Abstract
Aims: To evaluate the risk and predictors of death in a large population of patients with stable coronary disease treated with percutaneous intervention.
Methods and results: The study population comprised 1,276 patients with chronic angina or silent ischaemia who underwent elective coronary angioplasty. Baseline and in-hospital mortality data were prospectively collected for all patients during the index hospitalisation. Post-discharge outcome was assessed at out-patient clinic, by review of the patients’ records, or direct phone contact. Deaths were classified as cardiac and non-cardiac. Age, peripheral arterial disease, congestive heart failure with NYHA class ≥ III, triple-vessel disease, and procedural success (i.e. angiographic success for all lesions in the absence of peri-procedural infarction) remained as multivariate independent predictors of death. For the entire population 4-year cumulative all-cause and cardiac mortality were respectively 5.4% and 4.1%. Four-year mortality for patients without any multivariate predictor was 2.4%, while for patients with two or more predictors the death rate was 16.3% after four years.
Conclusions: Patients with stable coronary disease undergoing percutaneous treatment have an overall low mortality rate after four years. Nevertheless, stable patients comprise a heterogeneous population in terms of risk profile, ranging from patients at very low risk of late death to individuals with a poor long-term prognosis.
Introduction
Coronary angioplasty is globally accepted as a versatile therapeutic method, applicable to patients with virtually all clinical presentations of coronary disease, from silent ischaemia to acute myocardial infarction1,2. Today, percutaneous coronary intervention is the most frequently performed invasive method for both stable and unstable patients worldwide.
Over the last decades, a countless number of clinical trials have established the beneficial role of angioplasty for patients with acute coronary syndromes3,4. However, a disproportionate low number of studies have been conducted to specifically investigate the long term outcomes of stable patients, even though this subgroup accounts for a large proportion of the population treated with angioplasty5,6. Recent information on late outcomes of stable coronary patients undergoing angioplasty are mostly restricted to data derived from clinical trials7,8, whose risk profile frequently differs from patients treated in daily practice.
Patients with stable coronary syndromes are commonly regarded as “low risk”. Recently, results from the COURAGE trial have shown that selected stable patients may not benefit from percutaneous coronary intervention compared with optimal medical treatment8. However, it is widely recognised that the outcomes may vary considerably from patient to patient6 and, currently, it is poorly known which factors modulate the long-term prognosis of stable patients undergoing percutaneous intervention. Such knowledge may have important implications both for clinical decision-making as well as for the design of future interventional trials, particularly in the current era of drug-eluting stents, where device-related adverse events are expected to occur at low absolute frequencies.
In this study, therefore, we aimed to evaluate the long-term risk of death in a large population of consecutive patients with stable coronary disease treated with percutaneous intervention. Baseline characteristics were examined to evaluate their relation with the prognosis during the follow-up after coronary angioplasty.
Methods
Study population
From September 1998 to December 2003, a total of 4,289 consecutive patients from the National Public Health System living in Sao Paulo State, Brazil were treated with percutaneous coronary intervention in our institution. From these, 1,276 patients had stable coronary disease at presentation (defined as chronic ischaemic symptoms or silent ischaemia, with no acute coronary event within the last 30 days) and comprise the present study population.
Baseline and procedural characteristics were prospectively recorded in a dedicated database for all patients. Angiographic success was defined as normal antegrade flow at the end of the procedure, with a diameter stenosis <50% for balloon or <30% for stent. Procedural success was defined as angiographic success for all attempted lesions, in the absence of periprocedural myocardial infarction (new Q waves or elevation of cardiac markers > 3 X normal). Also, offline quantitative coronary angiographic analysis was performed for all treated lesions by a technician blinded to patient characteristics, utilising a validated computer-based edge-detection system (CAAS II, Pie Medical, Maastricht, The Netherlands)9.
The treatment strategy was entirely left at the discretion of the operator and followed current guidelines for optimal patient care2. Only bare metal stents were available during the enrolment period, and were unrestrictively used at operator’s choice. Ticlopidine or clopidogrel were typically administered for one month after the index procedure.
Clinical follow-up
In-hospital mortality data was prospectively collected for all patients during the index hospitalisation. Post-discharge evaluation was prospectively performed at our out-patient clinic. The life status was also assessed by reviewing all patients’ records for any new hospitalisation and visits in other specialties. Direct phone contact with the patients or their families was made whenever necessary. Deaths were classified into cardiac and non-cardiac. Cardiac death was defined as any death unless unequivocally related to a non-cardiac cause. All autopsies and medical records were carefully reviewed to ascertain the cause of death.
Statistical analysis
Categorical variables were presented as percentages and continuous variables were presented as their mean and standard deviation. The cumulative incidence of death was estimated according to the Kaplan-Meier method. Patients lost to follow-up were considered at risk until the date of last contact, at which point they were censored. Univariate and multivariate Cox proportional hazards models were used to identify predictors of mortality among baseline characteristics.
