
Abstract
Aims: Our aim was to investigate the strength of fractional flow reserve (FFR)-guided percutaneous coronary intervention (PCI) for stable coronary artery disease (CAD) in daily practice.
Methods and results: For this study, 3,512 patients with stable CAD and at least one 50-89% coronary stenosis were identified; those patients thought to require PCI (n=1,716) were selected. Of these, 962 (56%) were treated based on angiography (XA) alone, whereas 754 patients (44%) had an FFR-guided treatment. In the latter group, 321 patients (43%) were reallocated to another treatment, predominantly medical treatment. After propensity score matching, the number of indicated lesions was 957 in the XA-guided group and 947 in the FFR-guided group. FFR guidance resulted in PCI deferral in 462 lesions (48.8%). In a seven-day landmark analysis, the rate of periprocedural myocardial infarction (MI) was less than half in the FFR-guided group (p>0.05). For the eight-day to four-year follow-up period, FFR guidance resulted in a significantly lower rate of the combined endpoint of death/MI (hazard ratio [HR] 0.63) and MI-driven target lesion revascularisation (HR 0.35).
Conclusions: This large, retrospective study shows that performing FFR has a significant impact on therapeutic strategy and demonstrates the favourable long-term outcome of FFR-guided PCI in an “all-comers” population of patients with stable CAD in daily clinical practice.
Abbreviations
AP: angina pectoris
CABG: coronary artery bypass graft
CAD: coronary artery disease
FFR: fractional flow reserve
HR: hazard ratio
LAD: left anterior descending artery
MACE: major adverse cardiac events
MI: myocardial infarction
OMT: optimal medical treatment
PCI: percutaneous coronary intervention
PS: propensity score
RR: relative risk
TLR: target lesion revascularisation
XA: X-ray angiography
Introduction
The optimal therapeutic strategy for patients with stable coronary artery disease (CAD) remains a topic of vigorous debate. Myocardial revascularisation in the elective setting is considered appropriate when the expected benefits in terms of survival or health outcomes (symptoms, functional status, and/or quality of life) exceed the expected negative consequences of the procedure. According to the latest joint guidelines of the European Society of Cardiology (ESC) and the European Association of Cardio-Thoracic Surgery (EACTS), myocardial revascularisation – by either percutaneous coronary intervention (PCI) or coronary artery bypass graft (CABG) surgery – may be indicated in flow-limiting coronary stenoses to reduce myocardial ischaemia and its adverse clinical manifestations1.
Back in 1974, Gould and Lipscomb demonstrated that hyperaemic flow starts to decline in the presence of a ≥50% coronary stenosis2. Consequently, this cut-off value of 50% has been used to define CAD severity, risk stratify patients and justify revascularisation. Since 1996, however, an increasing number of studies have proven that fractional flow reserve (FFR) measured with a coronary pressure wire can identify flow-limiting stenoses more accurately than visual assessment of the coronary angiogram3,4.
In the randomised FAME trial5,6, use of invasive FFR measurements to guide PCI was shown to improve clinical outcomes in patients with multivessel disease. However, only scarce data validating the strength of FFR-guided revascularisation in routine daily practice are available7-10. Therefore, this study aimed to investigate the long-term outcome of FFR-guided PCI for stable CAD in routine daily practice – based on a propensity score (PS)-matched landmark analysis.
Methods
STUDY POPULATION
Between 1 July 2010 and 30 June 2014, a total of 16,718 patients underwent a diagnostic coronary angiography at Rigshospitalet, Copenhagen, Denmark. Of these, patients with stable angina pectoris (AP) and at least one 50-89% coronary stenosis were selected. All patients gave informed consent for the procedure. The study complies with the Declaration of Helsinki on ethical principles for medical research involving human subjects.
ANGIOGRAPHIC EVALUATION
Diagnostic coronary angiography was performed using 6 Fr catheters through the femoral or radial approach – according to patient suitability and the operator’s preference. All clinical and procedural data were prospectively stored in a dedicated electronic database and retrospectively analysed for the purpose of this study. The angiographic images were stored in a digital archive. For all patients evaluated by FFR, all coronary lesions were re-evaluated – blinded from the FFR values – by two independent PCI operators to grade lesion severity (% diameter stenosis) by visual assessment and indicate whether the lesion(s) should be treated by optimal medical treatment (OMT), PCI, or CABG. The final lesion severity (%) was calculated as the mean of the two values; in case of discordance regarding therapeutic choice, the opinion of a third PCI operator was obtained. Based on these data, a matrix reporting the post-XA (rows) and post-FFR (columns) decision was composed for those patients evaluated by FFR.
