Abstract
Aims: We aim to investigate the association between different types of statins, in particular simvastatin and atorvastatin, and long-term mortality after percutaneous coronary intervention (PCI).
Methods and results: Between 2000 and 2005, a prospective cohort was constituted of 5,647 patients who underwent PCI. Type and doses of statin use were collected after the PCI procedure. Survival status was obtained from municipal civil registries. The primary endpoint was all-cause mortality. Secondary endpoints were cardiac and cancer mortality. Median follow-up was 5.0 years (range three to nine years). During follow-up 738 patients (13.1%) died. In total, 4,970 patients (88%) were on statin therapy four weeks after PCI of whom the majority used either atorvastatin (34%) or simvastatin (29%). Cumulative survival rates at eight years in the atorvastatin group were 83%, and 79% in the simvastatin group (log-rank, p=0.004). After adjustment, statin use was associated with a 50% mortality reduction (HR 0.49, 95%CI 0.40-0.59) and atorvastatin use was associated with lower total mortality than simvastatin use (adjusted HR 0.77, 95% CI 0.61-0.97). This was largely driven by cancer mortality (adjusted HR 0.59, 95%CI 0.38-0.91).
Conclusions: In patients undergoing PCI the use of statins is associated with reduced mortality during prolonged follow-up. Patients using atorvastatin had a 23% lower mortality than those using simvastatin.
Introduction
Statins have been shown to reduce the risk of death and major cardiovascular events after acute myocardial infarction1,2 and percutaneous coronary intervention (PCI)3. Prescription of statins became routine clinical practice in patients with established coronary artery disease (CAD), since the placebo-controlled Heart Protection Study (HPS) demonstrated a mortality reduction4. Consequently, the various relevant clinical treatment guidelines all share general consensus in their propagation for statin use aimed at lowering LDL cholesterol to levels less than 100 mg/dL (2.5 mmol/L) or, in certain cases, even less than 70 mg/dL (1.8 mmol/L)5-10. None of these guidelines propagate a particular type of statin. Various studies with different statins have suggested that statins with higher potency may result in greater cardiovascular risk reductions. The PROVE-IT11 and A to Z12 trials enrolled patients with acute coronary syndromes (ACS), whereas the TNT13, just like the IDEAL14 trials, enrolled patients with stable CAD. The results of these trials appeared to conflict as the PROVE-IT and TNT trials did find significant differences, while the A to Z and IDEAL trials did not. Furthermore, it has been suggested that, besides the beneficial effects of statins on cardiac events, the use of statins may be associated with non-cardiac death or cancer death.15
Observational data, which reflect the outcome of statin use in real-world clinical practice rather than in a controlled trial setting with highly selected patients, are still rare. Therefore, we investigated the association between different types of statins, in particular simvastatin and atorvastatin, and long-term mortality in the daily clinical practice after PCI. The primary endpoint was all-cause mortality. Secondary endpoints were cardiac death and cancer death.
Methods
Study design and patient population
Between 1 January 2000 and 31 December 2005, 7,217 percutaneous coronary interventions were performed in our institution using either bare metal stents (BMS) or drug-eluting stents (DES). As we investigated the association between statin use and mortality during long-term follow-up, all patients who died within 30 days after the index procedure (n=88) were excluded. According to the standard policy in our laboratory, from January 2000 until 16 April 2002, 2,005 patients received bare metal stents (BMS). From 16 April 2002, until 23 February 2003, 969 patients received sirolimus-eluting stents (SES) (Cypher; Cordis Corp., Johnson & Johnson; Warren, NJ, USA), as part of the RESEARCH registry16. From 23 February 2003 to December 2005, interventions were performed in 2,673 patients with the exclusive use of paclitaxel-eluting stents (PES) (TAXUS Express2 or Liberté; Boston Scientific, Natick, MA, USA), as part of the T-SEARCH registry17. Although a total of 784 patients underwent multiple procedures only patients initially entered in one of the sequential cohorts (BMS, SES or PES group) were maintained for analytical purposes throughout the follow-up in the original cohort. A total of 5,647 patients fulfilled these criteria. This study was performed in accordance with the Declaration of Helsinki.
