Abstract
Background: A large, prospective, multicentre trial recently showed that fractional flow reserve (FFR) derived from coronary angiography (FFRangio) has an accuracy of 92% compared with conventional guidewire-based FFR (FFRwire); however, little is known about whether specific patient/lesion characteristics affect the diagnostic performance.
Aims: The primary goal of the present study was to investigate whether specific patient or lesion characteristics such as high body mass index (BMI), presentation with an acute coronary syndrome, or lesion location affect the diagnostic performance of FFRangio in patients enrolled in the FAST-FFR study.
Methods: FFRangio was measured in a blinded fashion in 301 patients (319 vessels) who were undergoing FFRwire assessment. Using an FFRwire ≤0.80 as a reference, the diagnostic performance of FFRangio was compared in pre-specified subgroups.
Results: The mean FFRwire and FFRangio were 0.81±0.13 and 0.80±0.12. Overall, FFRangio had a sensitivity of 93.5% and specificity of 91.2% for predicting FFRwire. Patient characteristics including age, sex, clinical presentation, body mass index, and diabetes did not affect sensitivity or specificity (p>0.05 for all). Similarly, lesion characteristics including calcification and tortuosity did not affect sensitivity or specificity (p>0.05 for all), nor did lesion location (proximal, middle, versus distal). Sensitivity was equally high across all target vessels, while specificity was highest in the LAD and lower (~85%) in the RCA and LCx (p<0.05).
Conclusions: FFRangio derived from coronary angiography has a high diagnostic performance regardless of patient and most lesion characteristics. The interaction of vessel on the specificity will need to be confirmed in larger cohorts.
Introduction
Although coronary physiologic assessment has been advocated as a Class I indication in the guidelines, its uptake in the cardiac catheterisation laboratory has been slower than expected1. This may be related to the time and effort required to introduce a coronary pressure wire down a vessel, issues with pressure wire drift, or the need for a hyperaemic agent. To address these limitations and to facilitate physiologic guidance in the cardiac catheterisation laboratory, systems have been developed to predict fractional flow reserve (FFR) based on coronary angiographic images alone, eliminating the need for a pressure wire or hyperaemic agent2.
Recent studies have shown that fractional flow reserve derived from coronary angiography (FFRangio) has excellent agreement with fractional flow reserve derived from a guidewire (FFRwire)3,4. Specifically, the most recent large-scale, multicentre, prospective observational study (FFRangio Accuracy versus Standard FFR [FAST-FFR]) enrolled 301 subjects and 319 vessels and showed a per-vessel sensitivity and specificity of 94% (95% confidence interval 88-97%) and 91% (86-95%)5.
However, it is not known whether specific patient or lesion characteristics affect this diagnostic performance. Accordingly, the primary goal of the present study was to investigate whether specific patient or lesion characteristics such as high body mass index (BMI), presentation with an acute coronary syndrome (ACS), or lesion location affect the diagnostic performance of FFRangio in patients enrolled in the FAST-FFR study.
Methods
STUDY DESIGN AND PATIENT POPULATION
The present study is a post hoc analysis of the FAST-FFR study. The detailed study protocol and the primary results of the FAST-FFR study have been published previously5.
In brief, the FAST-FFR study was a prospective, multicentre, international, observational study investigating the accuracy of FFRangio compared with FFRwire as a reference standard (NCT03226262). Patients presenting with stable angina, unstable angina, or non-ST-segment elevation myocardial infarction (non-STEMI), referred for coronary angiography and undergoing FFR measurement with a coronary pressure wire were eligible to be enrolled in the study.
Patients presenting with ST-segment elevation myocardial infarction (STEMI) or with a prior STEMI within the previous 12 months in the artery being interrogated were excluded from the study. Other major exclusion criteria included contraindication for hyperaemic agents, chronic total occlusion of the target territory, prior coronary artery bypass grafting/heart transplantation/valve surgery/transcatheter aortic valve replacement, known left ventricular ejection fraction (LVEF) <45%, Thrombolysis In Myocardial Infarction (TIMI) flow <3, >50% left main stenosis, severe aortic stenosis, percutaneous coronary intervention (PCI) in the target vessel within the past 12 months, severe diffuse disease, or a target vessel supplying major collaterals.