INTRACORONARY PRESSURE MEASUREMENTS
FFR was measured as previously described3. Briefly, after intracoronary administration of nitrates, a PressureWire™ Certus™ or Aeris™ (St. Jude Medical, St. Paul, MN, USA) was advanced distal to the coronary lesion. Hyperaemia was obtained after administration of intravenous adenosine (continuous infusion of 140 μg/kg/min). An FFR value ≤0.80 was considered “positive”, i.e., likely to induce reversible myocardial ischaemia.
CLINICAL FOLLOW-UP
The primary endpoint during follow-up was major adverse cardiac events (MACE), defined as a composite of death, myocardial infarction (MI), or any repeat revascularisation. The secondary endpoints were the individual components of MACE as well as the combined endpoint of death or MI. Death encompassed all-cause mortality. Myocardial infarction was defined as a ≥5-fold elevation of creatine kinase-myocardial band or new Q-waves in ≥2 contiguous leads of the electrocardiogram. Revascularisation encompassed both PCI and CABG of any coronary lesion(s) and was indicated being performed because of AP (AP-driven) or MI (MI-driven). Target lesion revascularisation (TLR) was defined as either percutaneous or surgical revascularisation in the follow-up period of any lesion thought to require PCI during the index procedure. Elective re-catheterisation because of angina was used as a surrogate parameter for recurrent AP or, inversely, angina-free status in this study.
STATISTICAL ANALYSIS
Continuous variables are summarised as mean±standard deviation. Categorical variables are summarised as absolute values and group percentage. Group comparisons are tested using the Student’s t-test for continuous variables and the Pearson’s χ2 test for categorical variables. In order to obtain comparable groups, PS matching was performed to match each patient of the FFR-guided group with one patient from the XA-guided group. Therefore, we derived propensity scores for all patients based on sex, age and all other variables which were associated with p<0.10 in multivariate analysis. Propensity scores were then matched with a maximal range of ±0.05 to obtain matched pairs of patients. Kaplan-Meier curves show the cumulative event-free survival rates for the different endpoints, and hazard ratios (HRs) were calculated with Cox proportional hazard models; a separate landmark analysis with the landmark (cut-off) set at seven days after the index procedure was performed. Stratified analysis was performed for the combined endpoint of death or MI and AP-driven revascularisation. All analyses were performed with SPSS, Version 20.0 (IBM Corp., Armonk, NY, USA).
Results
THERAPEUTIC REALLOCATION
A total of 3,512 patients presenting with stable AP and at least one 50-89% coronary stenosis were identified. Of these, 2,320 patients (66.1%) were treated based on the information from XA alone, whereas in 1,192 patients (33.9%) the final therapeutic decision was guided by additional FFR. In general, this FFR-based strategy resulted in reallocation of 373 patients (31.3%) to another therapy than the one initially chosen based on XA alone (Figure 1).
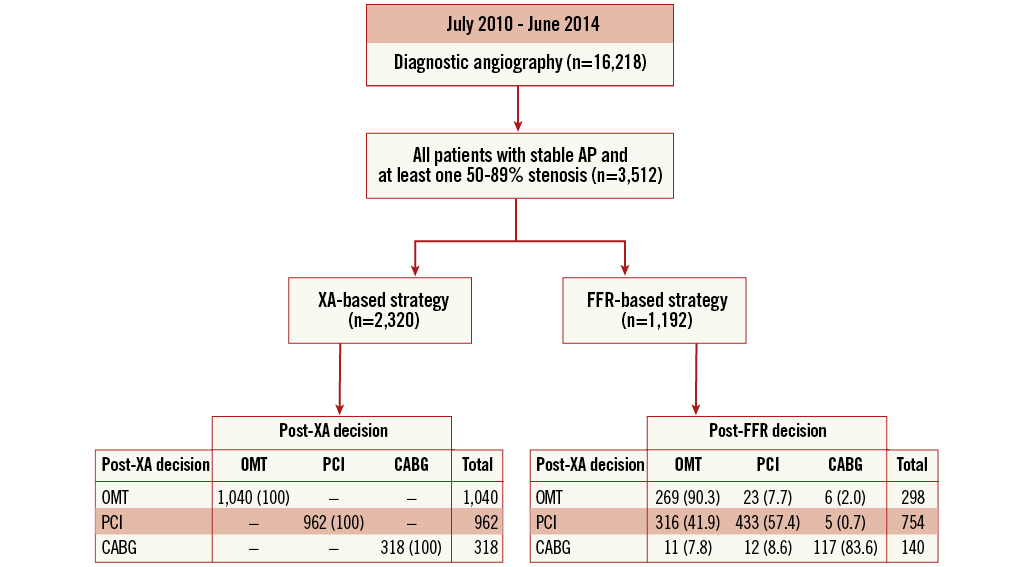
Figure 1. Study flow chart. AP: angina pectoris; CABG: coronary artery bypass graft; FFR: fractional flow reserve; OMT: optimal medical treatment; PCI: percutaneous coronary intervention; XA: X-ray angiography
In order to compare XA-guided PCI vs. FFR-guided PCI, those patients thought to require PCI based on XA alone were selected for further analysis, i.e., 962 patients in the XA-based subgroup and 754 patients in the FFR-based subgroup: this was our study population (n=1,716). In the latter FFR subgroup, 321 patients (42.6%) were reallocated to a treatment other than PCI, predominantly OMT (Figure 1).
