
Abstract
Aims: The aim of this study was to evaluate the safety and efficacy profile of new-generation, SYNERGY everolimus-eluting stents (S-EES) as compared to XIENCE everolimus-eluting stents (X-EES) with a durable polymer coating in “complex patients”.
Methods and results: We included 2,001 consecutive patients treated with S-EES (n=400) or X-EES (n=1,601) at two Italian centres between May 2013 and May 2015. We used propensity score matching to obtain two cohorts of patients with similar baseline risk profiles. Patients were stratified according to baseline complexity based on the EVOLVE II trial exclusion criteria. The primary outcome was major adverse cardiac events (MACE), defined as the composite of all-cause death, myocardial infarction (MI), and target lesion revascularisation (TLR), at one year. Among 391 matched pairs of patients treated with S-EES or X-EES, we identified 253 (63%) as complex. At one-year follow-up, among “complex” patients, MACE rates did not differ between the S-EES and X-EES groups (9.9% vs. 9.5%, p=0.830, HR 1.04, CI: 0.72-1.48). Similarly, death, MI, and TLR, stratified for complexity, were comparable between S-EES and X-EES treated patients at one year. Of note, no definite ST was observed in either the S-EES or the X-EES cohort.
Conclusions: New-generation S-EES with a bioresorbable polymer coating appear to be safe and effective irrespective of patient complexity as compared to X-EES.
Abbreviations
CI: confidence interval
DES: drug-eluting stent
DS: diameter stenosis
FDA: Food and Drug Administration
HR: hazard ratio
MACE: major adverse cardiac events
MI: myocardial infarction
MLD: minimum lumen diameter
PCI: percutaneous coronary intervention
PtCr: platinum-chromium
QCA: quantitative coronary angiography
RVD: reference vessel diameter
SD: standard deviation
S-EES: SYNERGY everolimus-eluting stent
ST: stent thrombosis
TLR: target lesion revascularisation
X-EES: XIENCE everolimus-eluting stent
Introduction
Technological advances in drug-eluting stent (DES) technology have shown a significant improvement in device safety and efficacy1. Based upon currently available evidence, DES is considered the standard of care for percutaneous coronary interventions (PCI)2. In spite of these findings, the persistence of polymer coating post drug elution remains of concern. Polymer coating at the site of the implanted stent has been associated with local inflammatory reactions and subsequent risk of thrombosis.
DES with biodegradable polymer coatings have been developed to address this concern. First-generation DES with a biodegradable polymer coating have been shown to improve long-term safety significantly as compared to the sirolimus-eluting CYPHER® stent (Cordis, Johnson & Johnson, Miami Lakes, FL, USA)3, and provide a similar safety and efficacy profile as compared to the XIENCE® everolimus-eluting stent (X-EES; Abbott Vascular, Santa Clara, CA, USA)4. The SYNERGY™ everolimus-eluting stent (S-EES; Boston Scientific Corp., Marlborough, MA, USA) is a new-generation, thin-strut, platinum-chromium device with a biodegradable polymer coating.
In the EVOLVE II trial, S-EES were shown to be safe and effective as compared to durable polymer EES in a selected patient population with rather simple clinical and angiographic characteristics5. Based on these data, the United States Food and Drug Administration (FDA) approved the use of S-EES. As patients with a higher clinical and angiographic complexity, such as those with recent acute myocardial infarction, left main disease, chronic total occlusion, long coronary lesion, multivessel coronary disease, in-stent restenosis, and coronary graft disease, were not included in this trial, the use of S-EES in these patients should be considered “off-label” according to the FDA approval5. Currently, no data are available on S-EES safety and efficacy in real-world “all-comers” patients.
The aim of this study was to evaluate the safety and efficacy profile of S-EES, as compared to X-EES, stratified by clinical and angiographic complexity, in a real-world population.
