial damage based on biomarkers were not registered, as they could not be identified as unstable angina or NSTEMI.
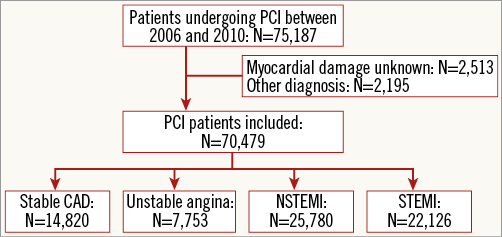
Figure 1. Flow chart. Selection of the patients with coronary artery disease undergoing PCI.
SCAAR DATA
The Swedish Coronary Angiography and Angioplasty Registry (SCAAR) documents all coronary angiography and PCI procedures performed at any of the 30 hospitals with a catheterisation laboratory in Sweden from 1989 onwards. SCAAR is part of the nationwide Swedish Web-system for Enhancement and Development of Evidence-based care in Heart disease Evaluated According to Recommended Therapies (SWEDEHEART) registry. Data are prospectively collected following the registration standards for clinical practice, and data are reported in a web-based interface14,15. Collection of data on baseline, angiographic and procedural characteristics is initiated at the catheterisation laboratory by the treating physician. The long-term follow-up data were available by merging data from national patient registers with SCAAR on the basis of the unique personal identification number which all Swedish citizens have. Vital status and date of death were obtained from the Swedish National Population Registry until December 31, 2011. Follow-up concerning hospitalisations for myocardial infarction (ICD 10th revision, codes I21 and I22) and heart failure (ICD 10th revision, codes I50, I97, I13.0, I13.2, K76.1) was derived from the National Patient Register, and was available only until December 31, 2010. Information regarding stent thrombosis and target lesion revascularisation (TLR) is registered in SCAAR itself at any coronary angiography performed in Sweden, irrespective of where in the country the initial PCI was performed, and follow-up was available until December 31, 2011. The merging with SCAAR was performed by the Epidemiologic Center of the Swedish National Board of Health and Welfare, and was approved by the ethics committee of Uppsala University.
INDICATIONS FOR PCI
In SCAAR, the indication for PCI in patients with CAD is registered as stable CAD, NSTE-ACS, or STEMI. Stable CAD is defined as symptoms of chest pain during exercise relieved by rest or nitroglycerine, described as definite angina or probable angina according to the Coronary Artery Surgery Study (CASS) classification16. NSTE-ACS is defined as typical symptoms of chest pain progressive during the last four weeks, occurring at rest or less responsive to nitroglycerine; new onset of symptoms during the last four weeks; or typical symptoms during the initial four weeks after myocardial infarction. In this study, patients classified as NSTE-ACS were divided into unstable angina and NSTEMI based on the elevation of biomarkers of myocardial damage (CK-MB or troponin-T level ≥2 times the upper limit of the normal range) before PCI. STEMI was defined as clinical suspicion of ongoing myocardial ischaemia with ST-elevation or new left bundle branch block on the qualifying ECG.
CLINICAL OUTCOMES
We investigated clinical outcome after PCI for the different indications during the first year after PCI and at the long-term follow-up after the first year. The pre-specified primary outcome was all-cause mortality. Secondary outcomes were myocardial infarction, stent thrombosis, TLR and admission for heart failure. For all secondary outcomes, we analysed the first time admission for the event after the index PCI. The first time admission for myocardial infarction ≥2 days after the index PCI was defined as new myocardial infarction. Stent thrombosis and TLR were assessed by the operator during coronary angiography at any subsequent angiography or PCI procedure. Stent thrombosis was confirmed on the coronary angiogram with angiographic signs of a thrombus or an occlusion in the stent combined with an acute clinical presentation17. TLR was defined as any revascularisation with PCI in the stented or dilated segment of the index procedure, in any subsequent procedure performed18.