FFRwire MEASUREMENT
FFRwire was measured with a commercially available 0.014-inch pressure sensor guidewire (either Abbott/St. Jude Medical, Philips/Volcano, or Opsens). After intracoronary injection of nitroglycerine, equalisation to the guide catheter pressure with the sensor positioned at the ostium of the coronary artery was performed and the pressure guidewire was then advanced beyond the stenosis in the target coronary artery. The sensor position was recorded to match the location of FFRangio analysis. To induce maximal hyperaemia, intravenous adenosine (140-180 µg/kg/min), intracoronary adenosine (200 µg for the left coronary artery and 100 µg for the right coronary artery), or intracoronary papaverine (12-15 mg for the left coronary artery and 10-12 mg for the right coronary artery) was administered. Simultaneous measurement of the mean proximal coronary pressure using the guide catheter and the mean distal coronary pressure using the pressure guidewire was performed. FFRwire was calculated as the ratio of the mean distal to proximal coronary pressure during steady-state hyperaemia. The invasive FFR tracings were sent to the coronary physiology core laboratory at the Cardiovascular Research Foundation (New York, NY, USA) which was blinded to the FFRangio data.
FFRangio MEASUREMENT
After obtaining the coronary angiogram, at least three DICOM images from different projections were selected and sent directly from the catheterisation laboratory system to the FFRangio console (CathWorks Ltd., Kfar Saba, Israel). Then, an independent operator from each hospital performed FFRangio analysis while blinded to the FFRwire value. Detailed theory and methodology have been published previously4,5. First, the operator input the mean aortic pressure, selected three projections within the recommended range for each target territory, and assigned target vessel and side branches for a three-dimensional reconstruction. The reconstruction is based on the known geometry of two or three projections with a minimum separation of 30° from single-plane angiograms and utilises epi-polar ray tracing together with mathematical constraints enforcing the tree’s structure. The system can construct each vessel separately such that each region/branch/lesion is not necessarily reconstructed from the same views, yet at the same time the tree topology is preserved and adheres to that reflected in all of the two-dimensional images. Based on these data, the coronary arterial network is modelled as an electrical circuit with each segment acting as a resistor. The vessel resistance is estimated based on its length and diameter. Each vessel’s contribution to flow is based on its impact on overall resistance depending on the arrangement. Normal maximal flow is estimated based on the volume of coronary vessels and total coronary length. FFRangio is then calculated as the ratio of the maximal flow rate in the stenosed artery compared with the maximal flow rate in the absence of the stenosis and was compared to the FFRwire at the same location as the pressure wire sensor4,5. The FFRangio analyses performed in each hospital were sent to the FFRangio core laboratory at CathWorks, blinded to the invasive FFR measurement, which was allowed to make post hoc exclusions of cases.
ENDPOINTS
The co-primary endpoints of the FAST-FFR study were the sensitivity and specificity of FFRangio using FFRwire as the reference standard. For both FFRangio and FFRwire, ≤0.80 was used as a cut-off to define a functionally significant coronary stenosis. In this substudy, sensitivity and specificity were compared between common pre-specified patient characteristics including age, sex, diabetes, clinical presentation, and BMI and lesion/procedural characteristics including main vessel, lesion location, FFRwire value (grey zone defined as 0.75-0.85 vs beyond grey zone), tortuosity, calcification, different FFRwire, and injection type of hyperaemia (intracoronary vs intravenous).
STATISTICAL ANALYSIS
Categorical variables are presented as counts and percentages and are compared using the chi-square test. Continuous variables are presented as mean and standard deviation or median and interquartile range and compared using the Student’s t-test (if normally distributed) or the Mann-Whitney U test (if non-normally distributed). A mixed effects model, including a random effect for multiple vessels within a patient, measured associations between FFRangio and predefined subgroups; an identity link and normal distribution was used for continuous measurements and logistic link for binary measurements. Testing the sensitivity of this model, a generalised estimating equations (GEE) model with compound symmetric covariance supported all conclusions made by mixed effects models. Statistical analyses were performed using SAS or Excel software, and type I error for all tests was held at 5%, considering a p-value less than 0.05 as being statistically significant.