Methods
STUDY DESIGN AND STUDY POPULATION
Patients treated with S-EES and X-EES at two tertiary centres in Italy (Humanitas Research Hospital, Milan, and Policlinico Umberto I, Rome) between May 2013 and May 2015 were consecutively included in an observational study. No limitations to the use of S-EES and X-EES were applied, and patients were included in the registry if either device was successfully implanted. Patients received a dual antiplatelet loading dose as per internal protocol: 75-100 mg of acetylsalicylic acid and 300-600 mg of clopidogrel or 180 mg of ticagrelor before the procedure. After PCI, patients were treated with dual antiplatelet therapy with acetylsalicylic acid plus clopidogrel, ticagrelor or prasugrel as per physician choice for 12 months or less in case of high risk. Patients’ data were collected by dedicated data coordinators. Clinical follow-up was performed by phone calls six months and one year after PCI by dedicated personnel. The respective institutional review boards approved the study, which involved collection of data at one year after PCI. The study complied with the Declaration of Helsinki.
CLINICAL OUTCOMES AND DEFINITIONS
The pre-specified primary outcome was major adverse cardiac events (MACE) – defined as the composite of all-cause death, myocardial infarction (MI), and target lesion revascularisation (TLR) – at one year. Secondary outcomes were the individual components of the primary outcome as well as definite stent thrombosis (ST), defined according to the Academic Research Consortium criteria6. MI was defined as a detection of a rise, exceeding the ninety-ninth percentile of the upper reference limit, and/or fall of cardiac troponin and clinical evidence of ischaemia. Post-PCI troponin was checked only when clinically required in case of prolonged chest pain, ischaemic ST changes, new pathological Q-waves, angiographic evidence of a flow-limiting complication, or imaging evidence of new regional wall motion abnormality. After PCI, MI was defined as elevations of troponin more than five times the ninety-ninth percentile of the upper reference limit if normal, or a rise of more than 20% if troponin values were elevated, within 48 hours of the procedure and with evidence of cardiac ischaemia. TLR was defined as any repeat revascularisation due to a stenosis within the stent or within a 5 mm border proximal or distal to the stent6.
The definition of clinical and angiographic complexity was based on the exclusion criteria of the EVOLVE II trial5. Patients were considered “complex” if they had at least one of the following characteristics: recent acute MI, left main disease, chronic total occlusion, coronary graft disease, in-stent restenosis, >2 vessels treated, and a lesion length >34 mm.
Chronic kidney disease (CKD) was defined as GFR ≥60 mL/min/1.73 m² for a duration of >3 months. Chronic total occlusion was assessed by angiographic evidence of 100% diameter stenosis and TIMI flow equal to zero for a time period of more than three months. Angiographic success was defined as angiographic evidence of <10% diameter stenosis post PCI as visually assessed by angiography.
QUANTITATIVE CORONARY ANGIOGRAPHY ANALYSIS
Quantitative coronary angiography (QCA) was performed in the first 180 S-EES patients and in the entire cohort of X-EES patients, for baseline and final angiograms. We used the validated QCA software (Integris Allura; Philips Healthcare, Best, The Netherlands). Two trained technicians, supervised by an interventional cardiologist, conducted QCA analysis.
The best frame angiogram was evaluated by automated contour detection, and adjusted by manual correction if necessary. Angiographic analysis was performed before and after PCI in the same projections. The coronary flow was classified according to the Thrombolysis In Myocardial Infarction (TIMI) classification. All measurements were performed on angiograms, and QCA measurements included lesion length, lesion location, calcification, TIMI flow, reference vessel diameter (RVD), pre and post percentage diameter stenosis (% DS), pre and post minimal lumen diameter (MLD), and acute gain. We tried to reduce any potential problems in data acquisition, such as vasomotor tone discrepancies, or catheter size deviations that may detrimentally affect our calibrations, by ensuring that the optimal images were utilised for analysis, and that inter- and intra-observer variability was tested for with a mean difference of less than 0.05 mm7.
STATISTICAL ANALYSIS
We performed a propensity score matching to adjust for confounders in this non-randomised study, and this was used to identify pairs of S-EES and X-EES treated patients, stratified by complexity, with similar baseline characteristics. The propensity score was estimated with the use of a multivariable logistic regression model, with S-EES used as a dependent variable, and sex, age, diabetes mellitus, hypertension, smoking, previous MI, previous coronary artery bypass grafting, previous PCI, ST-elevation myocardial infarction at presentation, left ventricular ejection fraction, number of diseased coronary arteries, at least one critical lesion on the left anterior descending coronary artery, long coronary artery lesion, chronic total occlusion, and in-stent restenosis as covariates. We measured the performance of the logistic models using the area under the receiver operating characteristic (ROC) curve c-statistic, and Hosmer-Lemeshow goodness-of-fit test. Matching was performed with the use of 1:1 nearest neighbour matching, without replacement, within a calliper of 0.28. Standardised differences were estimated for baseline covariates before and after matching to assess the imbalance pre and post matching, with an absolute standardised difference of less than 10.0% indicating a small imbalance9.