STATISTICAL ANALYSIS
The patients were divided into four groups based on the indication for PCI. Categorical variables are presented as frequency values and proportions, and differences between the indications were compared using the chi-square test. Continuous variables with a normal distribution are presented as mean±standard deviation (SD). Differences in continuous variables were evaluated using a one-way analysis of variance. The cumulative incidence of mortality, myocardial infarction, stent thrombosis, TLR and heart failure was presented by Kaplan-Meier event curves for the different indications of PCI. We performed landmark analyses at one year after PCI to provide a separate insight into the early and late risks of events. Cox regression analyses were performed to adjust for covariates. We adjusted for all observed significant differences in baseline, angiographic and procedural characteristics: age (continuous variable), gender, smoking status, prior medical treatment for diabetes, hypertension, hyperlipidaemia; history of myocardial infarction, PCI, CABG, heart failure, stroke and renal failure; number of diseased vessels, stent type19, number of stents (continuous variable) and the use of aspirin or P2Y12 inhibitors, GP IIb/IIIa inhibitors or bivalirudin. In addition, we adjusted for the year of the PCI procedure and hospital. For the endpoint of stent thrombosis, we also adjusted for mean total stent length and mean stent diameter as all patients received a stent. The adjusted hazard ratios are reported with corresponding 95% confidence intervals. STEMI patients were defined as the reference category. The log minus log test and the scaled Schoenfeld residuals were evaluated to test the proportional hazard assumption. For all analyses, two-sided p-values <0.05 were defined as significant. Statistical analyses were performed using SPSS, Version 20.0 (IBM Corp., Armonk, NY, USA).