Results
A total of 352 patients (376 vessels) were enrolled in this study. Among them, fifty-one subjects (57 vessels) were excluded from the analysis mainly due to data quality issues (14 vessels disqualified by FFRangio core lab and 21 vessels disqualified by FFRwire core lab, 3.7% vs 5.6%) for a total of 301 patients with 319 vessels which were included in the final analysis from 10 sites. The detailed reasons for exclusion and the flow chart of the FAST-FFR study have been published previously5.
Clinical and angiographic characteristics are summarised in Table 1. Overall, the mean age was 64.7±9.7 years with 74.1% being male. The mean BMI and LVEF were 28.9±4.8 kg/m2 and 58±6%, respectively. About one third of patients had diabetes mellitus (31.9%), and 41.9% of patients presented with an ACS (Table 1). About one half of lesions (54.2%) were located in the left anterior descending artery (LAD). The mean FFRwire was 0.81±0.13 and the mean FFRangio was 0.80±0.12 (Figure 1).
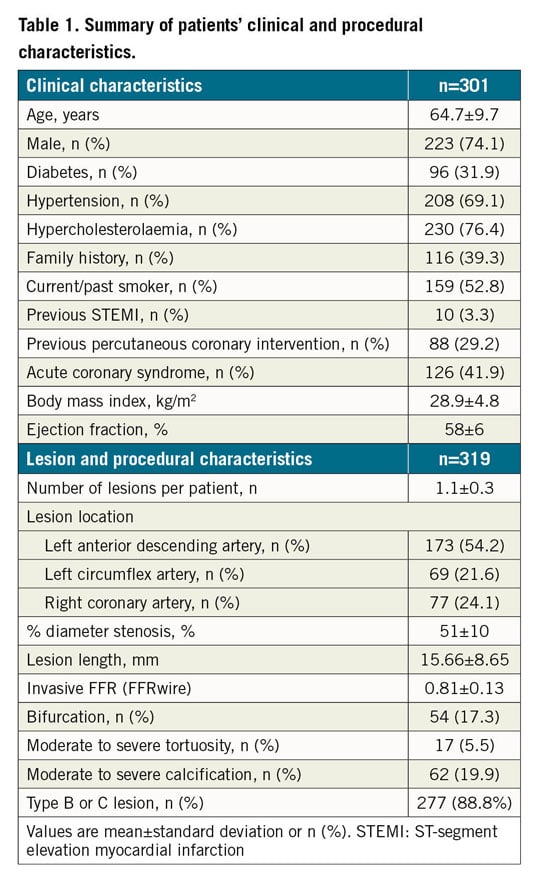
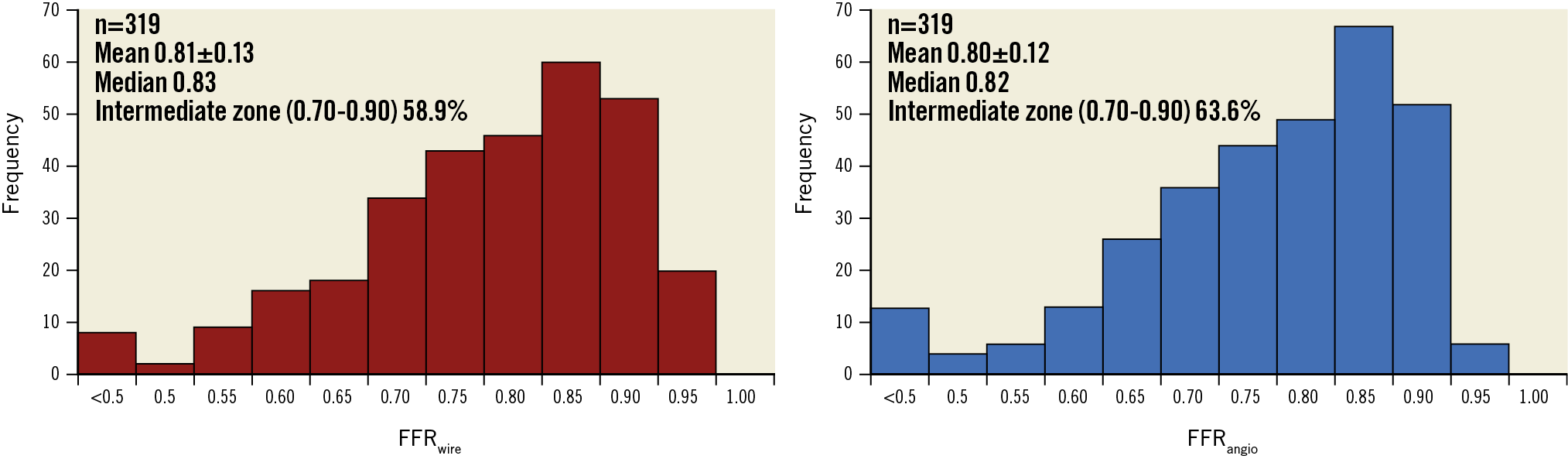
Figure 1. Histogram of FFRwire and FFRangio. Note, FFRangio values less than 0.50 are indicated as <0.50. FFRangio: fractional flow reserve derived from coronary angiography; FFRwire: fractional flow reserve derived from guidewire