Results
Baseline and procedural characteristics are shown in Table 1. Most patients had multivessel disease (52.5%). Treated diabetes was present in 28.5%, previous myocardial infarction in 36.8%, previous coronary surgery in 15.4%, and congestive heart failure in 13.7%.
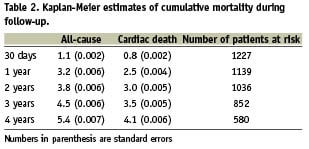
On average, patients were followed-up for 1448±731 days (median 1373 days; interquartile range 1000 – 1970 days). At least six months of follow-up data was available for 95.5% of patients. Table 2 shows the death rates during the follow-up period. After four years, the all-cause mortality was 5.4% and the cardiac mortality was 4.1% for the entire population. More than half of the deaths occurred during the first year (3.2% for 1-year all-cause mortality and 2.5% for 1-year cardiac mortality). Cardiac causes accounted for the vast majority of deaths throughout the follow-up period. The contribution of non-cardiac causes to the overall mortality was negligible at 30 days, and increased to approximately one fourth of all deaths at four years.
Patients were included during an enrolment period of approximately five years (between 1998 and 2003). There were no significant differences in mortality for patients treated in the early half and the last half of the inclusion period (4-year all-cause mortality: 4.5% vs 6.5% respectively; hazard ratio [HR] 1.42, 95% confidence interval [CI] 0.85 – 2.35; p=0.2). Also there were no differences in the rate of procedural success across the study period.
Independent predictors of all-cause death were age (continuous variable), peripheral arterial disease, congestive heart failure with NYHA class ≥ III, triple-vessel disease, and procedural success (i.e. angiographic success for all lesions in the absence of periprocedural myocardial infarction), as shown in Table 3. For illustrative purposes, mortality rates were estimated according to the presence and number of predictors (unsuccessful treatment for all lesions and categorised age > 67.4 years [higher tertile for the overall population] were considered as a risk factors, together with peripheral arterial disease, NYHA class ≥ III, and triple-vessel disease). Overall, 47.6% of all patients had at least one and 11.8% had two or more of risk factors at baseline. Four-year mortality was 2.4% for patients without multivariate risk factors, 6.3% for patients with one risk factor and 16.3% for patients with two or more predictors (Figure 1). Careful analysis of Figure 1 reveals that the mortality curves diverge throughout the entire follow-up period for patients at low, intermediate, or high risk of death. It is apparent, moreover, that patients with two or more risk factors had an especial propensity to fatal events early after the procedure.
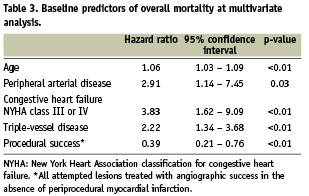
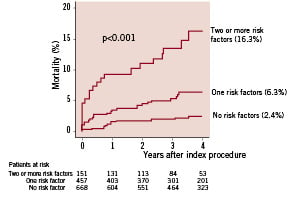
Figure 1. Kaplan-Meier mortality curves for stable patients according to the number of risk factors (age > 67.4 years, peripheral arterial disease, congestive heart failure with NYHA class ≥ III, triple-vessel disease, or unsuccessful treatment for all lesions).
Discussion
The present study demonstrates that patients with stable coronary syndromes treated with percutaneous intervention in the daily practice, in general, have low risk of early and late death. After four years, all-cause and cardiac mortality rates were 5.4% and 4.1% respectively. Our findings also show, however, that the group of patients with chronic stable coronary disease actually comprise a markedly heterogeneous population, with several subsets having distinctly different long-term prognosis. The risk of death was significantly influenced by baseline characteristics, mostly clinical parameters related to the severity of the atherosclerotic disease. Patients who did not present any multivariate predictor of death had an estimated low 4-year mortality of only 2.4%. Conversely, patients with two or more multivariate risk factors had an almost 7-fold increase in all-cause mortality (16.3%) after four years.
Patients with stable angina are generally considered to be at high risk if presenting a predicted annual cardiovascular mortality >2% and at low risk if their annual cardiovascular mortality is <1%6. In our series, the overall mortality during the first year after the procedure exceeded 2%. However, after that period, the annualised death rate decreased to approximately 1% per year between two and four years. It is especially of note that high risk patients had a disproportionately increased tendency to complications shortly after the procedure, as compared to patients with lower risk profiles. The ~5.0% death rate within 30 days was due to seven early deaths occurring among the 151 high risk patients (all early deaths occurred in the first nine days after the procedure). From these fatal episodes, four happened during the procedure or after emergency surgery due to acute complications during the angioplasty, two happened during the index hospitalisation due to multi-organ clinical complications (renal failure, lower limb ischaemia, sepsis, etc.), and one was a sudden unexplained death four days after the procedure. The low number of events precludes a more direct insight on the determinants of early death in this subset. To some extent, however, it may be most probably the reflection of procedural-related complications in patients at very high risk undergoing a palliative catheter-based intervention.