Results
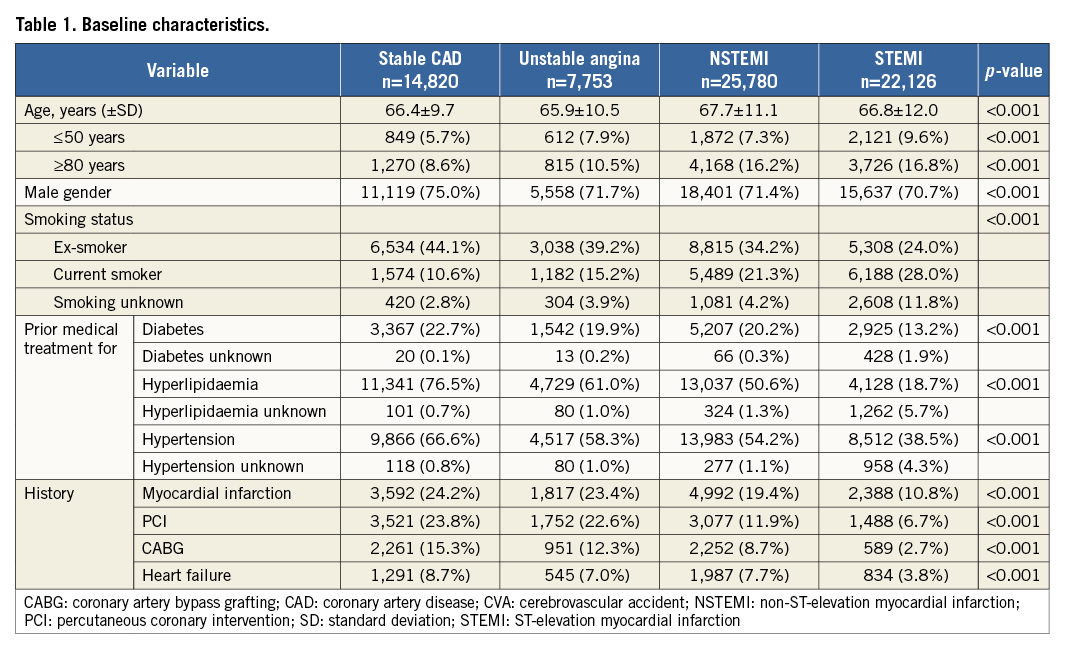
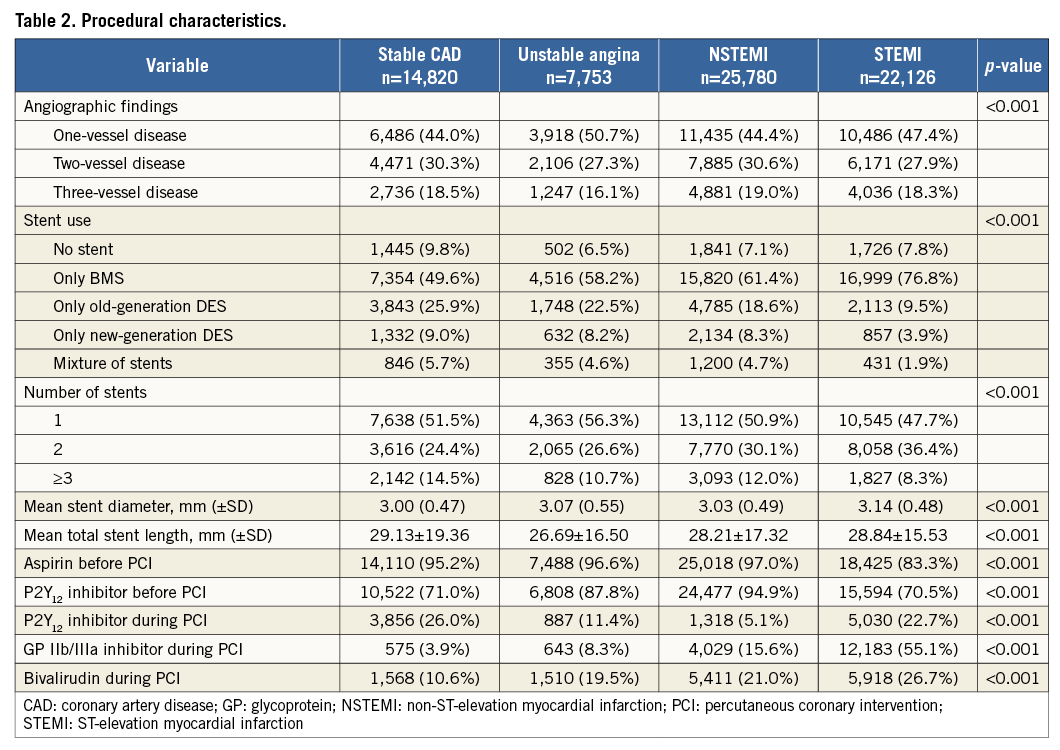
From January 2006 to December 2010, 75,187 unique patients underwent a PCI procedure (Figure 1). A total of 70,479 patients were identified who underwent a PCI procedure for stable CAD (21.0%), unstable angina (11.0%), NSTEMI (36.6%) or STEMI (31.4%). The proportion of NSTEMI patients of all patients who underwent PCI increased from 34.5% in 2006 to 39.2% in 2010. The proportion of STEMI patients increased from 28.7% in 2006 to 32.0% between 2007 and 2010. Depending on the analysis, a maximum of 0.3% of the patients had missing data. Baseline and procedural characteristics are shown in Table 1 and Table 2.
MORTALITY
The median time to end of follow-up (or death) was 3.25 years (interquartile range 1.96-4.62). During the six-year follow-up, there was a lower cumulative incidence of mortality in stable CAD, unstable angina and NSTEMI patients compared to STEMI patients (Table 3, Figure 2A). Mortality rates at one year after PCI were 2.0% (n=301) in stable CAD, 2.2% (n=168) in unstable angina, 4.7% (n=1,209) in NSTEMI and 9.6% (n=2,119) in STEMI patients. After adjustment for differences in baseline and procedural characteristics, there was a lower risk of mortality in patients with stable CAD, unstable angina, and NSTEMI patients compared to STEMI patients in the first year after PCI (Table 3, Figure 2B). Between one and six years after PCI, the risk of mortality was lower in patients with stable CAD and unstable angina as compared to STEMI patients, but there was no difference between NSTEMI and STEMI patients.