BASELINE CHARACTERISTICS
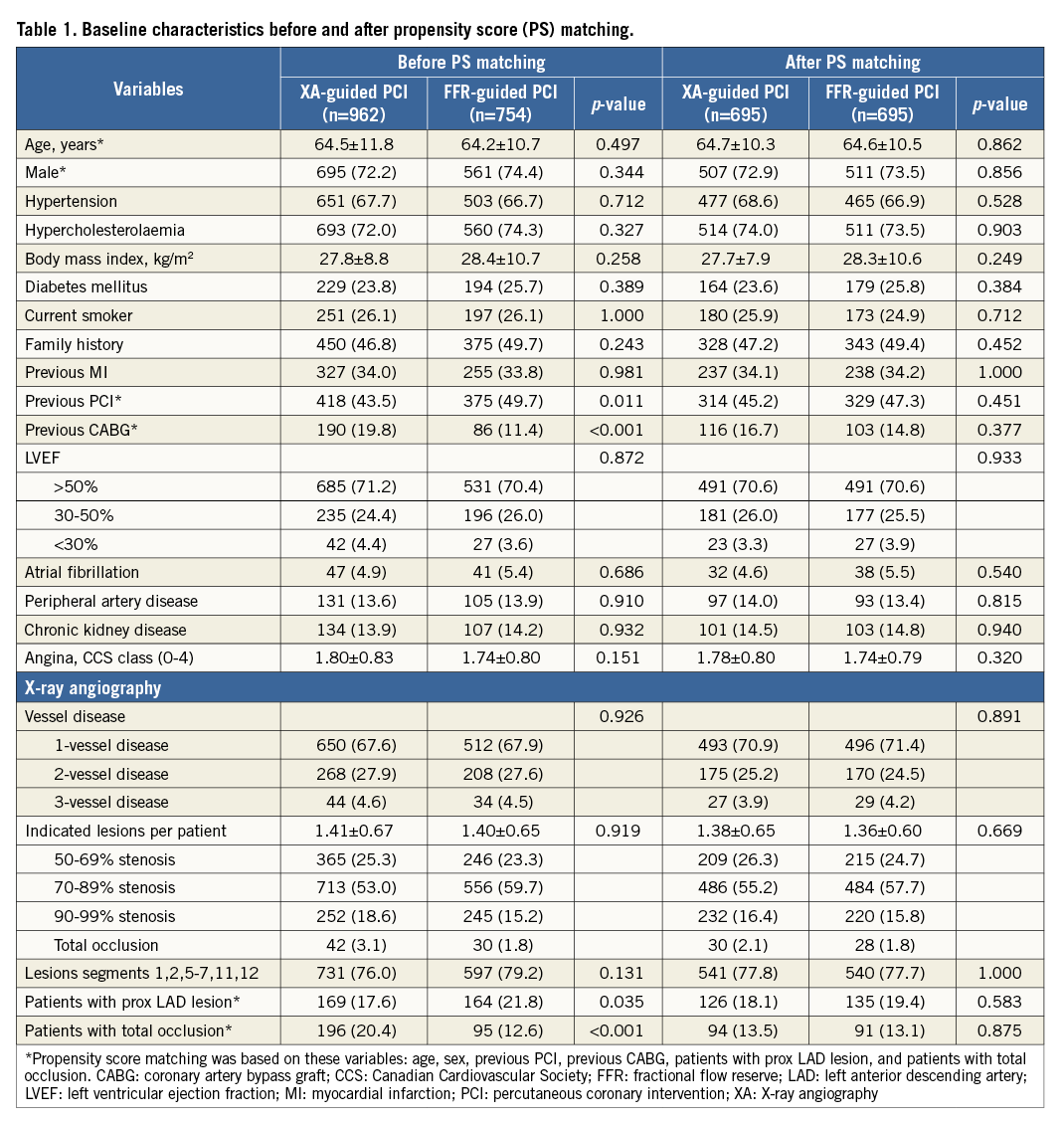
The baseline characteristics of both groups – before and after PS matching – are shown in Table 1. Before PS matching, patients from the XA-guided group (n=962) were more likely to have previous CABG, and hence chronic total occlusions. In contrast, patients in the FFR-guided group (n=754) were more likely to have previous PCI and a proximal LAD stenosis. After PS matching, baseline characteristics were similar between the two groups (n=695).
