
Abstract
Aims: The clinical performance of the SYNERGY drug-eluting stent (DES) in patients with acute myocardial infarction (MI) has not been investigated in detail. We sought to report on the outcomes after SYNERGY DES (Boston Scientific, Marlborough, MA, USA) implantation in patients with MI undergoing percutaneous revascularisation (PCI).
Methods and results: We included all consecutive patients with MI undergoing PCI with the SYNERGY DES and newer-generation DES (n-DES group) in Sweden. From March 2013 to September 2016, a total of 36,292 patients, of whom 39.7% presented with ST-elevation MI, were included. As compared to patients in the n-DES group (n=31,403), patients in the SYNERGY group (n=4,889) were older and presented more often with left main or three-vessel disease involvement, as well as with restenotic lesions (p<0.001 for all parameters). The Kaplan-Meier estimates of ST at two years in the SYNERGY and n-DES groups were 0.69% and 0.81%, respectively (adjusted HR 1.00, 95% CI: 0.69-1.46; p=0.99). Clinically relevant restenosis was encountered in 1.48% and 1.25% of patients in the SYNERGY and n-DES groups, respectively (adjusted HR 1.05, 95% CI: 0.81-1.37; p=0.72). No differences in the risk of all-cause death and recurrent MI were found between the two groups after adjustment (adjusted HR 1.12, 95% CI: 0.98-1.28; p=0.10, and adjusted HR 0.95, 95% CI: 0.82-1.10; p=0.49, respectively).
Conclusions: In a large and unselected cohort of patients with MI undergoing percutaneous revascularisation with the SYNERGY DES, stent performance and clinical outcomes did not differ compared with other n-DES up to two years.
Abbreviations
BP-DES: bioabsorbable polymer DES
CI: confidence interval
DAPT: dual antiplatelet therapy
DES: drug-eluting stents
HR: hazard ratio
ICD: International Classification of Diseases
MAUDE: Manufacturer and User Facility Device Experience
MI: myocardial infarction
n-DES: new-generation DES
PCI: percutaneous coronary intervention
PS: propensity score
RIKS-HIA: Swedish Register of Information and Knowledge about Swedish Heart Intensive Care Admissions
SCAAR: Swedish Coronary Angiography and Angioplasty Registry
ST: stent thrombosis
Introduction
Timely revascularisation by percutaneous coronary intervention (PCI) is advocated by current guidelines as a strategy to temper the negative consequences of prolonged myocardial ischaemia and improve prognosis in patients with acute myocardial infarction (MI)1. At variance with revascularisation performed in the setting of stable coronary artery disease, emergent or urgent PCI is associated with an increased risk of periprocedural complications, including stent thrombosis (ST)2,3. Enhanced platelet reactivity, the presence of thrombus trapped within stent struts potentially leading to late acquired malapposition and delayed endothelialisation have been well characterised for their association with stent failure and detrimental clinical outcomes in patients with MI4,5.
Developments in stent device technology have been key to improving both safety and efficacy of PCI in either the elective or emergent/urgent setting6. Drug-eluting stents with bioabsorbable polymers (BP-DES) represent one of the latest technological advances in the modern landscape of interventional cardiology devices7. A disappearing polymer over time offers the potential advantage of counteracting the pro-inflammatory and pro-thrombotic effects of permanent polymers which in turn may sustain the non-negligible rate of stent-related adverse events in the long term8. In the expanding arena of BP-DES technology, the SYNERGY™ stent (Boston Scientific, Marlborough, MA, USA) has demonstrated remarkable clinical results, with low rates of stent failure, in both randomised trials and real-life observational studies9,10.
Real-life observational studies, despite being limited by residual confounding when looking at causality, provide important complementary information to the results of randomised clinical trials. To date, the clinical performance of the SYNERGY DES in patients with MI has not been investigated in detail. Using data from a high-quality nationwide registry, we sought to investigate the outcomes after SYNERGY DES implantation in a large and unselected cohort of MI patients undergoing percutaneous revascularisation.
Methods
PATIENT POPULATION
This was an observational, nationwide and multicentre cohort study encompassing all consecutive patients with MI (ST-elevation MI and non-ST-elevation MI) undergoing emergent or urgent PCI with the SYNERGY DES in Sweden from March 2013 (date of the first SYNERGY DES implant in Sweden) to September 2016 so that all patients had complete one-year follow-up. Patients undergoing implantation of newer-generation DES (n-DES group) in the same period were also included. Only stents with at least 1,000 implants were included in this analysis. The standard recommendation for the duration of dual antiplatelet therapy (DAPT) was one year in all patients.