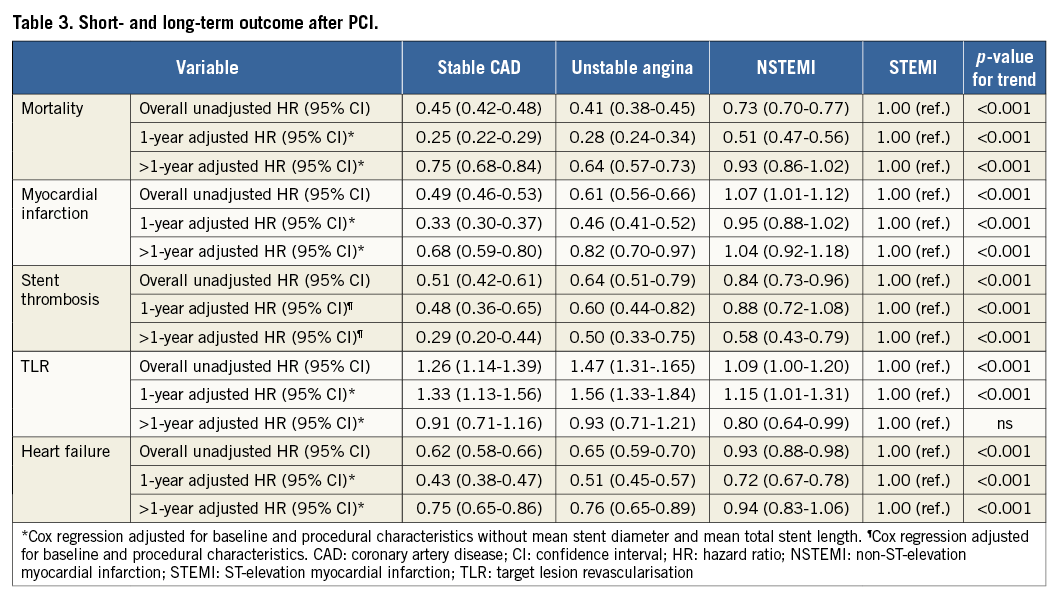
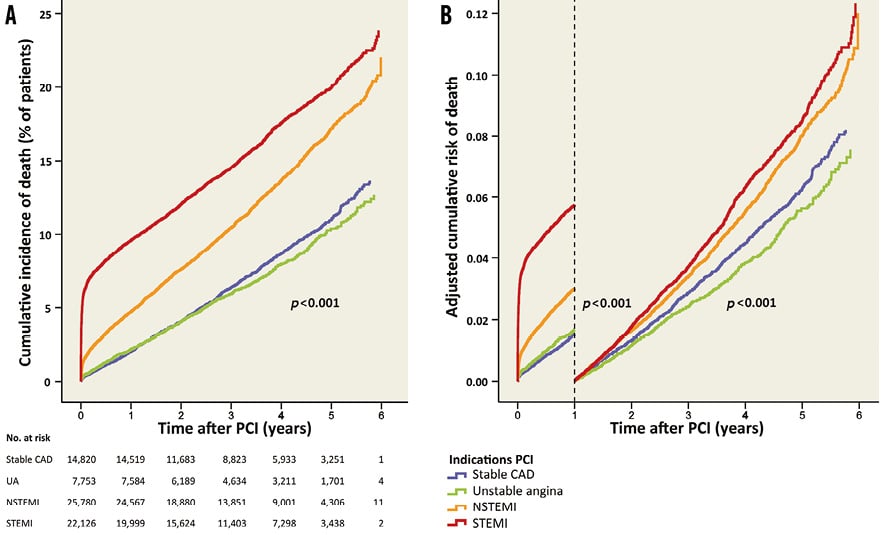
Figure 2. Mortality after PCI for different indications. A) Cumulative incidence of mortality for the different indications. B) Adjusted risk of mortality during and after the first year after PCI.
MYOCARDIAL INFARCTION
The median time to end of follow-up (or myocardial infarction) was 2.04 years (interquartile range 0.76-3.48). During the five-year follow-up, the cumulative incidence of myocardial infarction was higher in NSTEMI patients compared to STEMI patients, and was lower in stable CAD and unstable angina compared to STEMI patients (Table 3, Figure 3A). In the first year after PCI, myocardial infarction occurred in 4.1% (n=496) of patients with stable CAD, in 5.5% (n=353) of patients with unstable angina, in 11.0% (n=2,249) of patients with NSTEMI and in 10.3% (n=1,828) of patients with STEMI. The adjusted risk of myocardial infarction is shown in Table 3 and Figure 3B.
