In October 2009, I was asked a very simple question by an Indian doctor attending our monthly educational meeting just moments before coronary stent implantation during the live case demonstration: “Did you measure fractional flow reserve?”. I had little experience of fractional flow reserve (FFR) assessment at that time. Angiographically, a very discrete and tight (70~80% on my eyeball evaluation) stenosis in the mid right coronary artery was under discussion for angioplasty. Back then, I had no doubt concerning my decision for stent implantation for such an appealing lesion and was convinced that it would be clinically beneficial for the patient. Nevertheless, I measured FFR, probably with the idea of educating him (later, I realised that it was he who enlightened me!). Surprisingly, the FFR value was 0.84. I stopped the procedure and ordered a treadmill test and a myocardial perfusion single photon emission computed tomography (SPECT) scan because I could not believe the FFR results; however, the test results were all negative. Of course, the patient was treated medically. This was my first stunning experience of FFR which changed my daily practice thereafter.
After this impressive anecdotal experience, I started to evaluate the prevalence of “visual functional” mismatch (Figure 1) in a real-world scenario1. Previously, an angiographic substudy of the FAME I trial demonstrated that only 39% of significant (diameter stenosis >50%) angiographic stenosis had an FFR of ≤0.80. We also evaluated this crucial binary point based on our prospective IRIS-FFR registry. Among 1,129 lesions in 1,000 patients, the accuracy of angiographic diameter stenosis was only 66% in non-left main lesions and only 60% in left main lesions. We extended this finding to patients having intravascular ultrasound (IVUS) evaluation. Until then, an IVUS minimal lumen area (MLA) of <4 mm2 was considered the corresponding cut-off value for stent implantation. We found that a more appropriate