PROCEDURAL OUTCOMES
In the PS-matched cohorts, a total of 1,904 lesions were thought to require PCI, with 957 lesions in the XA-guided group and 947 lesions in the FFR-guided group. In the FFR-guided group, 764 (80.7%) of the 947 indicated lesions were evaluated by FFR; the other 183 lesions were considered significant based on XA alone. The total number of “perform PCI lesions” was 957 (100%) in the XA-guided group and – based on the FFR measurements – 485 (51.2%) in the FFR-guided group. Consequently, 462 (48.8%) lesions were deferred from PCI in the latter group (Table 2).
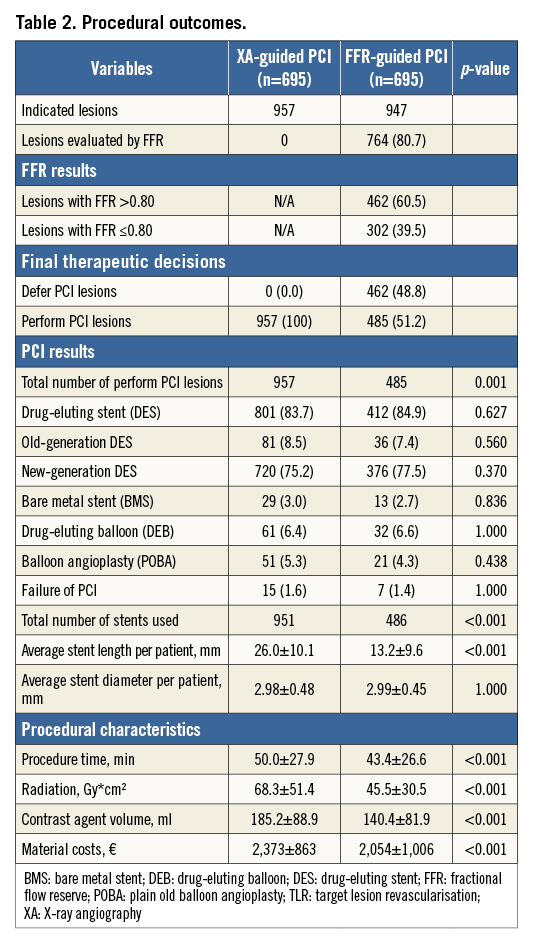
The treatment of “perform PCI lesions” was identical in both groups. In total, 1,255 (87.0%) of the “perform PCI lesions” were treated by stenting, of which 1,213 lesions were treated by drug-eluting stent (DES). Given the 48.8% of PCI deferrals in the FFR-guided group, markedly fewer stents were used in the FFR-guided group as compared to the XA-guided group (486 vs. 951, p<0.001). In addition, procedure time, radiation dose, contrast agent used, and total material costs were significantly lower in the FFR-guided group as compared to the XA-guided group (p<0.001) (Table 2).
CLINICAL OUTCOMES
The Kaplan-Meier curves in Figure 2 show the cumulative event-free survival rates for the different endpoints following XA-guided vs. FFR-guided PCI. The median follow-up duration was 21.3 months in the FFR-guided group and 23.2 months in the XA-guided group, which was not significantly different.
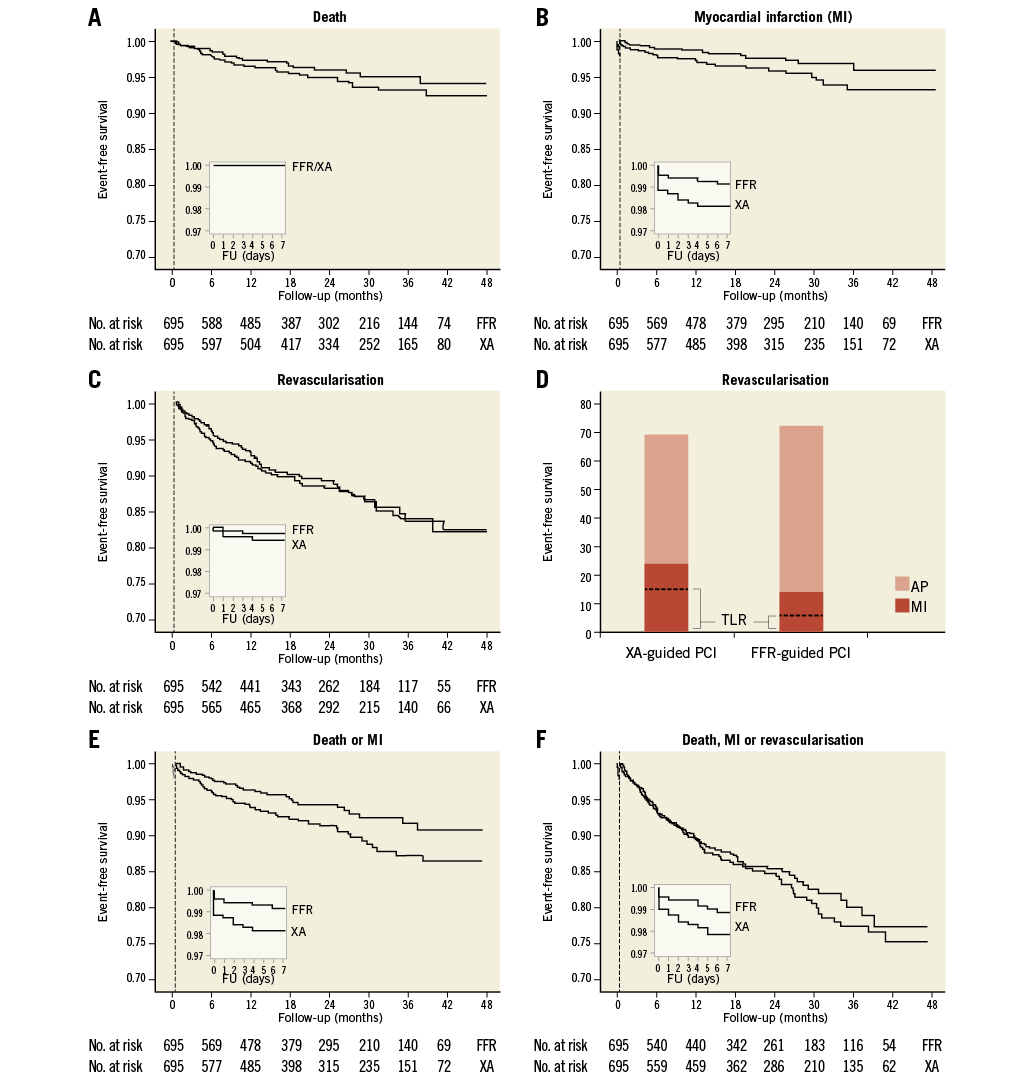
