
Abstract
Aims: Our aim was to investigate the impact of intravenous (IV) beta-blocker therapy on short-term mortality and other in-hospital events in patients with ST-segment elevation myocardial infarction (STEMI) treated with dual antiplatelet therapy (DAPT) and primary percutaneous coronary intervention (PCI).
Methods and results: Using the nationwide Swedish Web-system for Enhancement and Development of Evidence-based care in Heart disease Evaluated According to Recommended Therapies (SWEDEHEART) registry, we identified all patients with STEMI undergoing PCI between 2006 and 2013. Patients with cardiogenic shock and cardiac arrest at presentation were excluded. The primary endpoint was mortality within 30 days. Secondary endpoints were in-hospital events (mortality, cardiogenic shock and left ventricular ejection fraction [LVEF] <40% at discharge). We adjusted for confounders with a multivariable model and propensity score matching. Out of 16,909 patients, 2,876 (17.0%) were treated with an IV beta-blocker. After adjusting for confounders, the IV beta-blocker group had higher 30-day all-cause mortality (HR: 1.44, 95% CI: 1.14-1.83), more in-hospital cardiogenic shock (OR: 1.53, 95% CI: 1.09-2.16) and were more often discharged with an LVEF <40% (OR: 1.70, 95% CI: 1.51-1.92).
Conclusions: In this large nationwide observational study, the use of IV beta-blockers in patients with STEMI treated with primary PCI was associated with higher short-term mortality, lower LVEF at discharge, as well as a higher risk of in-hospital cardiogenic shock.
Abbreviations
BB: beta-blocker
BP: blood pressure
CABG: coronary artery bypass graft
COPD: chronic obstructive pulmonary disease
CPAP: continuous positive airway pressure
DAPT: dual antiplatelet therapy
HS troponin I: high-sensitivity troponin I
IV: intravenous
LAD: left anterior descending artery
LVEF: left ventricular ejection fraction
MI: myocardial infarction
PCI: percutaneous coronary intervention
PS: propensity score
RCA: right coronary artery
STEMI: ST-segment elevation myocardial infarction
SWEDEHEART: Swedish Web-system for Enhancement and Development of Evidence-based care in Heart disease Evaluated According to Recommended Therapies
Introduction
Beta-blocker therapy in the early phase of myocardial infarction (MI) has been shown to reduce infarct size and improve LVEF and survival over time1,2. Unless contraindicated, guidelines recommend starting beta-blocker therapy in all patients with STEMI within 24 hours and thereafter up-titrating treatment during and after hospitalisation as tolerated2,3. The role of IV beta-blocker therapy is more controversial and less clear. IV beta-blockade was studied nearly 40 years ago in animal models4. Shortly thereafter, Hjalmarson et al randomised patients to IV metoprolol or placebo and demonstrated a 36% reduction in mortality in the group randomised to IV metoprolol followed by oral metoprolol as a monotherapy5. A number of trials followed in both the pre-thrombolytic and thrombolytic era with varying results. Most of these studies investigated a combination of initial IV beta-blocker followed by long-term per oral treatment compared to placebo6-10. The suggested mechanisms for the observed benefit of beta-blockers included a reduction in myocardial oxygen demand and less malignant arrhythmia.
Studies on IV beta-blockade in the modern percutaneous coronary intervention (PCI) era have shown mixed results11-14. The METOCARD CNIC trial (effect of METOprolol in CARDioproteCtioN during an acute myocardial InfarCtion) showed a promising reduction in infarct size with IV beta-blocker therapy. However, these findings could not be replicated in the EARLY-BAMI trial (beta-blocker administration before primary PCI in patients with ST-elevation myocardial infarction trial)12-14.
In Sweden, the use of IV beta-blockers is highly variable in patients with acute myocardial infarction, ranging from 2% to 40% between centres. Most hospitals, however, use it in less than 20% of patients with acute myocardial infarction15.
The objective of this Swedish nationwide registry-based study was to assess the impact of IV beta-blocker therapy on short-term mortality as well as other in-hospital clinical events in patients with STEMI undergoing primary PCI.