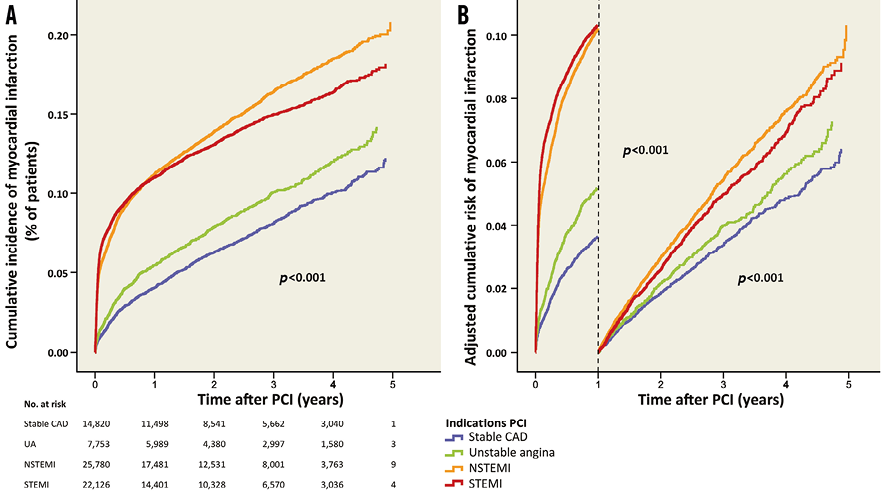
Figure 3. Myocardial infarction after PCI for different indications. A) Cumulative incidence of myocardial infarction for the different indications. B) Adjusted risk of myocardial infarction during and after the first year after PCI.
STENT THROMBOSIS
The median time to end of follow-up (or stent thrombosis) was 3.28 years (interquartile range 1.95-4.73). During the total follow-up of six years, the cumulative incidence of stent thrombosis was lower in patients with stable CAD, unstable angina and NSTEMI compared to patients with STEMI (Table 3, Figure 4A). In the first year after PCI, stent thrombosis occurred in 0.7% (n=96) of patients with stable CAD, in 0.8% (n=61) with unstable angina, in 1.3% (n=305) with NSTEMI and in 1.4% (n=290) with STEMI. The adjusted risk of stent thrombosis is shown in Table 3 and Figure 4B.
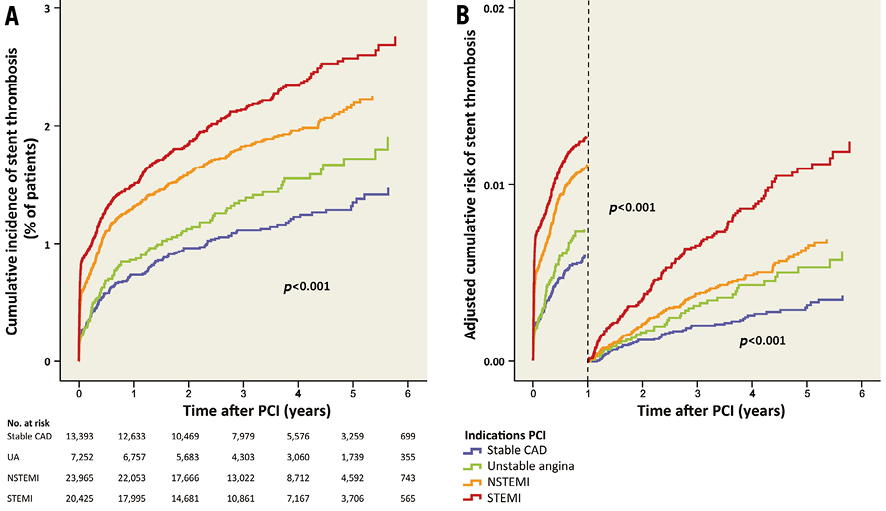
Figure 4. Stent thrombosis after PCI for different indications. A) Cumulative incidence of stent thrombosis for the different indications. B) Adjusted risk of stent thrombosis during and after the first year after PCI.
TLR
The median time to end of follow-up (or TLR) was 3.32 years (interquartile range 1.99-4.75). During the total follow-up of six years, the cumulative incidence of TLR was higher in patients with stable CAD and unstable angina compared to patients with STEMI (Table 3, Figure 5A). In the first year after PCI, TLR occurred in 3.4% (n=500) of patients with stable CAD, in 4.3% (n=329) of patients with unstable angina, in 3.1% (n=803) of patients with NSTEMI and in 2.7% (n=587) of patients with STEMI (Figure 5A). The adjusted risk of TLR is shown in Table 3 and Figure 5B.