Figure 2. Kaplan-Meier curves for the landmark analysis. Shown are the event-free survival curves for the different endpoints: A) death, B) myocardial infarction (MI), C) revascularisation (panel D shows the total number of revascularisation procedures for the entire follow-up period [0 days to 4 years] in the XA-guided and FFR-guided groups), E) death or MI, and F) major adverse cardiac events (MACE) in the two study groups – based on a landmark analysis with the landmark set at 7 days after the index procedure (vertical dashed line). The small inserts show the data for days 0 to 7. AP: angina pectoris; FU: follow-up; MI: myocardial infarction; TLR: target lesion revascularisation
When analysing the entire follow-up period without landmark (zero days to four years), FFR-guided PCI was associated with a significantly lower rate of MI (49% relative risk [RR] reduction), MI-driven TLR (59% RR reduction), and the combined endpoint death/MI (40% RR reduction) as compared to an XA-guided strategy. No statistical difference was shown for the primary endpoint MACE (12% RR reduction, p=0.382) (Table 3).
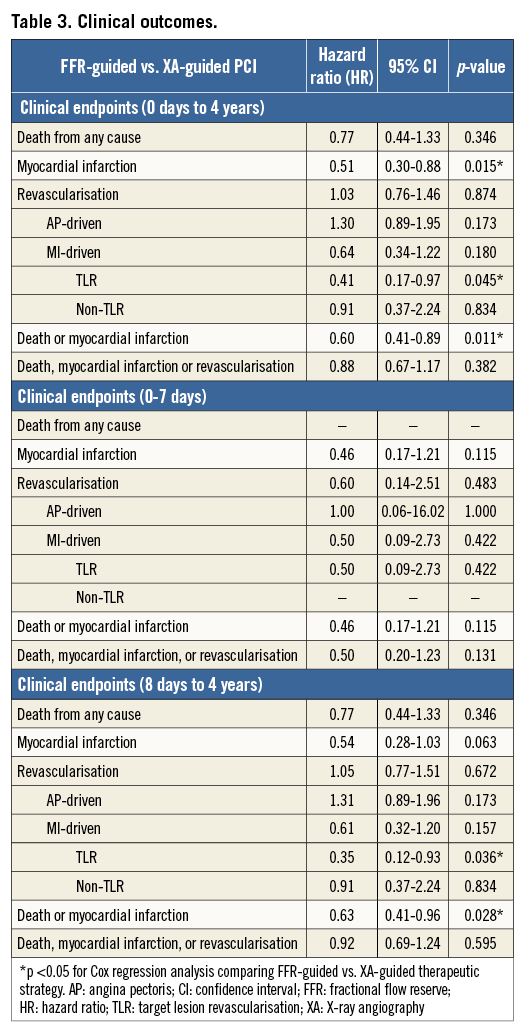
In the landmark analysis, the periprocedural MI rate was less than half in the FFR-guided group; however, statistical significance was not achieved due to the low absolute number of events (n=6 in the FFR group vs. n=13 in the XA group, p=0.115). For the eight-day to four-year period, FFR guidance resulted in a significantly lower rate of MI-driven TLR (65% RR reduction) and the combined endpoint death/MI (37% RR reduction). Accordingly, a 46% reduction was measured for the endpoint MI; however, statistical significance was not achieved (p=0.063). Revascularisation rates were similar in both groups (Table 3).