Methods
STUDY POPULATION
A total of 39,209 patients with STEMI were enrolled in the SWEDEHEART registry between 2006 and 2013 (Figure 1). Detailed information about the registry and variable definitions is available upon request16. In the present study, patients were excluded if they were not treated with PCI (n=4,390), if they did not receive upstream DAPT before the start of PCI with aspirin and a P2Y12 inhibitor (n=9,948), if they were on regular beta-blocker therapy at the time of hospitalisation (n=7,077) or presented with cardiac arrest or cardiogenic shock (n=801). If patients had more than one admission during the study period, later admissions were excluded (n=49). Patients with incomplete data regarding the use of IV beta-blockers during hospitalisation were also excluded (n=35). The remaining 16,909 patients constituted the final study population and were divided into two groups, those who received IV beta-blockers during hospitalisation (n=2,876) and those who did not (n=14,033). The Regional Ethical Review Board in Lund, Sweden, approved this study.
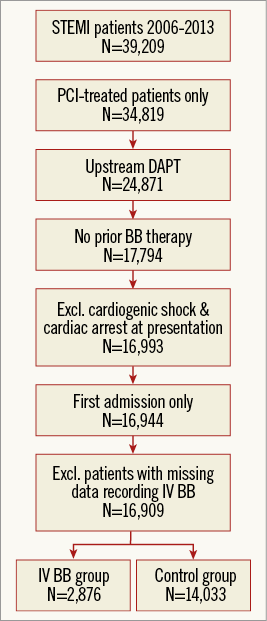
Figure 1. Study flow chart. Patients with STEMI who received upstream DAPT and PCI were included in this study. The numbers of patients remaining after each step of the inclusion and exclusion criteria are shown. BB: beta-blocker
ENDPOINTS
The primary endpoint of this study was all-cause mortality within 30 days from admission. The secondary endpoints were in-hospital events (mortality, cardiogenic shock) and LVEF <40% at a discharge echocardiogram. All subgroup analyses were predefined.
STATISTICAL ANALYSES
A detailed statistical analysis plan is available upon request. There were 7.2% individuals with missing values and they were therefore excluded from the primary analyses. All statistical analyses were performed using Stata version 14.1 for Mac (StataCorp, College Station, TX, USA). A two-sided p-value <0.05 was considered statistically significant.
Results
PATIENT CHARACTERISTICS
Patients treated with IV beta-blockers were younger (64.0 years, IQR 57-74 vs. 66.0 years, IQR 57-75) (Table 1). Comorbidities at presentation were similar in the groups. There was a longer system delay in the IV beta-blocker group; 73.4% of subjects in the IV beta-blocker group presented with a system delay of less than two hours compared to 78.3% in the control group. The IV beta-blocker group had a higher heart rate at presentation (80 bpm, IQR 68-95 vs. 73 bpm, IQR 62-85) as well as higher blood pressures (SBP 150 mmHg, IQR 130-170 vs. 140 mmHg, IQR 121-160). Atrial fibrillation in the presenting ECG was more common in the IV beta-blocker group (5.3% vs. 3.4%). The use of IV beta-blockers was significantly higher at the start of the study period (Figure 2), and 63.9% of IV beta-blocker administrations occurred between 2006 and 2009. The IV beta-blocker group more often presented with LAD lesions than the control group (55.8% vs. 41.6%), less often with right coronary artery (RCA) lesions (25.9% vs. 40.4%) and had higher peak cardiac enzyme values. A total of 1,517 cases and 1,517 controls remained after matching. Analysis on the matched population was performed with complete cases. Differences in patient characteristics after propensity score matching are shown in Table 1. Predictors of IV beta-blocker usage are available upon request.