Clinical characteristics
Data on patient and procedural characteristics were prospectively collected by the interventional cardiologists, technical staff and checked by research fellows. Available clinical variables were age, sex, indication for PCI (stable angina, unstable angina, acute myocardial infarction [AMI]), prior myocardial infarction (MI), prior PCI, prior coronary artery bypass graft (CABG) surgery, diabetes (defined as requirement for insulin or oral hypoglycaemic agents), hypertension (defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg or use of anti-hypertensive medication), current smoking, family history of coronary disease, multivessel disease and the use of statins, beta blockers, angiotensin-converting enzyme inhibitors, calcium antagonists, nitrates, diuretics, digitalis and anticoagulants.
Statins
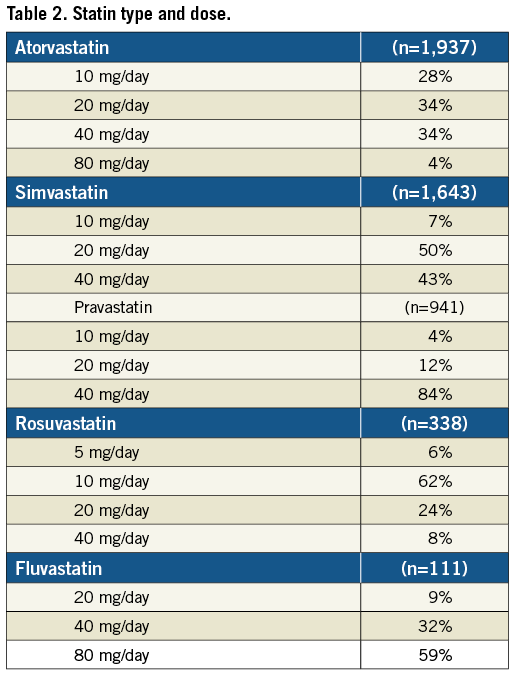
In general, statins were prescribed at the discretion of the treating physician. Data on pre-PCI statin use was collected during the procedure. Post-PCI, type and dose of statin were collected by review of hospital medical records and by questionnaires posted four weeks after the procedure and thereafter yearly to check patients’ compliance with statin use. For all instances of statin use, types of statin were recorded and daily dosages were retrieved by dose category and frequency of intake. Non-statin users were defined as those patients who did not use any statins one-month post-PCI. To compare statin therapy dosages, low dose statin use was defined as <25% and intensified dose as ≥25% of the maximum recommended therapeutic dose. According to the Dutch pharmacotherapeutic recommendations, a maximum recommended therapeutic dose of 40 mg for rosuvastatin and pravastatin and 80 mg for atorvastatin, simvastatin and fluvastatin was used.
Clinical follow-up and endpoints
Survival status was obtained from the municipal civil registries at one-month after PCI and yearly thereafter. Follow-up was performed by yearly, posted questionnaires and telephone interviews. Patients lost to follow-up were considered at risk until the date of last contact, at which point they were censored. Yearly, cardiac events were verified by contacting the patient’s referring hospital and cardiologist, and by reviewing the hospital medical records. The primary endpoint was all-cause mortality. Secondary endpoints considered were cardiac death and cancer death. Information on causes of death was collected from Statistics Netherlands. Cardiac death was classified according to the WHO’s International Classification of Diseases (ICD) and Related Health Problems, 10th revision (ICD-10, I00-I52). Cancer death and type of cancer were classified in the same manner (ICD-10, C00-C97). Sudden unexpected death occurring without any other explanation was included as cardiac death. In the case of lung, gastrointestinal, prostate, colorectal, kidney, bladder and liver cancers, the specific sites were recorded.
Statistical analysis
Continuous variables were compared using the Student unpaired t test, and are presented as mean±standard deviation. Categorical variables were compared using the Fisher’s exact test and are presented in percentages. The incidence of endpoints during long-term follow-up was studied according to the Kaplan-Meier method. Among patient subgroups (i.e., statin vs. non-statin use and atorvastatin vs. simvastatin use), the Mantel and Haenszel log-rank test was used to compare survival curves. Cox proportional hazard regression analysis was used to investigate the independent effect of statin therapy on outcome. Analyses were conducted for crude values and adjusted for age, sex, indication, prior MI, prior PCI, prior CABG, diabetes, hypertension, current smoking, family history of coronary disease, multivessel disease and the use of beta blockers, angiotensin-converting enzyme inhibitors, calcium antagonists, nitrates, diuretics, digitalis and anticoagulants. Statin therapy was entered in the multivariable model in several ways: 1) use of statin versus no use; and 2) use of simvastatin or atorvastatin. All analyses were also adjusted for statin dose. Proportionality of hazards was tested graphically based on visual inspection of log–log survival curves, and by performing a formal test of proportionality based on Schoenfeld residuals for each variable in the model. Cox regression analyses showed no statistically significant interactions with time. We calculated a propensity score for being prescribed a statin. Propensity scores were estimated for all patients and included in our constructed models. Sensitivity analyses were performed. We repeated the analyses described above among subgroups. All statistical tests were two tailed and a p-value of <0.05 was considered significant.