Results from the stratified analysis are shown in Figure 3. The favourable outcome obtained by FFR in terms of the combined endpoint death/MI remained valid across all subgroups (p for interaction >0.05). AP-driven revascularisation was more frequently needed in FFR-guided patients with one-vessel disease than in patients with multivessel disease (p for interaction=0.032).
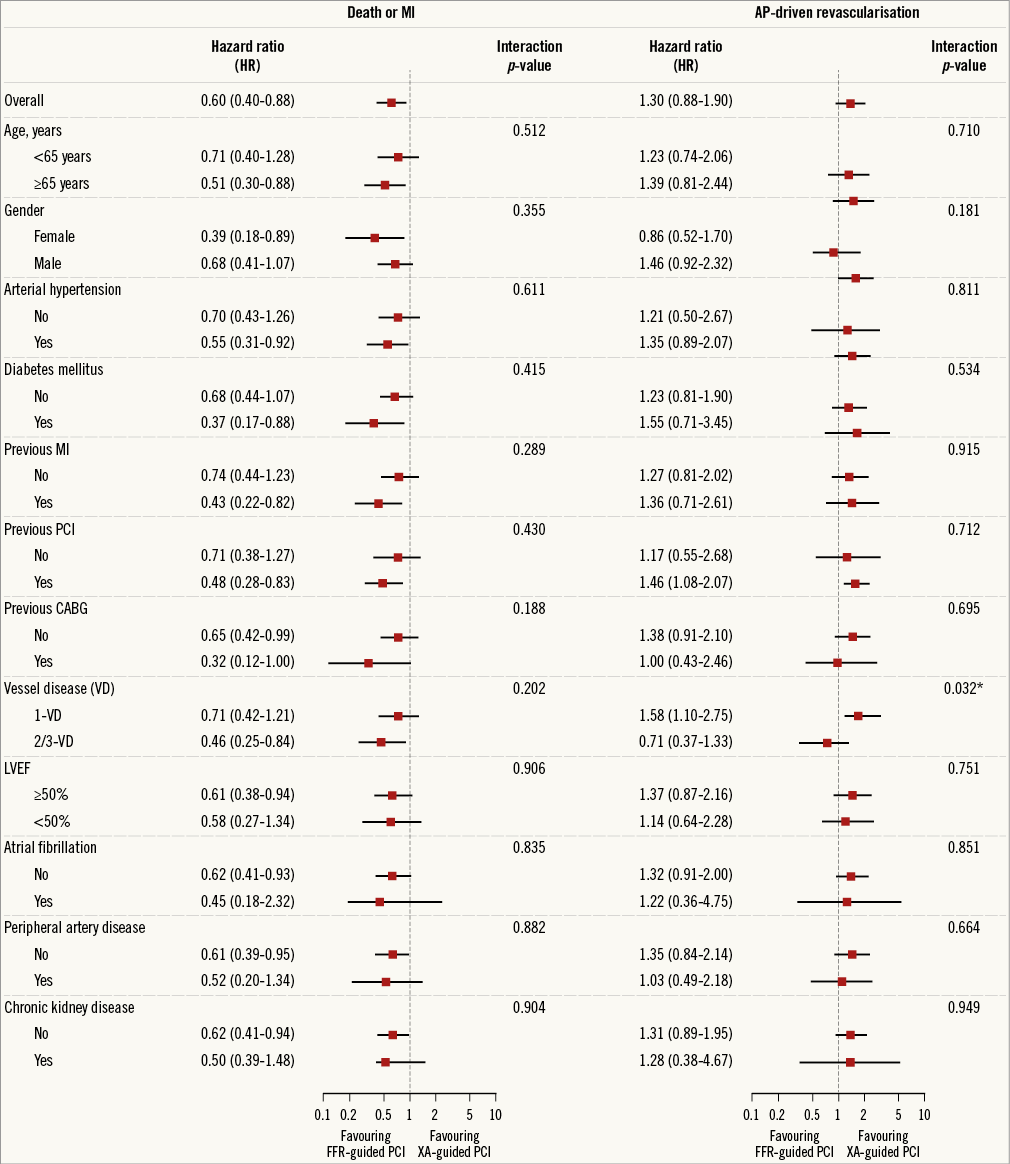
Figure 3. Stratified analysis for the combined endpoint death or MI, and AP-driven revascularisation, accompanied by tests for interaction between PCI strategy and patient characteristics. *p for interaction <0.05. AP: angina pectoris; FFR: fractional flow reserve; TLR: target lesion revascularisation; XA: X-ray angiography
Finally, at four-year follow-up, 84.6% of patients in the XA-guided group were free from elective (AP-driven) re-catheterisation as compared to 83.3% in the FFR-guided group (p=0.559) (Figure 4).