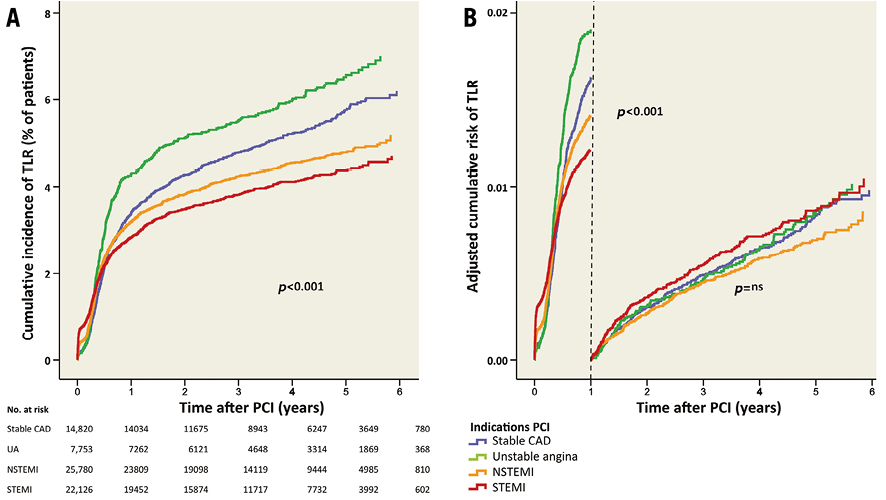
Figure 5. TLR after PCI for different indications. A) Cumulative incidence of TLR for the different indications. B) Adjusted risk of TLR during and after the first year after PCI. TLR: target lesion revascularisation
HEART FAILURE
The median time to end of follow-up (or heart failure) was 2.08 years (interquartile range 0.81-3.51). During the five-year follow-up, the cumulative incidence of heart failure was lower in patients with stable CAD and unstable angina compared to patients with STEMI, and did not differ between patients with NSTEMI and STEMI (Table 3, Figure 6A). During the first year after PCI, heart failure occurred in 5.0% (n=608) of patients with stable CAD, in 5.5% (n=354) with unstable angina, in 8.5% (n=1,735) with NSTEMI and in 9.4% (n=1,676) with STEMI. The adjusted risk of heart failure is shown in Table 3 and Figure 6B.
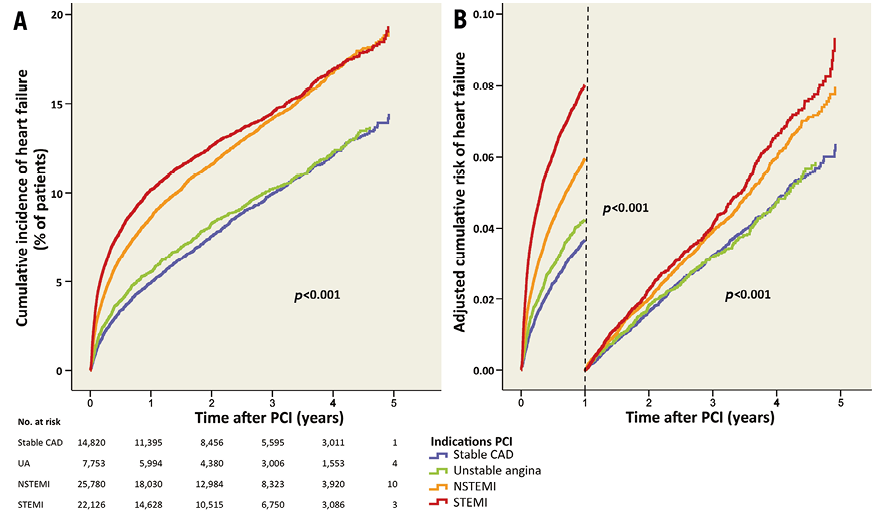
Figure 6. Heart failure after PCI for different indications. A) Cumulative incidence of heart failure for the different indications. B) Adjusted risk of heart failure during and after the first year after PCI.