Results
Median follow-up was 5.2 years (range three to nine years). Follow-up data were complete in 98.3%. Mean age was 62 years, with 73% being men (Table 1). Revascularisation for acute MI was performed in 26%. Fifty percent of the patients had multivessel disease and in 65% of the patients a drug-eluting stent was implanted. Aspirin was used by 95% of the patients; four out of five patients were using beta blocker therapy. ACE inhibitors were used by one-third of the patients. Non-statin users were five years younger than statin users (67 years vs. 62 years, p<0.05), less indicated for primary PCI (24% vs. 28%, p<0.05) and also were on less aspirin, beta blockers and ACE inhibitors.
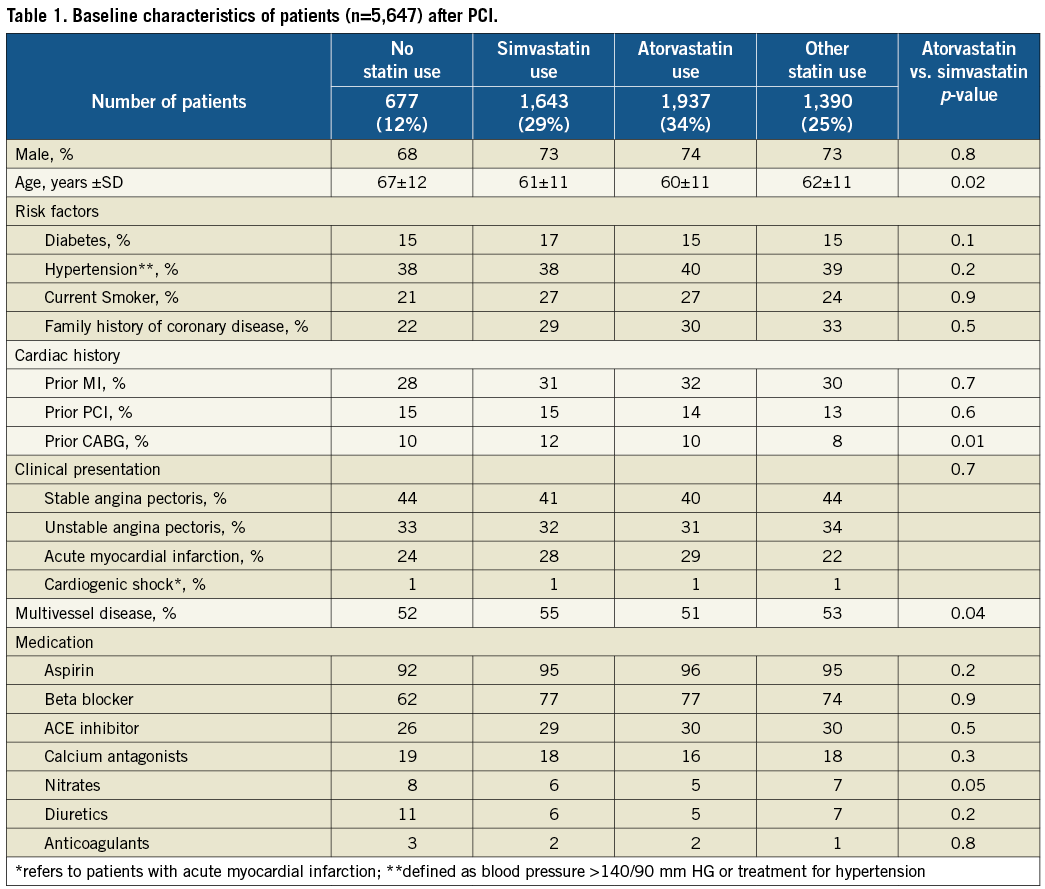
Of the 5,647 patients, 88% used statin therapy one month after the PCI procedure. The majority of patients used either atorvastatin (34%) or simvastatin (29%). Other statin types were pravastatin (17%), rosuvastatin (7%) and fluvastatin (2%). The simvastatin dose ranged from 10-40 mg/day, atorvastatin 10-40 mg/day, pravastatin 20-40 mg/day, rosuvastatin 5-20 mg/day and fluvastatin 40-80 mg/day. The median dosages of prescription were 20 and 40 mg/day for simvastatin and atorvastatin, respectively; 40 mg/day for pravastatin; 10 mg/day for rosuvastatin; and 80 mg/day for fluvastatin (Table 2). During longer follow-up 25% of the non-statin users started to use statins. In contrast, on average only 6% of the patients with baseline statin therapy stopped taking statins during follow-up. Patients’ compliance with statin use over the years was good. A yearly check showed that statin use ranged from 85% to 90%. Most baseline characteristics were similar among the baseline simvastatin users and baseline atorvastatin users (at four weeks) indicating that no distinct indication for the prescription of either type of statin existed in routine clinical practice.