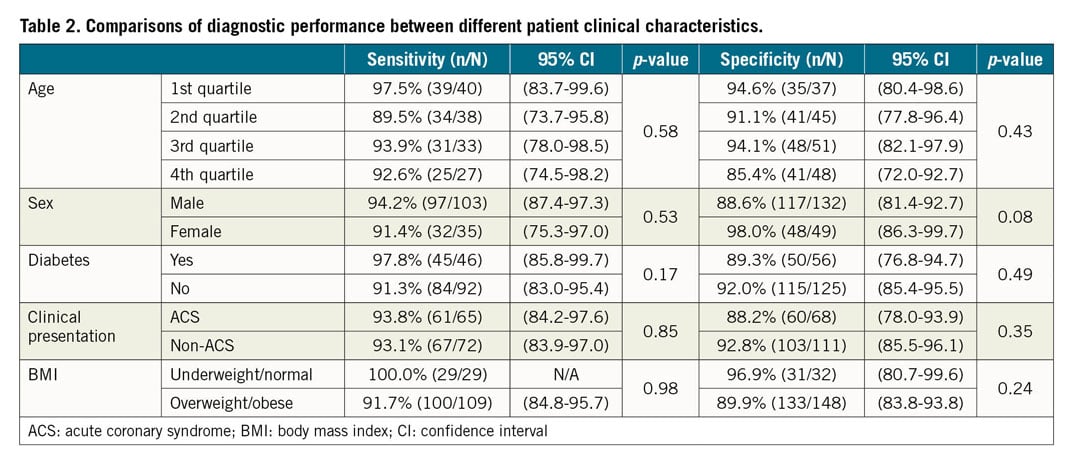
COMPARISONS OF DIAGNOSTIC PERFORMANCE BETWEEN DIFFERENT PATIENT CHARACTERISTICS
Comparisons of diagnostic performance between different patient clinical characteristics are summarised in Table 2. None of the patient clinical characteristics, including age, sex, the presence of diabetes, clinical presentation (ACS vs non-ACS), impacted on the sensitivity and specificity of FFRangio against FFRwire. Clinical presentation divided into stable angina, unstable angina, or NSTEMI did not impact on the sensitivity and specificity (p=0.99 for sensitivity and p=0.18 for specificity). BMI divided into <18.5 kg/m2, 18.5-24.9 kg/m2, 25.0-29.9 kg/m2, and >30 kg/m2 did not impact on the sensitivity and specificity (p=0.42 for sensitivity and p=0.47 for specificity). Similarly, there was no interaction for enrolling site geographic location (p=0.79 for sensitivity and p=0.19 for specificity).
COMPARISONS OF DIAGNOSTIC PERFORMANCE BETWEEN DIFFERENT LESION CHARACTERISTICS
Comparisons of diagnostic performance between different lesion and procedural characteristics are summarised in Table 3. Lesion and procedural characteristics including main branch/side branch, tortuosity, calcification, and the different FFRwire system did not impact on the sensitivity and specificity of FFRangio against FFRwire.