In the light of the low number of patients and events, we performed a sensitivity analysis using propensity matching with nearest neighbour matching of 1:2 (S-EES vs. X-EES) with a 0.2 calliper to reduce the sample variability of S-EES effect estimation10.
Categorical data are presented as frequencies and were compared using Fisher’s exact test. Continuous data are presented as mean±standard deviation (SD) and were compared using factorial ANOVA. Crude incidence rates (95% confidence interval [CI]) for MACE, as well as its individual components and ST, were determined using Kaplan-Meier estimation methods; differences between groups were assessed using the log-rank test. The Cox proportional hazards regression model was used to estimate the hazard ratio (HR) and 95% CI according to the type of treatment. Comparison between groups was performed stratifying by pair-matched patients. Statistical significance was accepted at the 95% confidence level (p<0.05) without adjustment for multiple comparisons. Statistical analyses were performed using Stata version 13.1 (StataCorp, College Station, TX, USA).
Results
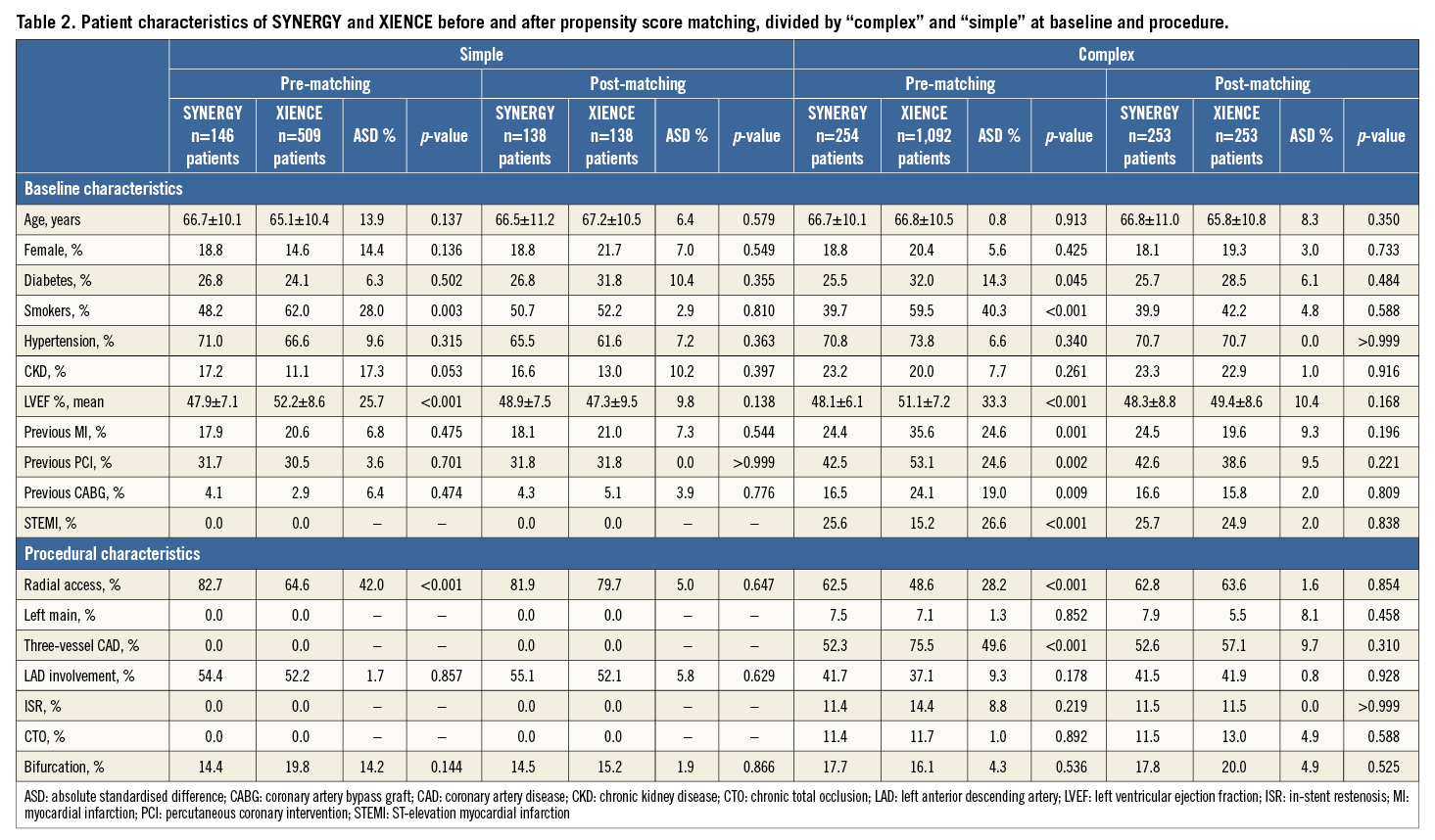
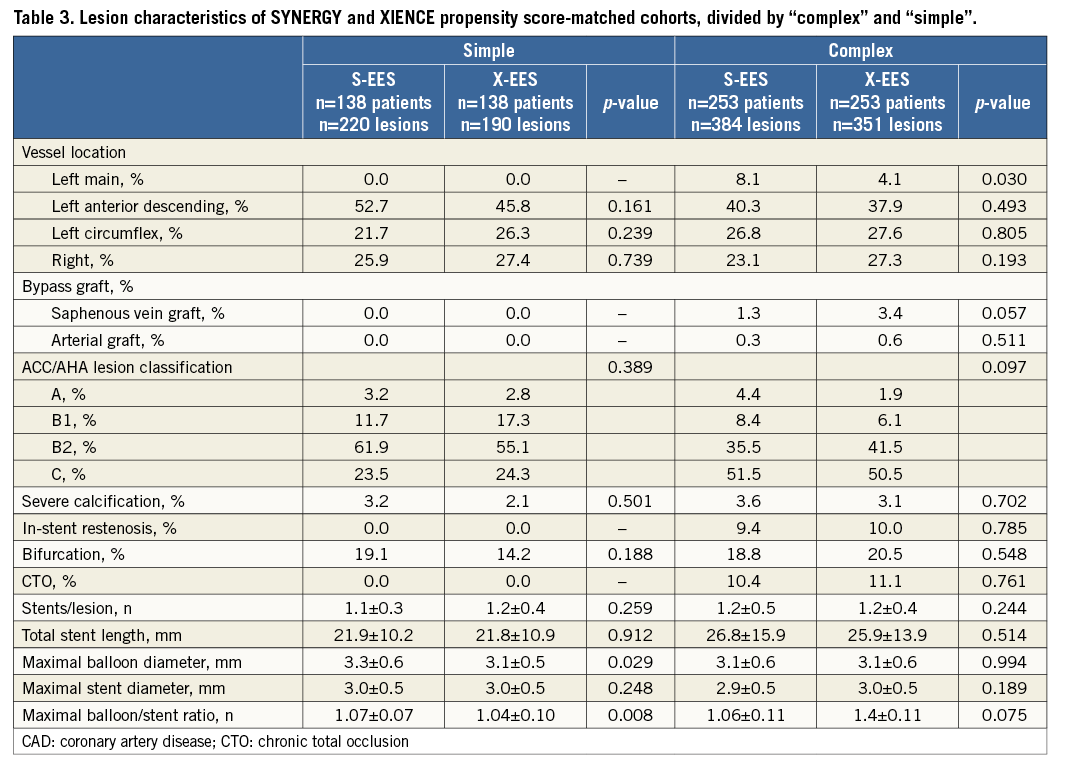
Among 2,001 patients included in this registry, 400 patients with 622 lesions were treated with S-EES, and 1,601 patients with 2,491 lesions were treated with X-EES. Baseline characteristics prior to propensity score matching are summarised in Table 1. After stratification by complexity (Figure 1), propensity score matching allowed the identification of 391 matched pairs of patients treated with S-EES or X-EES – 253 matched pairs were “complex”, and 138 matched pairs were “simple”. The c-statistic, as estimated by ROC, was 0.710 and 0.743 for the simple and complex models, respectively. The model fitted the data well, as examined by the Hosmer-Lemeshow goodness-of-fit test (p=0.368 and p=0.382 for simple and complex analysis, respectively). After matching, the absolute standardised differences were inferior to 10% for all variables (Table 2). Clinical, angiographic, and procedural characteristics were balanced between S-EES and X-EES among both “complex” and “simple” patients, as shown in Table 2-Table 4.