In total, 738 patients (13.1%) died during follow-up of whom 409 (55.4%) died from cardiac cause and 206 (27.9%) from cancer. The specific causes of death are listed in Table 3. At eight-years of follow-up, cumulative mortality rates of statin users versus non-statin users were 17% versus 40%, respectively (log-rank, p<0.0001) (Figure 1). Cumulative mortality rates at five- and eight-years in the atorvastatin group were 8% and 14%, respectively, and 12% and 20%, respectively, in the simvastatin group (log-rank, p=<0.001) (Figure 2). The five- and eight-year Kaplan-Meier estimates of cancer death were 2% and 3% in patients treated with atorvastatin and 3% and 6%, respectively, in the simvastatin group (p=0.001). At five- and eight-year follow-up cumulative survival rates of low dose statin use versus intensified dose statin use were similar.
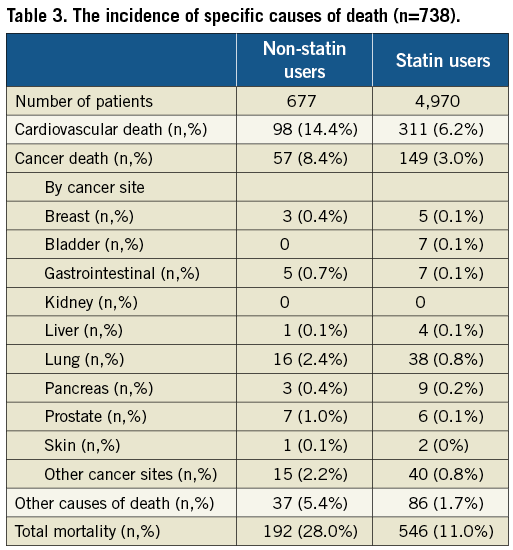
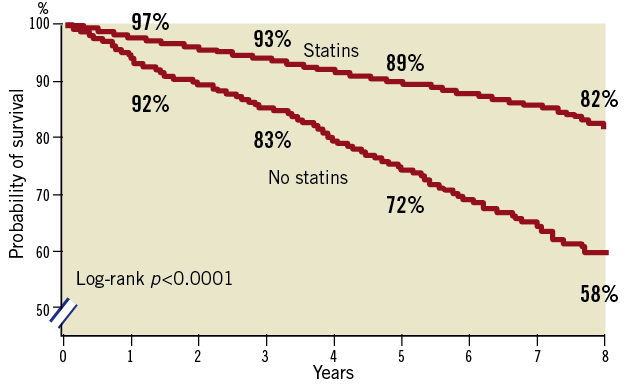
Figure 1. Unadjusted Kaplan-Meier curves for long-term survival according to statin users and non-statin users.
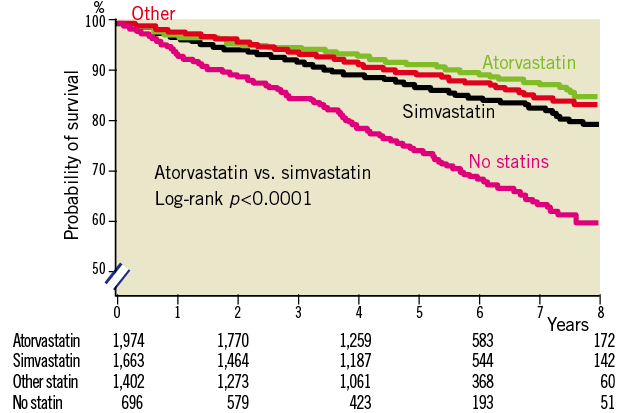
Figure 2. Unadjusted Kaplan-Meier curves for long-term survival according to types of statins.