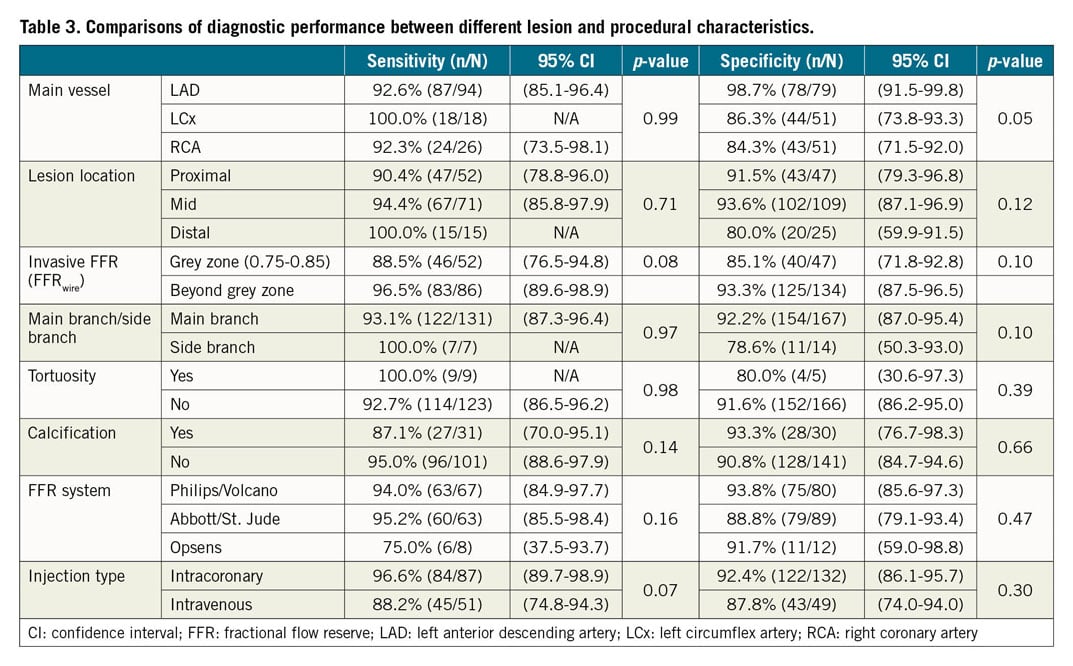
While lesion location (proximal, mid or distal) did not have any impact (p=0.71 and 0.12 for sensitivity and specificity, respectively), and the main vessel did not impact on the sensitivity (p=0.99), it did have a slight effect on the specificity (98.7% for LAD, 86.3% for LCx, and 84.3% for RCA; p=0.046). Comparisons of FFRwire and FFRangio values according to the different main vessel are shown in Figure 2. The FFRangio value was similar to FFRwire in the LAD territory (p=0.37), whereas the FFRangio value was lower in the LCx (p=0.03) and RCA (p=0.05) territories compared with FFRwire (Figure 2).