Discussion
We evaluated clinical outcome for the different indications of PCI in a contemporary population at short- and long-term follow-up. Baseline characteristics differed substantially between the indications for PCI, as well as the treatment strategy. During the first year after PCI, mortality risk was highest in patients with STEMI compared to NSTEMI, unstable angina and stable CAD. Between one and six years after PCI, the adjusted mortality risk was comparable in STEMI and NSTEMI patients but higher than in unstable angina and stable CAD patients.
Clinical outcomes after PCI have been widely investigated in patients with NSTEMI and STEMI. Previous studies often report that NSTEMI patients have an equal or higher mortality risk compared to STEMI patients in the long term after PCI6,7,11-13. However, comparisons are difficult as studies differ in evaluated time intervals, performance of landmark analyses and adjustments for covariates. Differences between our findings and previous studies are most likely influenced by patient selection. The complete registration of all consecutive PCI procedures in Sweden made it possible to analyse an unselected contemporary nationwide population.
Baseline characteristics and risks differed between the indications for PCI. Although the mean age did not differ substantially between the indications, the proportion of patients ≥80 years was twice as high in NSTEMI and STEMI patients compared to patients with stable CAD and unstable angina. NSTEMI patients presented more often with risk factors for CAD and a prior myocardial infarction or PCI compared to STEMI patients. This may indicate that NSTEMI patients have more extensive CAD than STEMI patients, as previous studies have suggested5,13. However, the number of diseased vessels did not differ substantially between NSTEMI and STEMI patients.
We did not observe a difference in the adjusted risk of mortality between STEMI and NSTEMI patients one to six years after PCI. However, there was a substantial difference in mortality risk between patients with and without elevated biomarkers of myocardial damage at short- and long-term follow-up. The great difference in mortality, especially between unstable angina and NSTEMI patients, confirms that myocardial damage is an important predictor of mortality, as previously reported at six months20. The one-year mortality in STEMI patients in this study was high compared to the TASTE study21. In TASTE, the STEMI population was more selected, based on the exclusion criteria.
Even after adjustment for type of stent, the stent thrombosis risk was higher in STEMI patients compared to all other indications, even in the long term. The activated coagulation may play a role, present in STEMI patients until months after an acute event22. In addition, undersizing of stents may contribute, due to vasoconstriction or mural thrombus in the acute phase of myocardial infarction23. An increase in the use of more potent platelet inhibitors and new-generation drug-eluting stents may contribute to a lower risk of stent thrombosis in STEMI patients in future clinical practice19,24.
Limitations
Data from observational registries typically have lower quality and there are more often missing data as compared to randomised clinical trials. In SCAAR, the completeness of the data has substantially improved over time, with almost no missing data after 2006. Furthermore, baseline and procedural characteristics differed between the indications for PCI, and greatly influenced the clinical outcomes. Therefore, we adjusted for all measured covariates. In addition, data about medical therapy and compliance were not available during the follow-up period.
Conclusions
In this contemporary, unselected population, mortality at one year after PCI was higher in STEMI patients compared to other indications. After the first year after PCI, we observed a substantial difference in the adjusted risk of mortality between patients with and without myocardial damage. The risk of stent thrombosis and heart failure was highest in STEMI patients, even in the long term.
| Impact on daily practice Mortality and clinical outcomes after PCI differ substantially between the different indications of PCI on short- and long-term follow-up. Our findings suggest that improvements in therapy are needed in the acute phase as well as in the long term. Specifically, therapies and strategies to reduce stent thrombosis and to oppose the development of heart failure appear to be most important for the reduction of mortality in patients with acute coronary syndromes undergoing PCI. |
Funding
SCAAR is sponsored by the Swedish Health Authorities and is independent of commercial funding. M. Fokkema receives non-commercial funding from Stichting Jo Kolk Studiefonds.
Conflict of interest statement
S. James has received research grants from AstraZeneca, Eli Lilly, Bristol-Myers Squibb, Terumo Inc., Medtronic, and Vascular Solutions, honoraria from The Medicines Company, AstraZeneca, Eli Lilly, Bristol-Myers Squibb, and Iroko, and is a consultant/on the advisory board for AstraZeneca, Eli Lilly, Merck, Medtronic, and Sanofi. P. Eriksson has received honoraria from AstraZeneca, Eli Lilly, Boston Scientific and Iroko Cardio. The other authors have no conflicts of interest to declare.