All patients were part of the prospective Swedish Coronary Angiography and Angioplasty Registry (SCAAR), whose details have been previously reported11. Briefly, the SCAAR registry prospectively collects data on baseline clinical, angiographic and procedural characteristics of patients from all 29 cardiac catheterisation centres performing coronary angiography and PCI in Sweden. Data are collected using an internet-based interface at all centres and are then transferred to a central server located at the Uppsala Clinical Research Center. Follow-up data are obtained by merging the SCAAR database with other nationwide registries using the unique personal identification number of each Swedish citizen. Merging is performed by the Epidemiologic Centre of the Swedish National Board of Health and Welfare and approved by the local ethics committee at Uppsala University. This allows an almost complete administrative follow-up of all patients included in the SCAAR.
STUDY DEVICES
SYNERGY is an everolimus-eluting DES with a 4 μm biodegradable poly(lactic-co-glycolic acid) coating located on the abluminal side of the struts. Everolimus (100 μg/cm²) is eluted within three months while polymer bioresorption is completed within four months. Depending on the stent size, the metallic part is composed of a 74 μm (for sizes ≤2.5 mm) to 81 μm (for 4.0 mm stents) platinum-chromium alloy12.
The n-DES group included the Resolute Integrity® and Resolute Onyx™ (Medtronic, Minneapolis, MN, USA), XIENCE Xpedition® (Abbott Vascular, Santa Clara, CA, USA), PROMUS Element™ Plus and Promus PREMIER™ (Boston Scientific), Orsiro (Biotronik AG, Bülach, Switzerland), BioMatrix™ (Biosensors Interventional Technologies Pte Ltd., Singapore), Ultimaster® (Terumo Corp., Tokyo, Japan).
OUTCOMES OF INTEREST AND DEFINITIONS
The co-primary outcomes of interest for this analysis were definite ST and clinically relevant restenosis up to two years. Secondary outcomes of interest were all-cause death and recurrent MI.
In keeping with the Academic Research Consortium13, definite ST is defined as symptoms suggestive of an acute coronary syndrome and angiographic evidence of ST. ST occurring during the index hospitalisation are tracked in the registry and were included in the cumulative analysis of ST during follow-up.
Restenosis is defined as a newly detected stenosis in a previously stented segment as assessed by angiography (>50% diameter stenosis) or in the presence of demonstrated functional ischaemia with fractional flow reserve values below 0.80. Of note, in the SCAAR registry, if a patient undergoes repeat coronary angiography or PCI for any indication, operators are informed by the system about the characteristics of the previous procedure(s) and implanted stent(s), if any. At that time, the system mandates the compilation of any occurrence of restenosis or ST in the previously treated segments.
MI has been defined as any rehospitalisation after the index procedure registered in the Swedish Register of Information and Knowledge about Swedish Heart Intensive Care Admissions (RIKS-HIA) with International Classification of Diseases (ICD) codes I21 and I22. There were no substantial changes to the definition of MI during the course of the study. In addition, all data entered in SCAAR and RIKS-HIA, including ICD codes, are regularly and randomly monitored for quality. Consistency with source clinical files is above 95%.
Finally, data on all-cause mortality were obtained from the National Population Registry. Patients who received a mixture of stents during their index procedure were excluded from the analysis of MI and all-cause death.
STATISTICAL ANALYSIS
Continuous parameters are reported as mean and standard deviation while dichotomous variables are reported as frequencies and percentages. Continuous variables were compared using the Student’s t-test or the Mann-Whitney U test, as appropriate. The chi-square test was used to compare categorical variables. The rate of missing baseline values, if any, is shown in Supplementary Table 1. ST and restenosis were analysed at stent level while all-cause death and MI were analysed at patient level. Time-to-event curves were plotted using the Kaplan-Meier method and the adjusted hazard ratios (HR) for the outcomes of interest were calculated by multivariable Cox proportional hazard regression models. We addressed the issue of potential bias in treatment assignment by using the propensity score (PS) technique. Further details about the PS and Cox proportional hazard regression models are reported in the Supplementary Appendix and Supplementary Table 2.