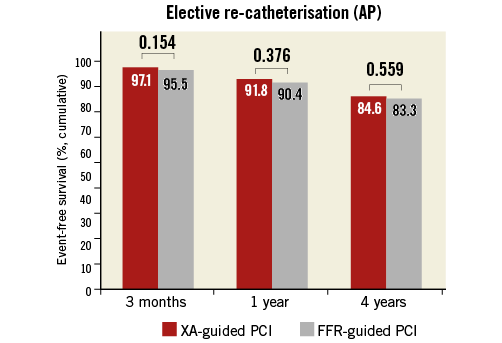
Figure 4. Control of angina. Bar charts showing event-free survival rates for elective (AP-driven) re-catheterisation in the two study groups at three months, one year, and four years after the index procedure. FFR: fractional flow reserve; XA: angiography
Discussion
This large, retrospective study shows that performing FFR has a significant impact on therapeutic strategy and demonstrates the favourable long-term outcome of FFR-guided PCI in an “all-comers” population of patients with stable CAD in daily clinical practice – based on a PS-matched landmark analysis.
Based on our data, we report that the use of FFR in patients with stable CAD leads to a change in revascularisation strategy in 31% of all patients and in 43% of those considered for PCI. Recently, three other studies (FFR-R3F11, RIPCORD12, FAMOUS-NSTEMI13) also addressed this issue, reporting that the management plan based on XA alone was changed in, respectively, 43%, 22% and 26% of all patients and 56%, 19% and 29% of those initially planned for PCI (Figure 5). Importantly, the FFR-R3F and FAMOUS-NSTEMI studies also (or only) included unstable patients – as such, we should be careful comparing these studies. Still, it can be concluded that the use of FFR has an important influence on patient management.
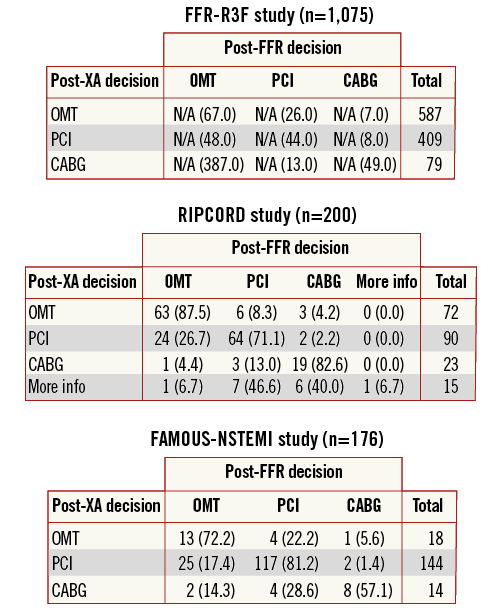
Figure 5. Therapeutic reallocation. Reallocation to another treatment following invasive FFR measurement, as reported in the FFR-R3F, RIPCORD, and FAMOUS-NSTEMI studies.
Based on the concept of the FAME trial, this study aimed to investigate the outcome of FFR-guided PCI vs. XA-guided PCI in real-life clinical practice. A previous report by Li et al7 has tried to address the same question; however, there were several important limitations as this study was performed in a non-selected population with very unequal baseline characteristics. In addition, patients in whom FFR was performed to “confirm OMT” rather than to evaluate real intermediate stenoses were not excluded; in our study, this group represents 25% of all patients (Figure 1). Although Cox regression analysis adjusted for several variables was performed, the reported results in the study by Li et al7 should be interpreted with caution and left many questions unanswered. In this study, a maximal effort was made to obtain two comparable groups with a similar baseline profile: this was done by excluding those patients in whom FFR was performed to “confirm OMT” as well as by PS matching both groups.
In the FFR-guided group, roughly 50-60% of all coronary lesions were deferred from PCI based on an FFR value >0.80. This number of PCI deferrals is similar to that in the DEFER14 study (56%, although with a cut-off of >0.75) but higher than that in the FAME5,6 trial (39%). As a consequence of the lower number of PCIs performed in the FFR group, we also noted a significant reduction of procedure time, radiation exposure and amount of contrast agent used, all of which may reduce procedure-related risks, both in the short and in the long term. In line with previous works15,16, we also measured a significant lower material cost in the FFR-guided group. Unfortunately, we cannot provide data about the incremental costs of the two different approaches. Nevertheless, our data indicate that an FFR-based strategy can contribute to a more rational use of economic resources.
As reflected by the ESC/EACTS guidelines on myocardial revascularisation1, the DEFER and FAME trials have led to a paradigm shift in the evaluation and management of patients with CAD. However, a thorough study confirming the favourable outcome of FFR-guided PCI in a real-life setting of stable CAD has been missing.