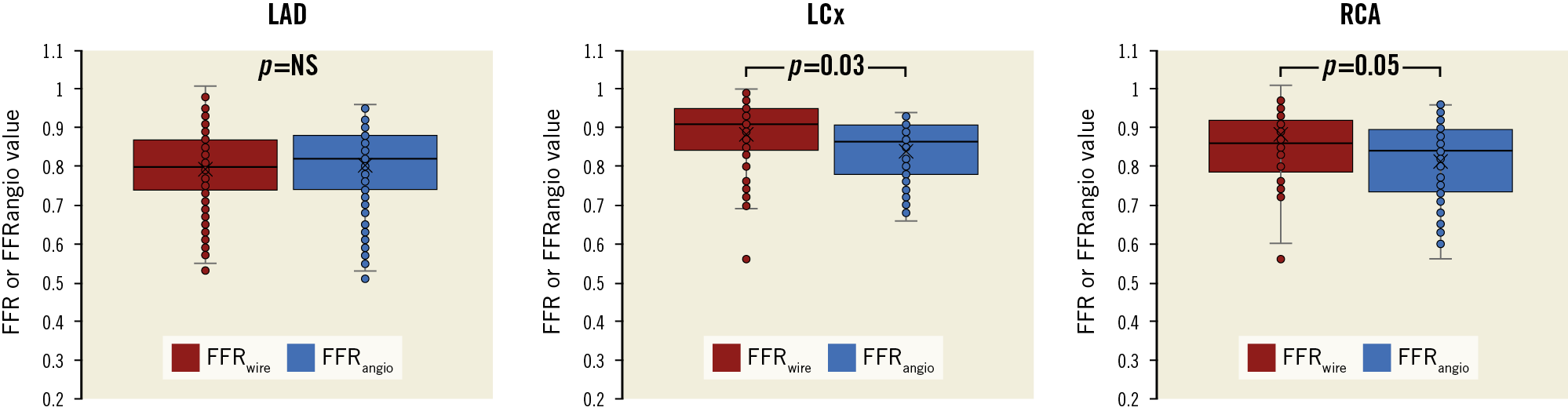
Figure 2. Comparison of FFRwire and FFRangio values according to vessels. FFRwire and FFRangio values were similar in the LAD territory whereas FFRangio was significantly smaller than FFRwire in the LCx (p=0.03) and RCA (p=0.05). FFRangio: fractional flow reserve derived from coronary angiography; FFRwire: fractional flow reserve derived from guidewire; LAD: left anterior descending artery; LCx: left circumflex artery; RCA: right coronary artery
When FFRwire was separated into the cut-off zone (defined as 0.75-0.85) and beyond the cut-off zone (<0.75 or >0.85), both sensitivity and specificity tended to be numerically better beyond the cut-off zone (96.5% vs 88.5%, p=0.08 for sensitivity, and 93.3% vs 85.1%, p=0.10 for specificity).
Discussion
A number of factors appear to limit widespread adoption of wire-based coronary physiologic assessment including the additional time it takes, wire handling characteristics, issues with pressure wire drift, potential side effects from intravenous adenosine, the small risk of placing a wire in the coronary vessel and the cost. By eliminating the need for a coronary pressure wire and a hyperaemic agent, FFRangio overcomes most of these issues. The FAST-FFR trial demonstrated that FFRangio has high diagnostic accuracy compared with FFRwire5; however, it was unclear if these results applied to all patient and lesion subtypes. In the current study we found that FFRangio has a high diagnostic performance in a wide range of patient, lesion, and procedural characteristics, although there may be some variability depending on the vessel interrogated.
The principal finding of the present study is that the diagnostic performance of FFR derived from angiography (FFRangio) is similar to conventional FFR derived from a pressure wire (FFRwire), irrespective of most patient and lesion characteristics. These results suggest that the strategy of FFRangio-guided PCI can possibly be used in a wide variety of patients and lesions evaluated in the cardiac catheterisation laboratory.
Of note, there is a greater prognostic importance of lesions located in the proximal portion of a major epicardial vessel and particularly those located in the left main or LAD, because of the larger extent of the myocardium at risk. In a recent study, a significant interaction between FFR strata and major adverse cardiac events (MACE) was found in patients with stenoses located in proximal coronary segments, unlike in patients with stenoses located in distal coronary segments6. Earlier studies which evaluated the diagnostic performance of non-hyperaemic pressure ratios when compared with FFRwire found that they were lower in the left main or LAD compared to the LCx or RCA, and the larger amount of myocardium supplied by the left main or LAD resulted in a greater discrepancy between resting and hyperaemic coronary flow7. In contrast to these data, the diagnostic accuracy of FFRangio was shown here to be preserved across lesion location (proximal vs distal), and the specificity of FFRangio compared with FFRwire was significantly higher across lesions in the LAD compared with the LCx or RCA. This is probably due to the fact that FFRangio simulates hyperaemic status, whereas non-hyperaemic pressure ratios are derived from resting status; therefore, careful interpretation of our results is needed. The lower specificity in the LCx and RCA may be related to an overestimation by FFRangio of the degree of maximal hyperaemia that can be achieved in these territories with smaller myocardial mass. In this current study, we provided granular data by looking at sensitivity and specificity separately, which are not affected by the prevalence of disease (i.e., prevalence of FFRwire positive lesions). On the other hand, in a recent pooled analysis with 700 lesions, overall diagnostic accuracy among three different territories was not statistically different (LAD 91.0% vs LCx 94.6% vs RCA 87.5%, p=0.095)8. Therefore, the effect of territories on specificity needs to be investigated further.