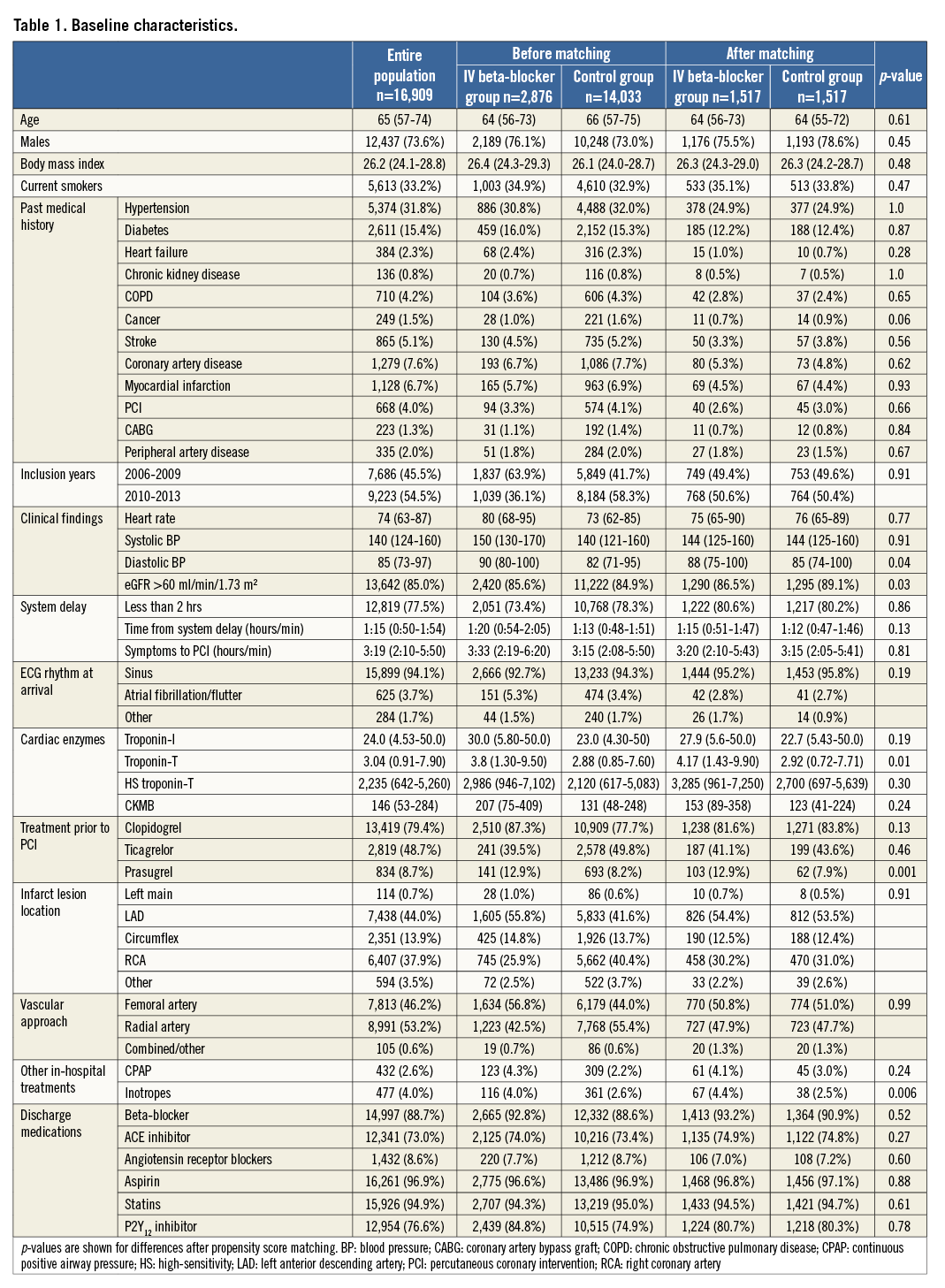
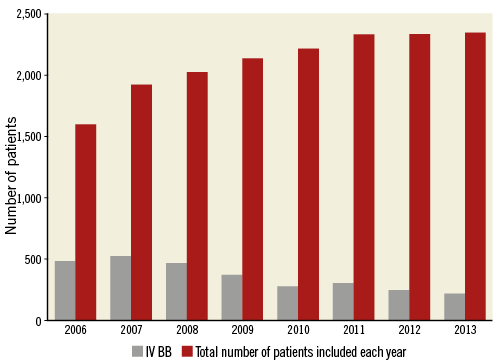
Figure 2. Trend of intravenous beta-blocker (IV BB) administration during the study period.
ENDPOINTS
The Kaplan-Meier event rate for all cause-mortality within 30 days was statistically significantly higher in patients treated with an IV beta-blocker (3.6% compared to 2.6% [unadjusted HR: 1.37, 95% CI: 1.10-1.70]) (Figure 3). Results were consistent and statistically significant after multivariable adjustments and propensity score (PS) matching (Table 2). In-hospital mortality was numerically higher in the IV beta-blocker group (Kaplan-Meier event rates: 2.7% vs. 2.0%, [unadjusted HR: 1.13, 95% CI: 0.88-1.46]). In-hospital cardiogenic shock was more frequent in the IV beta-blocker group (1.9% vs. 1.3%, [unadjusted OR: 1.45, 95% CI: 1.07-1.95]). The proportion of patients discharged with an LVEF <40% was statistically significantly higher in the IV beta-blocker group (31.4% compared to 18.1%, [unadjusted OR: 2.07, 95% CI: 1.88-2.29]). Results of the secondary endpoints were consistent after multivariable adjustment and propensity score matching adjustment except for cardiogenic shock (Table 2).