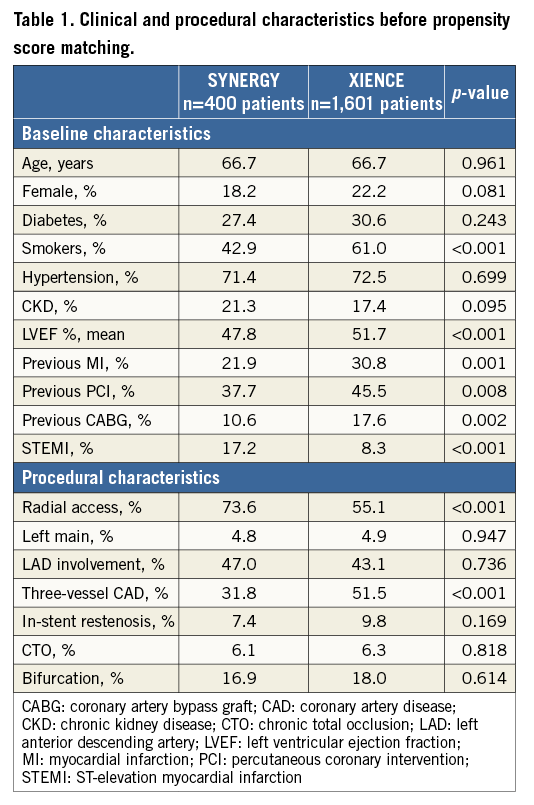
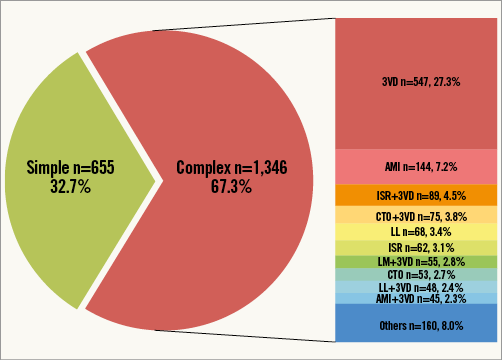
Figure 1. Pie chart reporting the distribution of “simple” and “complex” patients pre propensity score matching in the overall study population. The bar graph reports the rates of complex characteristics sorted by frequency. AMI: acute myocardial infarction; CTO: chronic total occlusion; ISR: in-stent restenosis; LL: long lesion; LM: left main; 3VD: three-vessel coronary artery disease
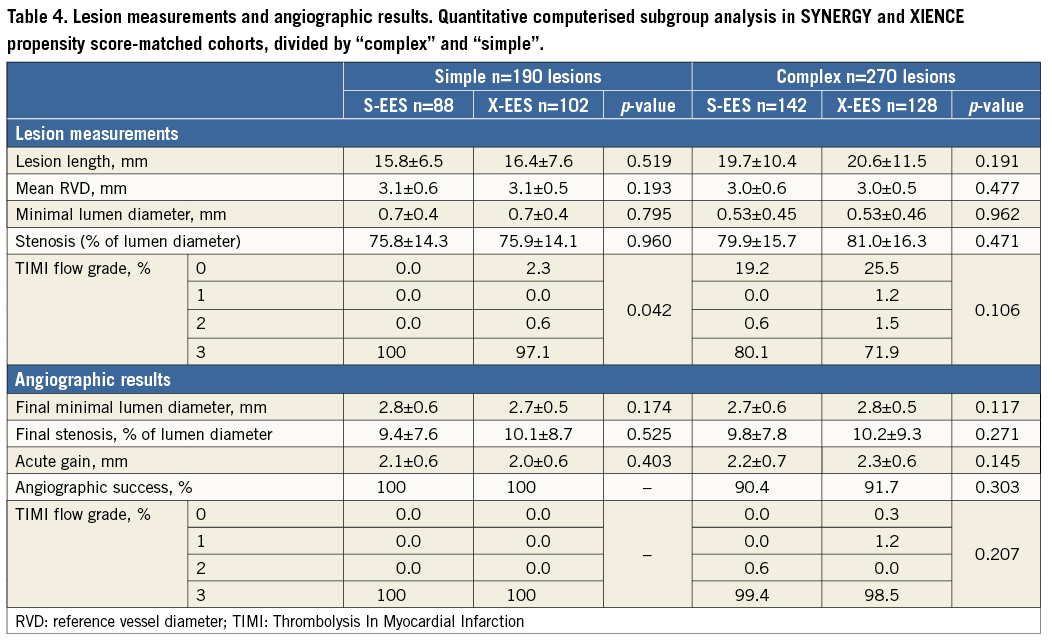
ANGIOGRAPHIC OUTCOMES
Quantitative angiographic outcomes showed comparable results for S-EES and X-EES treated patients, in both “complex” and “simple” subgroups (Table 4).