cut-off value to predict an FFR of ≤0.80 was an IVUS MLA of 2.4 mm2, a figure much smaller than previously reported. More importantly, using even stricter criteria of MLA, 30% of analysed lesions had an MLA <2.4 mm2 but an FFR of >0.80. The overall diagnostic accuracy of MLA was only 69%. More recently, advanced artificial intelligence technology considering various measured and unmeasured factors has improved the diagnostic accuracy of IVUS parameters (80-90%), but is still far from perfect. Therefore, I completely agree with the current guidelines which recommend direct FFR evaluations unless the stenosis is very tight (>90%). In my opinion IVUS and anatomical evaluations cannot replace FFR for deciding on revascularisation and should be used to optimise stenting outcomes. Since then, I have never relied on my eyeball evaluation and have routinely measured FFR.
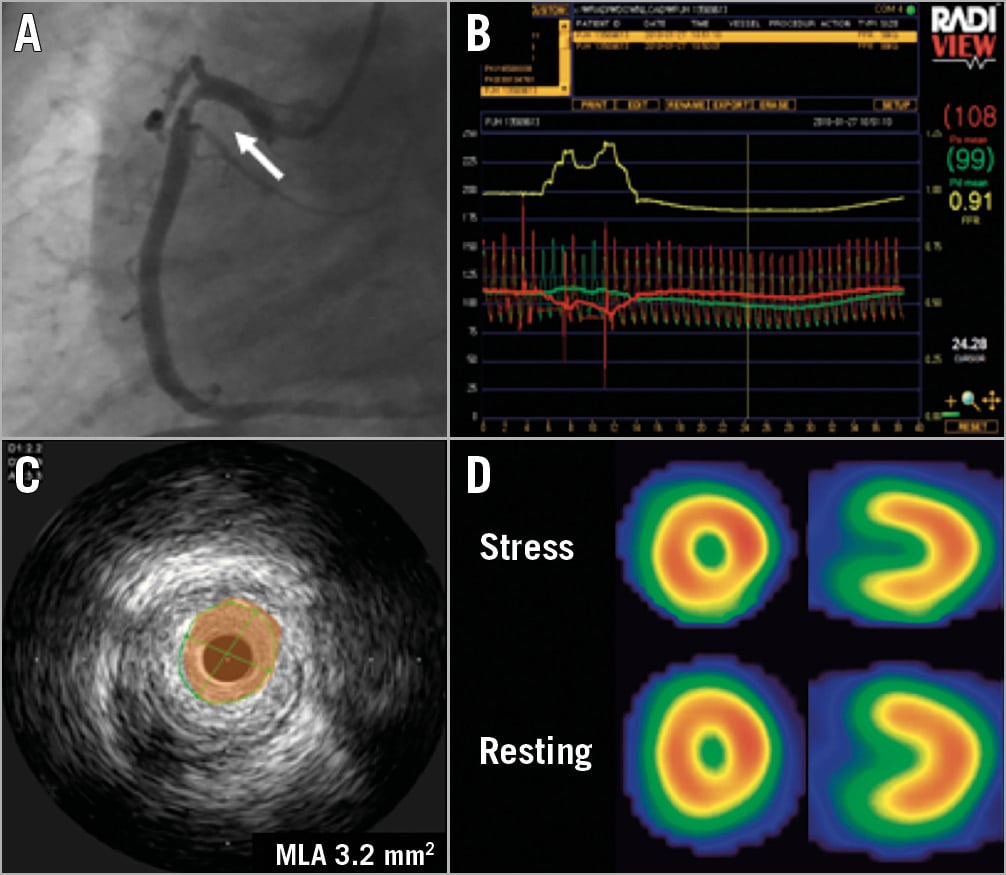
Figure 1. Examples of “visual-functional mismatch”. A) Coronary angiography. B) Fractional flow reserve. C) Intravascular ultrasound. D) Thallium SPECT1.
From January 2010, clinical consensus was reached among percutaneous coronary intervention (PCI) operators at the Asan Medical Center regarding routine FFR measurement prior to stenting in case of no evidence of ischaemia. This was supported by the FAME I trial demonstrating the advantage of FFR-guided PCI over coronary angiography (CAG)-guided PCI in multivessel disease as well as the growing interest in the appropriate use of coronary stenting among various cardiology societies across the world. The FFR penetration rate increased rapidly in our daily PCI practice from 1.9% between 2008 and 2009 (before routine FFR use) to 50.7% between 2010 and 2011 (after routine FFR use). This provided a valuable opportunity to evaluate the overall benefit of FFR-guided PCI in real practice (Figure 2),2. During four years, a total of 5,097 patients underwent PCI in our centre – 2,699 patients before and 2,398 patients