Sensitivity analyses were conducted with stratification of all models by the treating centre, as well as by adding the treating centre as a frailty term. In addition, in order to exclude the potential influence of different overlapping stent types, a sensitivity (stent-level) analysis was conducted by assessing the cumulative incidence of definite ST and restenosis in patients implanted with only the same type of stent at the index procedure.
Results
BASELINE CLINICAL CHARACTERISTICS
The final study population consisted of 36,292 patients encompassing a total of 61,066 implanted stents. Stents were grouped, as follows: SYNERGY, N=8,876 (14.5%); BioMatrix, N=1,310 (2.1%); Orsiro, N=4,031 (6.6%); PROMUS Element Plus, N=1,500 (2.5%); Promus PREMIER, N=14,520 (23.8%); XIENCE Xpedition, N=5,352 (8.8%); Resolute Onyx, N=11,948 (19.6%); Resolute Integrity, N=12,233 (20.0%); Ultimaster, N=1,296 (2.1%). A total of 2,458 patients were implanted with multiple stent types during the index procedure.
Clinical and procedural characteristics of patients in the SYNERGY and n-DES groups are presented in Table 1 and Table 2, respectively. Non-ST-elevation MI was encountered in 60% of subjects in both groups. As compared to the n-DES group, patients in the SYNERGY group were older and presented more often with hypertension, previous MI and previous revascularisation by PCI. Left main or three-vessel disease involvement as well as restenotic lesions were more frequently seen in the SYNERGY DES group. Stenting with SYNERGY was more frequently performed in the left main, right and circumflex coronary arteries and vein graft lesions. Mean stent length was significantly increased with SYNERGY while mean stent diameter was significantly increased in the n-DES group. Adjunctive thrombectomy and direct stenting were more frequently used in the n-DES group.
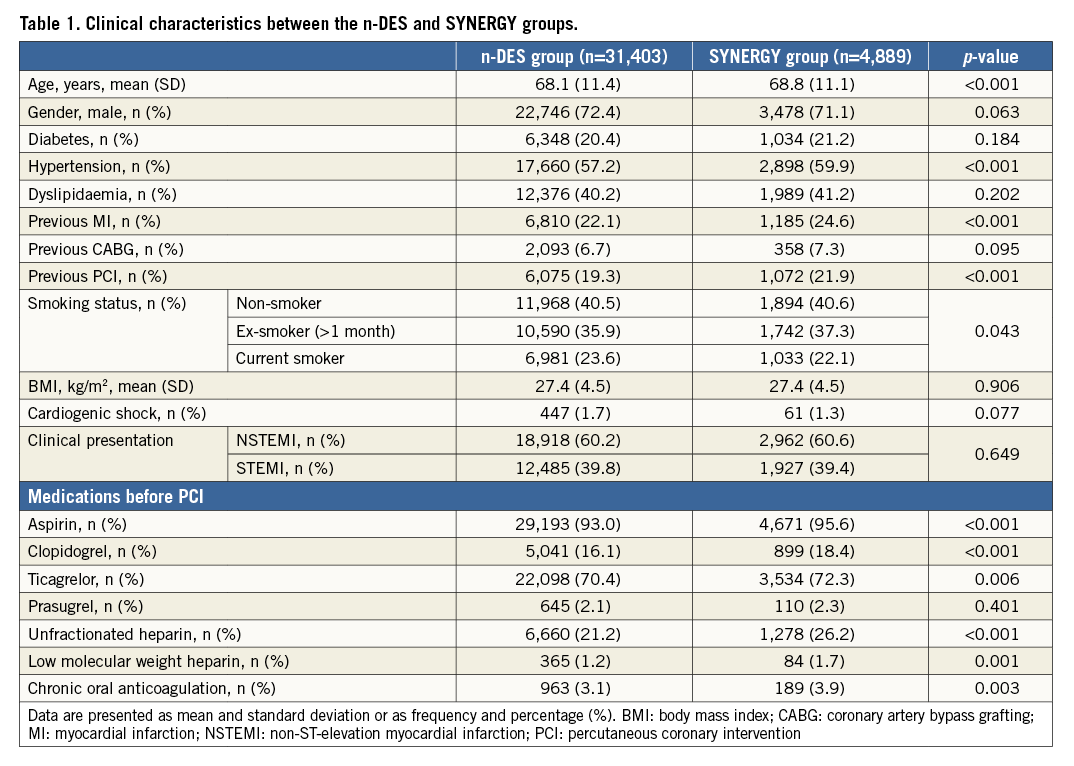
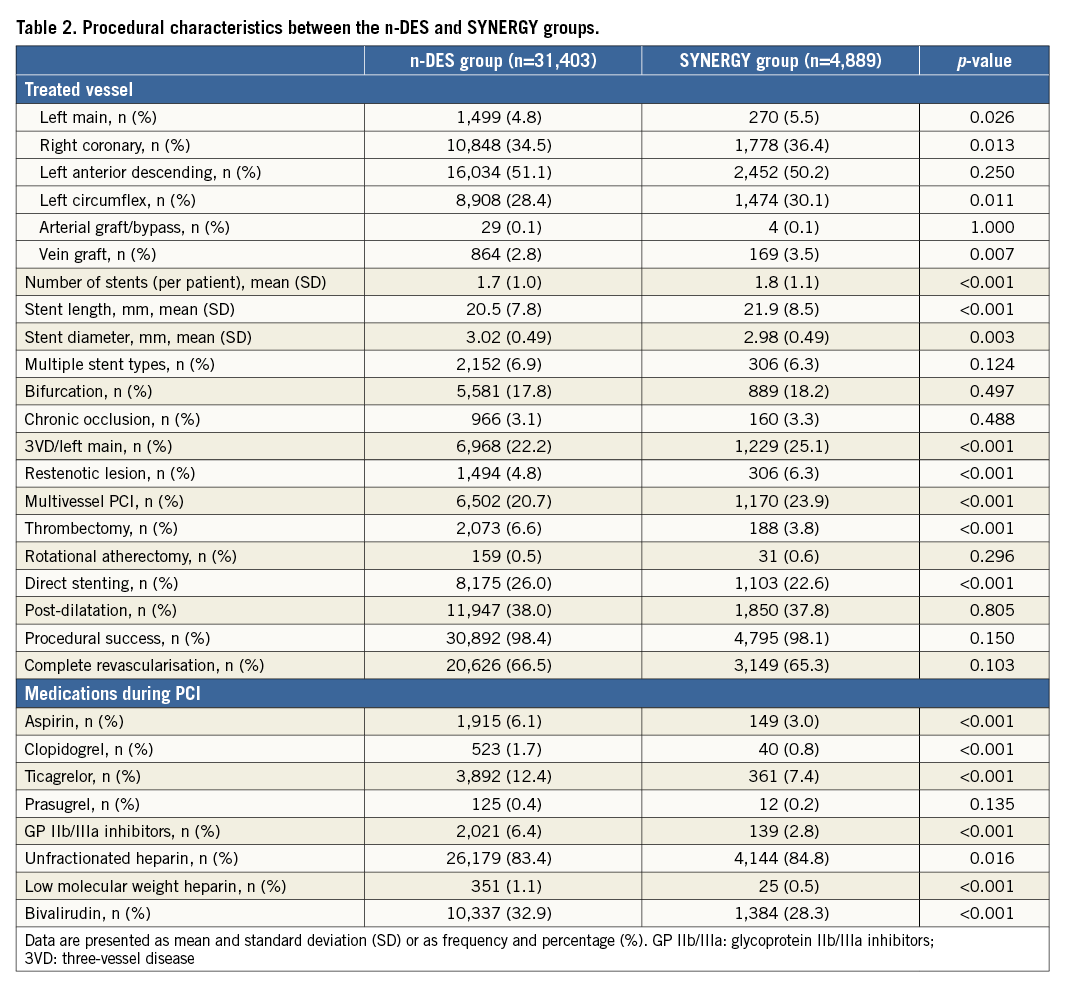
STENT THROMBOSIS AND RESTENOSIS UP TO TWO YEARS
At two years, 440 ST and 702 restenoses were encountered in the overall population. The cumulative incidences of ST and restenosis in the SYNERGY and n-DES groups are presented in Figure 1. The Kaplan-Meier estimates of ST in the SYNERGY and n-DES groups were 0.69% and 0.81%, respectively. Restenosis was encountered in 1.48% and 1.25% of patients in the SYNERGY and n-DES groups, respectively.