After adjustment for all baseline clinical and demographic characteristics the risk of all-cause mortality was significantly lower in the baseline statin users compared with non-baseline users (adjusted hazard ratio [HR] 0.49, 95% Confidence interval [95%CI] 0.40-0.59) (Table 4). This was both driven by a reduction in cardiac mortality (adjusted HR 0.51, 95%CI 0.39-0.67) as well as a reduction in cancer mortality (adjusted HR 0.48, 95%CI 0.34-0.67).
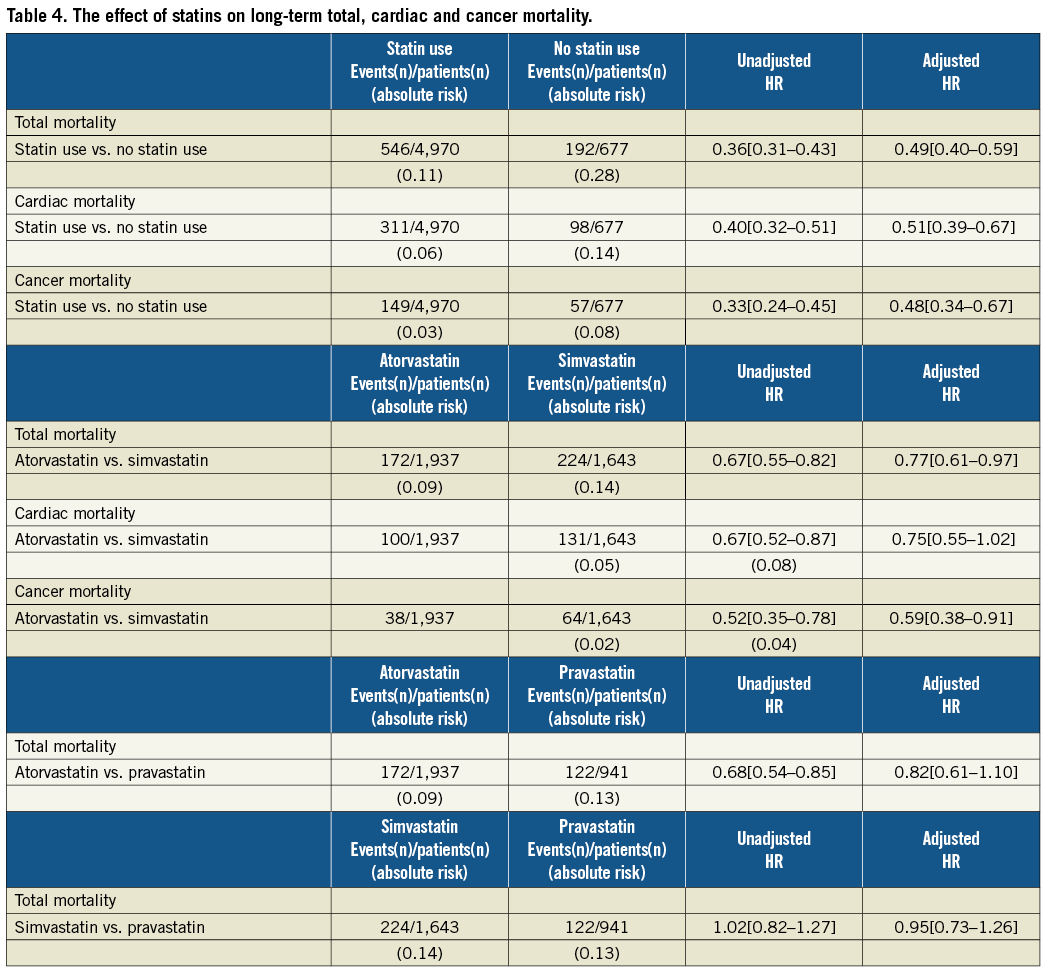
Baseline atorvastatin use was associated with lower total mortality than baseline simvastatin use (adjusted HR 0.77, 95%CI 0.61-0.97). This was largely driven by a difference in cancer mortality (adjusted HR 0.59, 95%CI 0.38-0.91) and a reduction in cardiac mortality (adjusted HR 0.75, 95%CI 0.55-1.02). By placing a propensity score in the model the results did not change. Furthermore, results from sensitivity analyses did not defer markedly from the results of our multivariable analyses, which included all available potential confounders. Mortality rates were similar when comparing pravastatin use versus simvastatin use, and pravastatin use versus atorvastatin use.