after routine FFR use (2,178 pairs after propensity matching). Among patients with routine FFR use, stent implantation rates were reduced from 2.1 stents to 1.5 stents (29% reduction), with a significantly lower incidence of the one-year composite outcomes of cardiac death, myocardial infarction and repeat revascularisation (from 8.6% to 4.8%, p<0.001). This was mainly driven by avoidance of unnecessary stenting and a subsequent decreased risk of periprocedural myocardial infarction and repeat revascularisation. Our registry study extended the practical role of FFR to the real-world setting. This didactic experience convinced me that we were on the right track.
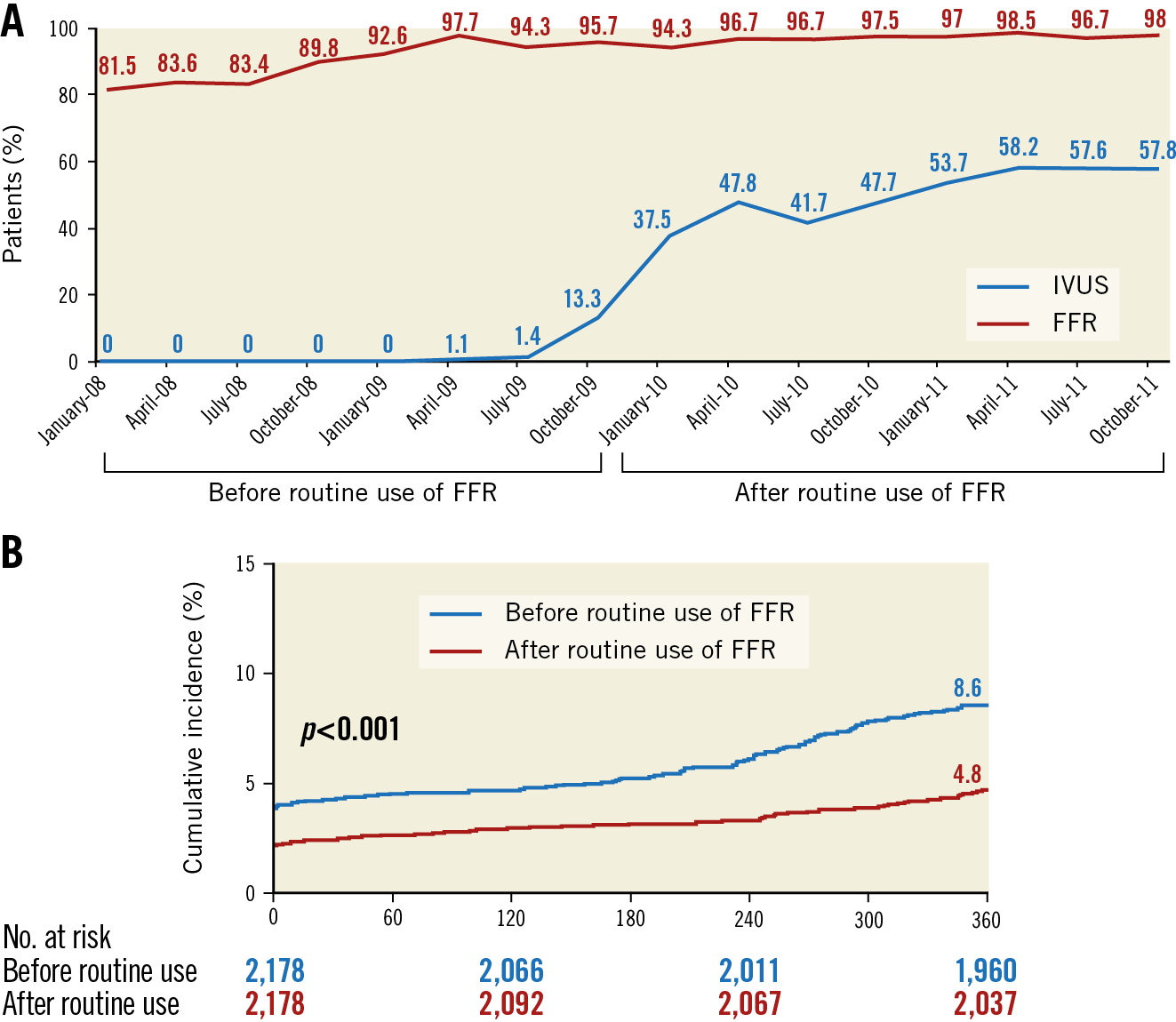
Figure 2. Penetration rate of fractional flow reserve use between 2008 and 2011 in the Asan Medical Center (A), and the temporal trend in outcomes of the composite of death, myocardial infarction, and repeat intervention (B)2.
More interesting changes were observed in patients with severe coronary artery disease such as left main or triple vessel disease, in whom coronary artery bypass graft (CABG) surgery was the preferential treatment strategy. After routine FFR use, the proportion of patients receiving CABG significantly decreased from 54% to 43%. This was probably due to the fact that FFR measurement reduced the complexity of angiographically diagnosed coronary artery disease. When comparing the clinical outcomes between the PCI group and the CABG group, before routine FFR use, the PCI group showed a significantly higher event rate than the CABG group (7.4% versus 4.1%, p=0.029), whereas after routine FFR use the difference in outcomes between the two strategies disappeared (5.5% versus 4.5%, p=0.58)3. PCI outcomes have improved significantly following FFR-guided PCI in severe coronary artery disease, and subsequently became comparable to CABG. I await the results of the FAME 3 trial, comparing FFR-guided PCI with CABG in multivessel disease, with optimism.