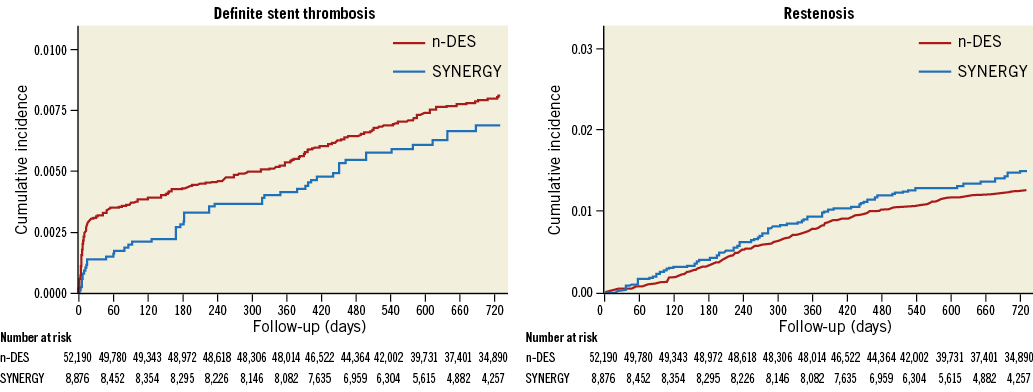
Figure 1. Cumulative rates of stent thrombosis and restenosis up to two years in SYNERGY versus other n-DES.
In adjusted analyses, there were no differences in the risk of ST (adjusted hazard ratio [HR] 1.00, 95% confidence interval [CI]: 0.69-1.46; p=0.99) and restenosis (adjusted HR 1.05, 95% CI: 0.81-1.37; p=0.72) between SYNERGY and n-DES stent groups.
RECURRENT MI AND ALL-CAUSE DEATH UP TO TWO YEARS
The cumulative incidences of recurrent MI and all-cause death in SYNERGY and n-DES groups are presented in Figure 2. After excluding patients implanted with different stent types at the index procedure (n=2,458), a total of 1,948 patients had recurrent MI and 3,002 subjects died at two years. The cumulative incidence of MI at two years was 6.49% and 6.32% in the SYNERGY and n-DES groups, respectively (adjusted HR 0.95, 95% CI: 0.82-1.10; p=0.49).
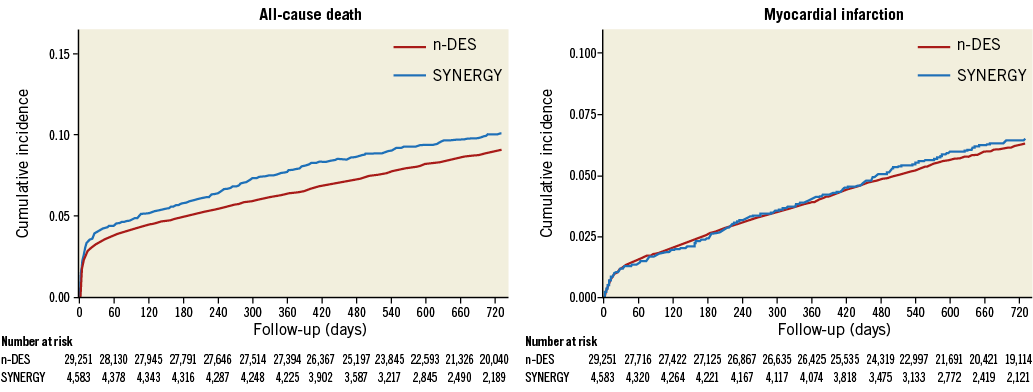
Figure 2. Cumulative rates of all-cause death and recurrent MI up to two years in SYNERGY versus other n-DES.
The Kaplan-Meier estimates for the cumulative incidence of mortality were 10.1% and 9.1% in the SYNERGY and n-DES groups, respectively (adjusted HR 1.12, 95% CI: 0.98-1.28; p=0.10).
SENSITIVITY ANALYSES
Results were consistent in analyses assessing the potential influence of the treating centres on clinical outcomes (Supplementary Appendix, Supplementary Table 3). Cumulative incidence curves after stratification of the overall study population into patients presenting with ST-elevation or non-ST-elevation MI are shown in Figure 3 and Supplementary Figure 1. No significant interaction between different stent groups and clinical presentation (ST-elevation or non-ST-elevation MI) was found with respect to all investigated outcomes (p-values for interaction >0.05). The cumulative incidence of definite ST and restenosis in patients implanted with the same type of stent at the index procedure is presented in Supplementary Figure 2. Results were consistent with the main analysis.