The role of invasive (surgical or percutaneous) treatment for stable patients has been under intense debate in the last years. The large randomised COURAGE trial8 showed no additional mortality benefit of angioplasty in comparison to a more conservative medical approach for non-acute patients. However, a recent meta-analysis with data from 17 randomised trials (7,513 patients) comparing angioplasty with medical for non-acute coronary syndromes has shown a significant reduction in all-cause mortality for invasively treated patients (odds ratio 0.80; 95% confidence interval: 0.64 to 0.99)10. Accordingly, in a sub-study of the previous MASS trial, diabetics with multivessel disease have been shown to be at increased mortality risk after the first year when left on medical treatment alone, when compared to patients treated percutaneously or surgically11.
The present study population from the so-called “real world” differs conceivably from patients enrolled in randomised trials. In our series, all included patients were referred by the attending physician to be treated with percutaneous coronary intervention, a therapeutic choice that must be seen as the result of a complex and multifactorial decision sequence. The Euro Heart Survey on Coronary Revascularisation that included 2,936 patients with stable angina pectoris, has shown that treatment decisions are modulated by multiple factors12. Deviations from general guidelines “recommendations” were more often observed in patients with extensive coronary disease, impaired left ventricular function, and diabetes12 - all of them subsets where a case-by-case analysis must be frequently applied in clinical practice to ascertain the best therapeutic option. The influence of an individualised therapeutic plan is readily apparent when analysing the differences between the outcomes of patients in some randomised trials and the outcomes of patients with similar characteristics included in concomitant registries, whose final treatment was not dictated by the rigid randomised protocol. In the randomised BARI trial, diabetics treated with balloon angioplasty had a higher mortality than diabetics undergoing surgery13. Conversely, in the BARI registry, patients with diabetes treated with surgery had similar unadjusted mortality compared to the subgroup chosen to be treated with balloon angioplasty13. Also, in the AWESOME study, patients with previous coronary surgery had similar outcomes when treated with surgery or angioplasty in the randomised cohort14. In the patient-choice registry, however, the 36-month survival rate was significantly better for patients treated with angioplasty, compared to re-operation in patients with prior bypass surgery14.
Recently, the clinical impact of late (> 30 days) stent thrombosis after drug-eluting stenting has been perceived as a major point of concern. Observational studies have demonstrated that delayed stent thrombosis occur at low, although sizeable, rates in the daily practice15. In the present series, where only bare metal stents were utilised, the death rate between 30 days and four years was 4.3% for the entire population. However, for patients at very low risk of death (i.e. patients without any negative predictors at baseline), the mortality rate between 30 days and 4 years was 2.1%, while 11.6% of patients at very high risk (i.e. patients with two or more predictors at baseline) died during the same period. It seems clear that, in case drug-eluting stents are to be used for similar patients, the relative impact of late stent thrombosis must be interpreted in the light of the markedly different prognosis presented by the subsets that comprised this heterogeneous population. Also importantly, in case coronary bypass surgery should be considered as an option for low-risk stable patients, the inherent surgical mortality must be carefully weighted against the expected therapeutic benefits, keeping in perspective the low predicted death rate of this subgroup. Curiously, even though the inclusion period was relatively prolonged (between 1998 and 2003), we did not observe any difference in the survival rates over time between patients treated in the early and in the late phases of the enrolment. Possibly, this lack of difference reflects the fact that the whole study population was managed according to similar guidelines, treated in a stent era where aggressive risk factor reduction had been already extensively shown to be of benefit.
Our results are in line with the current concept that chronic patients treated with percutaneous coronary intervention have a better collective prognosis than patients with acute coronary syndromes7. It was possible, however, to document that some subgroups of stable patients are at a very high risk of death, even higher than in many historical cohorts of patients with acute coronary disease7. Our findings emphasise the need for risk assessment for patients with stable coronary disease. Patients at a high estimated risk should be promptly recognised and may benefit from aggressive risk factor reduction and disease-specific treatment strategies.
Conclusions
Patients with stable coronary disease undergoing percutaneous treatment have an overall low mortality at 4-year follow-up. Stable patients with poor long-term prognosis can be readily identified at baseline and should be considered for aggressive therapeutic strategies aiming to reduce their risk profile. Nevertheless, very low risk patients are also easily identifiable and comprise the most numerous subset, for which coronary angioplasty is a safe therapeutic method at early and late follow-up.