In this study, we could roughly confirm results from the FAME trial in our routine daily practice. FFR-guided PCI was shown to be associated with a significant reduction of the hard endpoint death or MI. Most importantly, this result was still observed in the landmark analysis (eight days to four years), thereby tackling one main criticism of the FAME trial that the difference in patient outcome could just be attributed to a reduction of periprocedural events17.
In an attempt to clarify and extend these results, all infarctions which occurred in this eight-day to four-year follow-up period in the XA-guided (n=22) and FFR-guided (n=12) groups were scrutinised. Interestingly, the incidence of definite stent thrombosis was more than double in the XA-guided group (n=10) as compared to the FFR-guided group (n=4) (Table 4). Based on these results, we can conclude that PCI on all angiographic stenoses regardless of their ischaemic potential exposes patients to an increased risk of stent-related problems and additional morbidity.
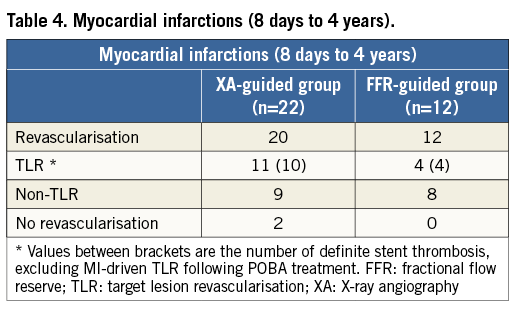
Concerning “revascularisation” in its totality, there was no difference between the therapeutic strategies in our study. In contrast, the FAME trial5 reported a tendency towards less revascularisation in the FFR-guided group, in particular at one-year follow-up (HR 0.68, p=0.08). A possible explanation for this (relatively) higher revascularisation rate in our FFR cohort could be the difference in study populations. In the FAME trial5,6, only patients with multivessel disease were included, whereas 2/3 of our patients had one-vessel disease. As our stratified analysis indicates that AP-driven revascularisation was more common in FFR-guided patients with one-vessel disease, this could be an explanation for the higher AP-driven revascularisation rate observed in our FFR cohort.
The reason for this higher revascularisation rate in this FFR subgroup with one-vessel disease is not known. However, we speculate that this may be related to the fact that, in patients with one-vessel disease, PCI deferral most often implies a therapy with OMT alone, whereas in multivessel disease patients are often still treated by PCI of another lesion. Being aware of an intermediate lesion without any kind of invasive treatment apparently makes patients more likely to return for re-catheterisation. Also, if a patient deferred from PCI is referred for elective re-catheterisation, the threshold to perform PCI seems to be low. Our data confirm that 2/3 of AP-driven revascularisations in previously PCI-deferred lesions are performed without prior (non-) invasive testing.
Finally, interaction analysis showed that the favourable outcome as seen with FFR-guided PCI was maintained across all subgroups. In the DEFER14 trial, only patients with a single stenosis >50% in a native coronary artery were included, whereas, in the FAME5,6 trial, only patients with multivessel CAD were included, excluding patients with one-vessel disease (which was the largest group in our study) as well as patients with previous CABG. The results reported in this study indicate that the concept of performing PCI only in case of “FFR-proven ischaemia” is valid for all subgroups, and also for those patients with one-vessel disease, diabetes mellitus, prior CABG, or a reduced left ventricular function.
Limitations
There are several limitations in this study including its retrospective design, single-centre site and reliance on electronic medical records. FFR measurements were performed at the operator’s discretion. Thus, a selection bias cannot be excluded. One of the main predictors for the use of FFR was the age (or experience) of the operator, with the most liberal FFR use among the younger PCI operators. Besides this observation, the baseline characteristics of the XA- and FFR-guided groups before PS matching suggest that FFR was rather randomly used. In addition, data concerning functional status and medical treatment in the follow-up period are missing. Instead, elective re-catheterisation because of AP was used as a surrogate parameter for recurrent angina or, inversely, angina-free status in this study.
Conclusions
This large retrospective study is the first to show the favourable outcome of FFR-guided PCI for patients with stable CAD in daily clinical practice – based on a strict PS-matched landmark analysis. Most importantly, our data indicate that FFR guidance minimises inappropriate PCI, and hence stent-related complications and cardiovascular morbidity, this without compromising control of patients’ symptoms.
| Impact on daily practice This retrospective study demonstrates the favourable long-term outcome of FFR-guided PCI in an “all-comers” population of patients with stable CAD in daily clinical practice. FFR guidance results in a significant number of PCI deferrals, thereby minimising inappropriate revascularisation and stent-related complications and morbidity. Based on our own as well as international data, we have to conclude that FFR is still underutilised in daily clinical practice. |
Conflict of interest statement
T. Engstrøm has received grants from and is a member of the advisory board of Eli Lilly, Novo Nordisk and Zealand Pharma. The other authors have no conflicts of interest to declare.