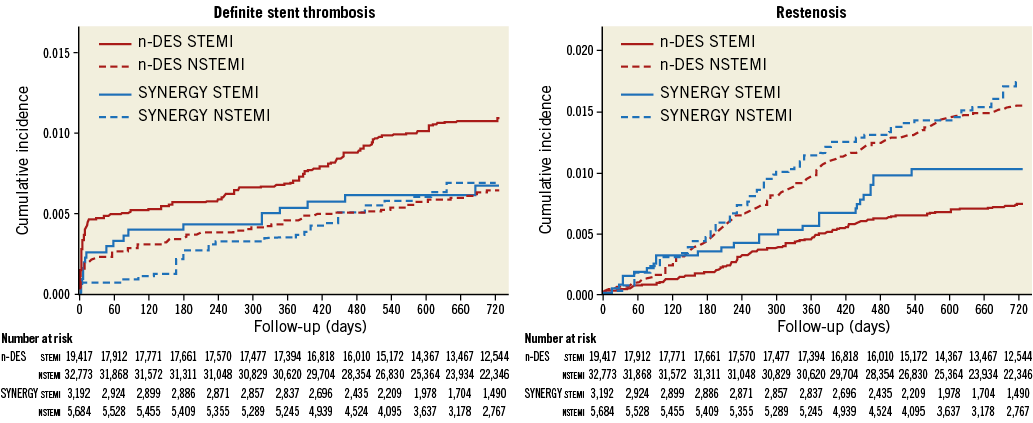
Figure 3. Cumulative rates of definite stent thrombosis and restenosis up to two years in patients presenting with ST-elevation or non-ST-elevation myocardial infarction.
Discussion
The principal findings of this analysis can be summarised as follows: a) patients undergoing urgent/emergent PCI for acute MI in Sweden who were implanted with the SYNERGY DES had a higher burden of clinical and anatomical complexity as reflected by more advanced age, higher prevalence of risk factors, longer stented lesions or multivessel PCI performed at the time of the index procedure; b) after addressing confounding by taking into account such clinical and anatomical differences, performance of the SYNERGY DES did not differ with respect to other n-DES in terms of both stent-related performance and clinical outcomes over an extended follow-up duration of up to two years.
Different factors may have prompted a more selective use of the SYNERGY DES in less favourable clinical and anatomical contexts in this study. One of the potential advantages conveyed by a rapidly reabsorbing polymer is that of favouring the healing process with rapid endothelialisation of the stent struts. Actually, after the polymer bioresorption process has been completed, the equivalent of a bare metal stent is left in place. These characteristics could be particularly useful in frail patients (i.e., elderly subjects) when a shorter duration of DAPT administration may become necessary due to heightened bleeding risk. Thinner strut thickness could also be advantageous in complex anatomies (i.e., restenotic lesions and small vessels) for potential reduction of the burden of stent failure. Lastly, this stent technology is supposed to improve the long-term efficacy of PCI by reducing permanent polymer-related inflammation14.
The rates of ST and restenosis were reassuringly low with both SYNERGY and n-DES devices in the current analysis. Indeed, definite ST was below 1% at two years by Kaplan-Meier estimates in both treatment arms. Low rates of ST in patients with acute MI and complex lesions in this study differ from concerns about ST with SYNERGY recently highlighted in the Manufacturer and User Facility Device Experience (MAUDE) in the USA. Indeed, Khan et al raised concerns about an increased risk of ST, in particular acute ST with SYNERGY as compared to n-DES with permanent polymers15. In our opinion, findings from MAUDE should be cautiously interpreted since intrinsic limitations can be present in the data collection and analysis. The MAUDE database is based on a voluntary reporting of adverse events with medical devices approved for clinical use in the USA. Reporting bias, skewed reporting (more events reported with newer devices) and incompleteness can represent possible shortcomings in the collection process of adverse events. In addition, concerns raised by the authors about the possibility of an increased risk of ST with the SYNERGY stent were based on the simple calculation of the ratio of ST events to the totality of adverse events reported in the database, without taking into account the total number of implanted stents.