CLINICAL OUTCOMES
Clinical outcomes up to one year are reported in Table 5. The follow-up rate was 88% and 76% in the overall population at six and 12 months, respectively. The rate of those lost to follow-up, excluding patients with an ineligible timing for follow-up, was 1%, 4%, 1%, and 2% at six months, and 10%, 15%, 4%, and 11% at 12 months in S-EES simple, S-EES complex, X-EES simple, and X-EES complex patients, respectively. MACE occurred with similar rates in “complex” patients (9.9% vs. 9.5%, p=0.830, HR 1.04, CI: 0.72-1.48), as well as “simple” patients (4.5% vs. 3.0%, p=0.791, HR 0.91, CI: 0.45-3.229) treated with S-EES and X-EES (Figure 2). Similarly, the risk of death (complex: 1.8% vs. 0.9%, p=0.201, HR 0.51, CI: 0.17-1.54; simple: 1.5% vs. 0.0%, p=0.158), and TLR (complex: 4.4% vs. 4.5%, p=0.987, HR 0.99, CI: 0.57-1.72; simple: 0.0% vs. 0.0%) did not differ between S-EES and X-EES treated patients. No MI occurred in “simple” patients, while in the “complex” population the MI rate was higher among patients treated with X-EES (S-EES 0.4% vs. 6.1%, p=0.004, HR 3.30, CI: 1.18-9.23). Specifically, there was neither any post-procedural MI, nor any stent-related MI during follow-up. Of note, no definite ST was observed in either S-EES or X-EES treated patients at one-year follow-up.
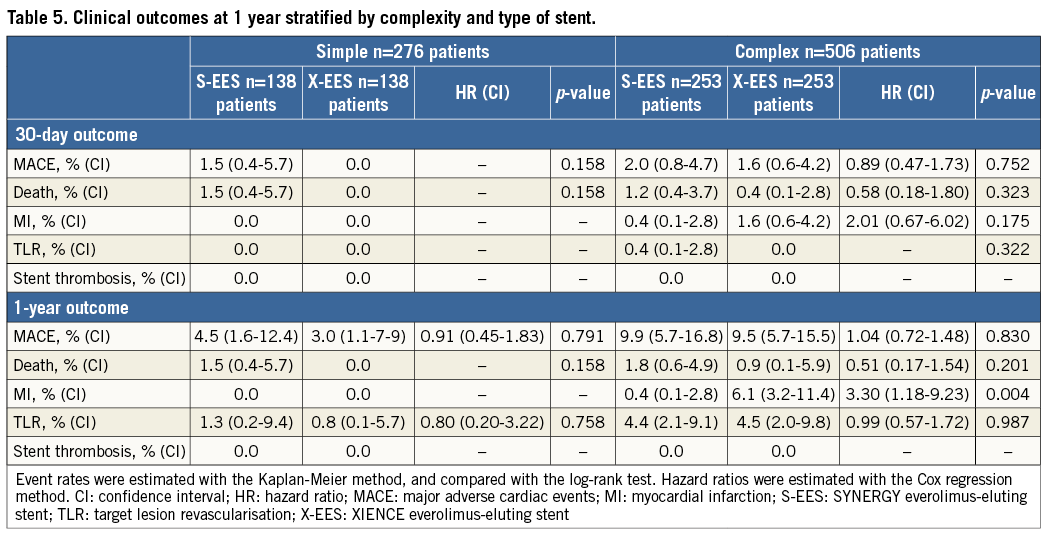
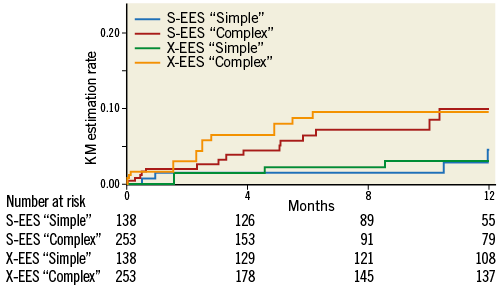
Figure 2. Kaplan-Meier estimation rate of major adverse cardiac events as a composite endpoint of all-cause mortality, myocardial infarction, and clinically driven target vessel revascularisation, at one-year follow-up, stratified by complexity and stent type. KM estimation rates, log-rank p-values, and hazard ratios are reported in Table 5. S-EES: SYNERGY everolimus-eluting stent; X-EES: XIENCE everolimus-eluting stent
A similar risk of events was estimated in the 1:2 sensitivity propensity score matching for MACE, death, and TLR, while the risk of MI was similar between S-EES and X-EES at one month and one year.
Discussion
To the best of our knowledge, this is the first real-world, observational registry evaluating the safety and efficacy profile of the newer-generation abluminal bioabsorbable polymer S-EES as compared to the current gold standard X-EES, according to patient and lesion complexity. Our main findings may be summarised as follows:
1) Among “complex” patients, the risk of MACE was similar for S-EES and X-EES up to one-year follow-up.
2) S-EES and X-EES yield similar procedural and angiographic results in “complex” and “simple” patients.
3) No ST was reported in either S-EES or X-EES in an all-comers “complex” and “simple” population at one year.