Both sensitivity and specificity of FFRangio tended to be lower in lesions with FFR values around the cut-off of 0.80, as one would expect with any test near its cut-off. In fact, every test has an accuracy of 50% at its cut-off value9. Reports of diagnostic accuracies of other non-conventional FFR methodologies exist and include 71.3% for the 0.75-0.84 grey zone for quantitative flow ratio (QFR)10, and 46.1% for the 0.70-0.80 grey zone for fractional flow reserve derived from computed tomography (FFRCT)11. In the ADVISE II study, the accuracy of instantaneous wave-free ratio (iFR) was similar to that of FFRangio only when using the hybrid approach with adenosine12. However, in the case of FFRangio it is impressive how high the sensitivity and specificity remain near the cut-off for FFRwire. The reason for this result needs to be investigated further in future studies.
Limitations
There are some limitations to our study. First, although this is the largest study comparing FFRangio and FFRwire to date, the original study was not specifically designed for this substudy. Therefore, some parameters such as sex and the administration route of adenosine might yield statistically significant difference if a larger number of cases were enrolled. Second, it is possible that variables not accounted for in this study could affect the diagnostic performance of FFRangio. Third, this is an observational study comparing the diagnostic performance between the two modalities; we do not have clinical outcome data regarding FFRangio-guided PCI. Fourth, for some of the presented subgroups, differences in sensitivity and specificity may partly be masked by reference standard misclassification and not by using the absolute numerical difference between FFRangio and FFRwire as an outcome. Finally, because this study is a post hoc analysis of the FAST-FFR study, the results of the present study should be interpreted as hypothesis-generating.
Conclusions
FFRangio derived from coronary angiography has a high diagnostic performance regardless of most patient and lesion characteristics. Although the sensitivity was similar, the specificity of FFRangio in the LCx and RCA, while reaching 85%, was lower than in the LAD in this study. This finding will require confirmation in larger cohorts.
|
Impact on daily practice FFRangio derived from coronary angiography may be reliably used in a wide variety of patient/lesion characteristics. The interaction of vessel on the specificity will need to be confirmed in larger cohorts. |
Funding
The FAST-FFR trial was funded by CathWorks.
Appendix. Study collaborators
Allen Jeremias, MD; Department of Cardiology, St. Francis Hospital, Roslyn, NY, USA. Gabriel Greenberg, MD; Department of Cardiology, HaSharon Medical Center, Petach Tikva, Israel. Rami Jubeh, MD; Department of Cardiology, Shaare Zedek Medical Center, Jerusalem, Israel. Daniel M. Kolansky, MD; Division of Cardiovascular Medicine, University of Pennsylvania School of Medicine, Philadelphia, PA, USA. Thomas McAndrew, MD, PhD; Ovidiu Dressler, MD; Mitsuaki Matsumura, BS; Cardiovascular Research Foundation, New York, NY, USA. Akiko Maehara, MD; Cardiovascular Research Foundation, New York, NY, and Columbia University Medical Center, New York, NY, USA.
Conflict of interest statement
Y. Kobayashi reports a consulting relationship with ACIST Medical. T. Engstrøm reports consulting relationships with Boston Scientific, Abbott, Bayer, Novo Nordisc, and AstraZeneca. R. Kornowski reports being a co-founder and shareholder of, and having intellectual property in CathWorks. R. Jubeh reports a consulting relationship with Pfizer and Bayer. D. Kolansky reports institutional research support from CathWorks. A. Maehara receives institutional research support from Abbott and Boston Scientific. A. Kirtane reports institutional grants to Columbia University or Cardiovascular Research Foundation from Medtronic, Boston Scientific, Abbott Vascular, Abiomed, CSI, CathWorks, Siemens, Philips, and ReCor Medical. W. Fearon receives institutional research support from Abbott, Medtronic, and CathWorks, has a consulting relationship with Boston Scientific, and has minor stock options with HeartFlow. The other authors/study collaborators have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