Our findings of a low ST rate are corroborated by the results of a comprehensive Bayesian network meta-analysis of 147 trials which showed improved safety with everolimus-eluting BP-DES regarding definite or probable stent thrombosis at one year as compared with all other stent types16. Moreover, recent findings of the BIO-RESORT trial, which investigated the comparative performance of SYNERGY and Orsiro versus a durable polymer zotarolimus-eluting DES, showed an almost identical rate of definite ST (0.3%) across different stent types up to one year. Interestingly, the majority of patients (70%) presented an acute coronary syndrome as their referral diagnosis in the BIO-RESORT trial17. Finally, the final five-year follow-up of the landmark EVOLVE II trial showed no cases of ST in a selected cohort of patients treated with SYNERGY for de novo coronary lesions18.
Notwithstanding, the rate of ST in our population of patients with MI is slightly higher as compared to the previous report of SCAAR in unselected patients undergoing PCI with the SYNERGY stent, which showed a cumulative rate of ST at one year of 0.4%9. However, this was an expected finding since, as mentioned above, different factors may favour ST incidence in patients with MI19,20.
We also observed an increased rate of all-cause death and recurrent MI in our study as compared with previous reports on the real-life performance of the SYNERGY DES9,21. Such differences are a consequence of the higher clinical risk profile of patients included in this study since we restricted our analysis to patients who presented only with acute MI.
Finally, we did not identify differences in the adjusted risk of mortality between different stent groups. However, besides no significant differences in the risk of MI and ST, we found a numerical increase in mortality in the SYNERGY group. In our opinion, this finding further supports the understanding of a high-risk clinical profile of patients treated with the SYNERGY in SCAAR and also reflects the possible presence of residual confounding in adjusted analyses.
Limitations
There are different limitations of our study that should be acknowledged. Rotational atherectomy was used as a proxy for lesion calcification but the penetration of this technique is low in daily clinical practice. The definition of MI in this study did not include periprocedural MI, thus raising possible concerns for underestimation of MI rates. In addition, MI evaluation based on ICD codes has limitations, since more sensitive definitions, based on different laboratory assays (i.e., troponin or CK-MB assays), currently represent the gold standard diagnostic tool for MI assessment both in the early postoperative phases and during follow-up. This aspect is important in our study since the MI definition might have penalised the performance of the SYNERGY DES. Indeed, by using a more sensitive definition of MI which also included periprocedural MI, the BIOFLOW-V study found a significant reduction of target vessel MI rates with an ultra-thin, sirolimus-eluting bioabsorbable polymer stent (Orsiro) as compared to XIENCE22. Finally, no information was available in the registry concerning adherence to prescribed medical therapy, varying duration of DAPT administration, or the implementation of secondary preventive measures.
Conclusions
In a large and unselected cohort of patients with MI undergoing percutaneous revascularisation with the SYNERGY DES, rates of ST and clinically relevant restenosis were low and did not differ as compared to other new-generation DES.
| Impact on daily practice From a large and consecutive cohort of patients undergoing percutaneous coronary intervention for acute myocardial infarction, the current analysis provides evidence on the performance of the SYNERGY DES as compared to other n-DES. Up to two-year follow-up, the angiographic and clinical outcomes did not differ between patients implanted with SYNERGY versus other n-DES. These findings may be useful to support a more informed and evidence-based stent selection process in daily clinical practice. |
Funding
SCAAR is funded solely by the Swedish government and the Swedish Association of Local Authorities and Regions. This study has been financially supported by Boston Scientific with an institutional research grant. The funder did not influence the collection, analysis and interpretation of the data, nor did they participate in drafting and reviewing the manuscript.
Conflict of interest statement
S. James has received institutional research grants and honoraria from Boston Scientific. The other authors have no conflicts of interest to declare.
Supplementary data
Supplementary Appendix. Statistical methods.
Supplementary Figure 1. Cumulative rate of all-cause death and recurrent myocardial infarction up to two years in patients presenting with ST-elevation or non-ST-elevation myocardial infarction.
Supplementary Figure 2. Cumulative rates of definite stent thrombosis and restenosis (stent-level analysis) up to two years in patients implanted with the same type of stent.
Supplementary Table 1. Rates of missing baseline values.
Supplementary Table 2. Variable list for patient- and stent-level propensity score models.
Supplementary Table 3. Sensitivity analyses across treating centres.
To read the full content of this article, please download the PDF.