Our clinical observations appear to validate the angiographic and clinical findings of non-inferiority from the EVOLVE I and EVOLVE II trials, which compared S-EES to the PROMUS Element™ everolimus-eluting stent (Boston Scientific Corp.)5,11. The EVOLVE II trial showed that the safety and efficacy of S-EES is at least comparable to a second-generation durable polymer-coated EES. However, EVOLVE II did not include “complex” clinical and angiographic characteristics, as is common practice in a trial for regulatory approval for novel devices. In order to validate the results of the EVOLVE II trial in “complex” patients, we evaluated the safety and efficacy outcomes of S-EES in a real-world population, stratified by patient complexity, defined according to the exclusion criteria of the EVOLVE II trial. Two thirds of the patients included in our study were “complex”, and were characterised by a fourfold increase in the risk of MACE as compared to “simple” patients at one-year follow-up. Such a difference is not unexpected and may be explained by a higher rate of negative prognostic characteristics such as clinical presentation of acute MI, low left ventricular ejection fraction, multivessel CAD, and “complex” angiographic features12. Notably, event rates were low considering the complexity of the patients, and were similar to previous studies on new-generation DES implanted in “complex” patients13. The favourable safety and efficacy outcomes observed with newer-generation DES in “complex” patients in our real-world investigation may have significant implications for the comparison between surgical and percutaneous revascularisation strategies – a hypothesis that is currently being investigated by ongoing trials (EXCEL trial - ClinicalTrials.gov Identifier: NCT01205776, and SYNTAX II trial - A Trial to Evaluate a New Strategy in the Functional Assessment of 3-vessel Disease Using the SYNTAX II Score in Patients Treated With PCI- ClinicalTrials.gov Identifier: NCT02015832).
Our findings indicate a similar safety and efficacy profile for S-EES and X-EES, regardless of patient complexity. The risk of MACE as well as its individual components and ST was comparable in matched patients treated with S-EES and X-EES, with the exception of MI rates in the complex group, which may be explained as an incidental difference of rare events, as confirmed by sensitivity analysis with 1:2 propensity score matching. Moreover, the similar efficacy of S-EES and X-EES stents in “complex” and “simple” patients is supported by angiographic findings of post-procedural diameter stenosis percentage and acute gain. These results indicate that the procedural, angiographic, and clinical outcomes of the EVOLVE and EVOLVE II trials hold true in more complex clinical and anatomical settings11.