Discussion
This prospective observational study in PCI patients observed that in daily clinical practice a 51% mortality reduction by statin therapy occured. Simvastatin and atorvastatin are the two most frequently prescribed statins in daily clinical practice throughout the Netherlands. This study observed for the first time that patients taking atorvastatin have a 23% significantly lower mortality compared to patients taking simvastatin therapy. This beneficial effect was largely driven by a reduction in cancer death and cardiac death. Findings from observational studies like this one, which reflect the use of medicines in real-world clinical practice rather than in a controlled trial setting, may help treating physicians to improve clinical outcome.
Our study demonstrates a much larger reduction in mortality than the 12% proportional reduction in five-year all-cause mortality from the Cholesterol Treatment Trialists’ (CTT) Collaborators meta-analysis15, but is in accordance with a large retrospective cohort study from Israel18. This difference might be caused by selection bias of randomised controlled trials (RCT) or by hidden confounders. Earlier findings in our registry suggest that only a small subpopulation would have been eligible for inclusion in a RCT19.
Several studies such as the CURVES study showed a greater efficacy of atorvastatin therapy in lowering total cholesterol compared to simvastatin therapy after eight weeks of statin therapy in patients with hypercholesterolaemia20. An open-label randomised controlled clinical trial (the IDEAL study) compared a high dose of atorvastatin (80 mg/d) with a usual dose of simvastatin (20 mg/d) in a stable outpatient clinic population of patients with a history of acute MI11. This study found a 11% non-significant reduction of major cardiac events, defined as cardiac death, non-fatal MI or cardiac arrest (p=0.07). The PROVE-IT-TIMI 22 trial12, comparing atorvastatin 80 mg/day with pravastatin40 mg/day, in which the primary endpoint comprised any cardiac event including revascularisation, demonstrated a significant 16% reduction (p<0.01). Nevertheless, in contrast to our observational study, none of these landmark studies demonstrated differences in all-cause mortality with the remark that none of these trials were specifically designed to find any difference in all-cause mortality.
The effect of statin therapy on cancer is studied frequently, as preclinical studies have shown that statins influence critical cellular functions that are involved in tumour initiation, growth and metastasis21. Associations between statin use and cancer have been reported earlier15,22-24. Some have indeed noticed a decreased risk of cancer22,23, however two large meta-analyses did not find any relation between statin use and cancer15,24. In our study however, the use of statins in post-PCI patients was remarkably associated with a 52% reduction of cancer death. Our results are partly in line with the results of earlier studies22,23. Although Karp et al suggest that only the use of lipophilic statins at a sufficiently high dose might be associated with a clinically important 25% reduction in the incidence of cancer in a population of 30,000 post-acute myocardial infarction patients22, our study did not find any difference on the basis of the solubility profile of statins.
Limitations
As with all observational studies, this study is subject to certain limitations. Patients were not randomly assigned to statin therapy. The reasons why 12 % of the patients did not receive a statin after the PCI procedure were unclear. Furthermore, patients were not randomised to atorvastatin or simvastatin, but nevertheless the baseline characteristics were similar in the two groups indicating that there were no particular reasons for choosing either treatment. The two treatment arms were adjusted for all known factors. By sending yearly questionnaires, we were able to estimate the patient’s adherence to statin therapy accurately. Being a tertiary centre, the majority of the patients are directly referred to a peripheral hospital after the PCI procedure. Moreover, as this study was originally not designed to evaluate statin therapy efficacy, hidden confounding could have been introduced. Referral cholesterol levels are not routinely measured anymore and LDL cholesterol values prior and after the PCI treatment were only available for approximately five percent of the patients. Therefore, no adjustments for LDL cholesterol levels in the analyses were done. We did not have any information on side effects like rhabdomyolysis or liver enzyme elevation, but may conclude that these did not occur more frequently than reported elsewhere. Rhabdomyolysis occurs rarely with the most commonly prescribed statins at currently accepted doses. In the meta-analysis of Baigent et al only 0.023% of the patients treated with statins and 0.015% of the patients treated with placebos reported rhabdomyolysis15.
Conclusion
In patients undergoing PCI the use of statins is associated with reduced mortality during prolonged follow-up. Patients using atorvastatin had a 23% lower mortality than those using simvastatin.
Conflict of interest statement
All authors have no conflict of interest to declare in relation to this paper.