Traditionally, non-invasive functional studies have been used in the diagnosis of stable angina by objectively documenting the presence of ischaemia during the myocardial stress test, but it required additional time, resources and costs. The unmet need of “on table” functional assessment led to the development of the concept of FFR. The initial cut-off value of 0.75 was validated against three different non-invasive functional studies. Currently, an FFR of 0.80 has been applied as a revascularisation threshold to avoid a few significant stenoses being left untreated. However, there had been concerns regarding the cut-off value of FFR. We derived the threshold value of FFR for revascularisation decision making using the clinical outcome data from the IRIS-FFR registry. From 5,846 patients, we compared the risk of cardiac events between 6,468 deferred lesions and 2,165 stented lesions according to the FFR value during 1.9-year follow-up4. When the hazard ratio lines were plotted, the lines of risk of deferred lesion events and stented lesion events intersected at an FFR value of 0.79, which meant that lesions with an FFR <0.79 favoured stent implantation and lesions with an FFR >0.79 favoured deferral with medical treatment (Figure 3). The results of this study strongly supported the contemporary cut-off value of FFR and decision-making metrics in the catheterisation lab.
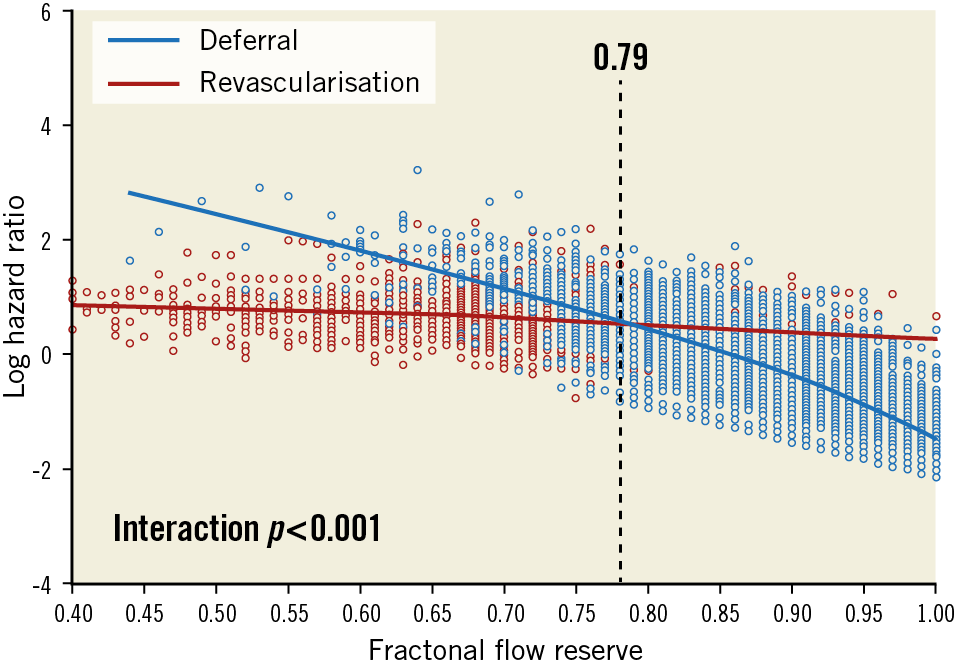
Figure 3. Outcome-derived fractional flow reserve threshold for revascularisation4.
More recently, after two randomised trials showing the non-inferiority of instantaneous wave-free ratio-guided PCI to FFR-guided PCI, several non-hyperaemic pressure ratios (NHPR) were introduced. At present, the physiologic connotations of such indices need critical appraisal; “many” indices appeared to make the coronary physiology world more complex. However, as all indices were numerically equivalent to each other, the treating physician can use any NHPR according to their circumstance, especially when inducing hyperaemia is not easily available or may be inappropriate. This may conceivably become more advantageous for the general propagation of coronary physiology and appropriate stenting.
FFR is now a standard index to guide the decision on revascularisation based on multiple robust clinical trials. As for my current clinical practice, I have tried to validate FFR-guided PCI from concept to real-world practice which arose from a very simple, albeit crucial, question. The positive clinical feedback from FFR-guided PCI outcomes observed in my clinical experience has further reinforced my trust and made me become a firm believer in FFR. Unless new standards emerge and new data redefine the role of FFR, I would measure FFR in my everyday clinical practice. Encouragingly, the adoption of intracoronary physiology has been increasing more rapidly of late in more parts of the world than ever.
Acknowledgements
This research was supported by the Ministry of Trade, Industry & Energy (MOTIE), Korea Institute for Advancement of Technology (KIAT) through the Encouragement Program for The Industries of Economic Cooperation Region.
Conflict of interest statement
The authors have no conflicts of interest to declare.