It is noteworthy that, in this real-world population, no ST event was observed in either S-EES or X-EES treated patients during one year of follow-up. Due to low precision in decimal digit estimation of rare event risk, our results might be consistent with randomised trials and contemporary registries on new-generation DES, in which ST rates at one year range from 0.08% to 0.9%14,15.
The favourable antithrombotic properties of fluoropolymer-coated X-EES during short- and long-term follow-up have been suggested by numerous reports16. Conversely, limited data are available on the thrombogenicity of S-EES. Bioresorbable polymer-based DES have been associated with a reduced risk of very late ST as compared with durable polymer-based early-generation DES. On the other hand, direct comparisons of bioresorbable polymer-based biolimus-eluting stents and X-EES have shown no difference in terms of ST during long-term follow-up4.
Multiple factors appear to be implicated in DES acute thrombogenicity and long-term vascular healing. These factors include platform material and stent strut thickness as well as polymer biocompatibility, composition, distribution, and, in the case of bioresorbable polymers, duration of bioresorption2,17. The S-EES was designed to expedite stent vascular healing by the use of a thin-strut (74 μm) platinum-chromium (PtCr) platform with an ultra-thin (4 μm) abluminal poly(D,L-lactide-co-glycolide) polymer, which is reabsorbed within four months18. Taking these observations into consideration, we are of the opinion that the newer-generation DES included in the present study may further improve upon the efficacy profile of the early-generation DES, without compromising safety, which is of the utmost importance among “complex” patients. The absence of ST events in “complex” as well as “simple” patients is an extremely promising finding for S-EES. This will need to be confirmed in larger-scale studies with long-term follow-up.
Limitations
Our study needs to be interpreted in the context in which it was conducted. First, this was an observational study and is, therefore, subject to the intrinsic limitations of this type of analysis. However, “complex” patients are often excluded from randomised controlled trials. For such reasons, observational studies can be used as complementary forms of research to verify that findings from clinical trials can be translated into benefits in the real-world population19. Of note, we attempted to minimise bias using propensity score matching for a wide range of variables. The propensity score was defined as the probability of patients receiving an S-EES depending on pre-treatment covariates that summarise the patient baseline risk profile. Second, the size of our study does not allow definitive conclusions with respect to the analysed endpoints. In addition, single events, as well as MI, might have been underestimated due to the absence of routine assessment of post-procedural troponin. However, the consistency of the observed findings across all analysed endpoints, and in the sensitivity analysis, supports the similar safety and efficacy profile of S-EES and X-EES. Third, our study is limited to one-year follow-up, while theoretical differential clinical outcome between the compared technologies might be observed during long-term follow-up; moreover, the follow-up rate at 12 months was 76%. Larger-scale studies are needed to confirm our findings. However, at this point in time, our study represents the first available evaluation of the S-EES in “complex” patients.
Conclusions
In conclusion, this multicentre, real-world, propensity score-matched analysis indicated that the new-generation S-EES with a bioresorbable polymer coating appears to be safe and effective as compared to the X-EES, irrespective of patient complexity.
| Impact on daily practice In the EVOLVE trials, S-EES were shown to be safe and effective as compared to durable polymer EES in a selected and “simple” patient population. Based on these data, the FDA recently approved the use of S-EES. Our clinical observations appear to validate the angiographic and clinical findings of non-inferiority from the EVOLVE trials. In fact, S-EES seem to be safe and effective as compared to X-EES, irrespective of patient complexity, in our real-world population. In the light of our findings of favourable outcomes in a “complex” patient cohort, we suggest that S-EES might be a favourable candidate to be compared with surgical revascularisation in patients with complex clinical and anatomical features. |
Acknowledgements
We would like to acknowledge Daniela Cattani, Melania Scatturin, Monica Genta and Natascia Perego (Cardiovascular Department, Humanitas Research Hospital, Rozzano-Milano, Italy) for their assistance in data collection and management.
Conflict of interest statement
M. Mennuni and P. Presbitero have received consultancy fees from Boston Scientific. G. Stefanini has received a research grant to the institution from Boston Scientific and speaker/consultancy fees from B. Braun, Boston Scientific, and Edwards Lifesciences. The other authors have no conflicts of interest to declare.