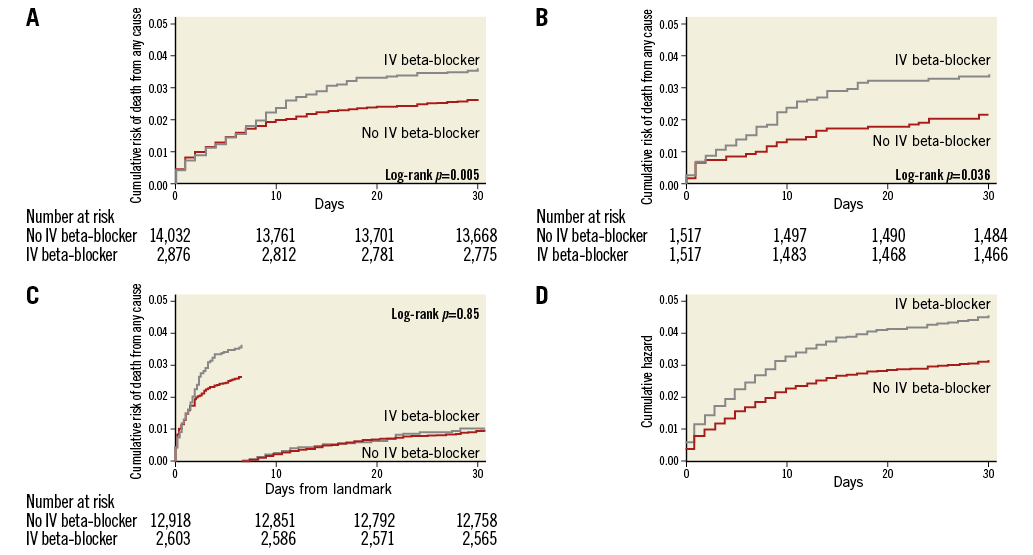
Figure 3. Survival graph for all-cause mortality within 30 days, landmark analysis and Cox proportional regression model. A) Failure estimates for the entire population. B) Failure estimates for the PS-matched population. C) Landmark analysis. D) The cumulative hazard for a Cox proportional regression model. IV: intravenous
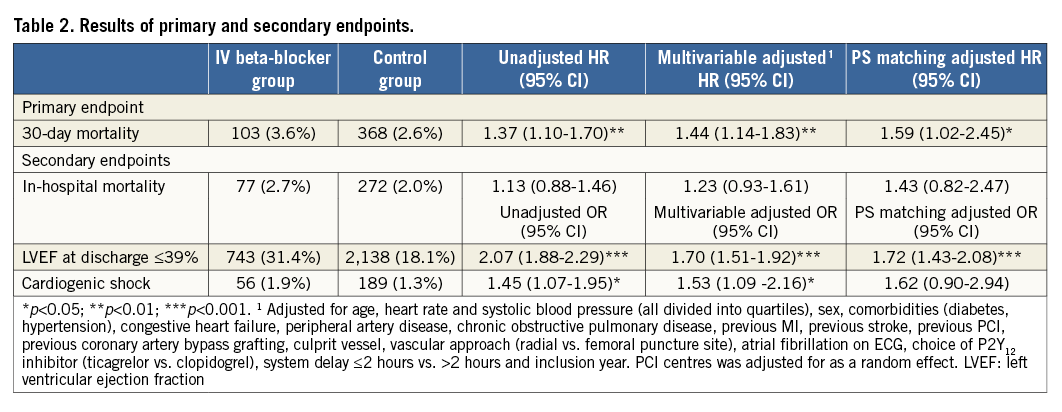
SUBGROUP ANALYSES
Results of the primary endpoint, all-cause mortality within 30 days, were consistent across a wide range of subgroups (Figure 4). A statistically significant p-value for interaction was observed for patients with heart rate ≥100 beats per minute at presentation, with a greater hazard in patients with higher heart rate.
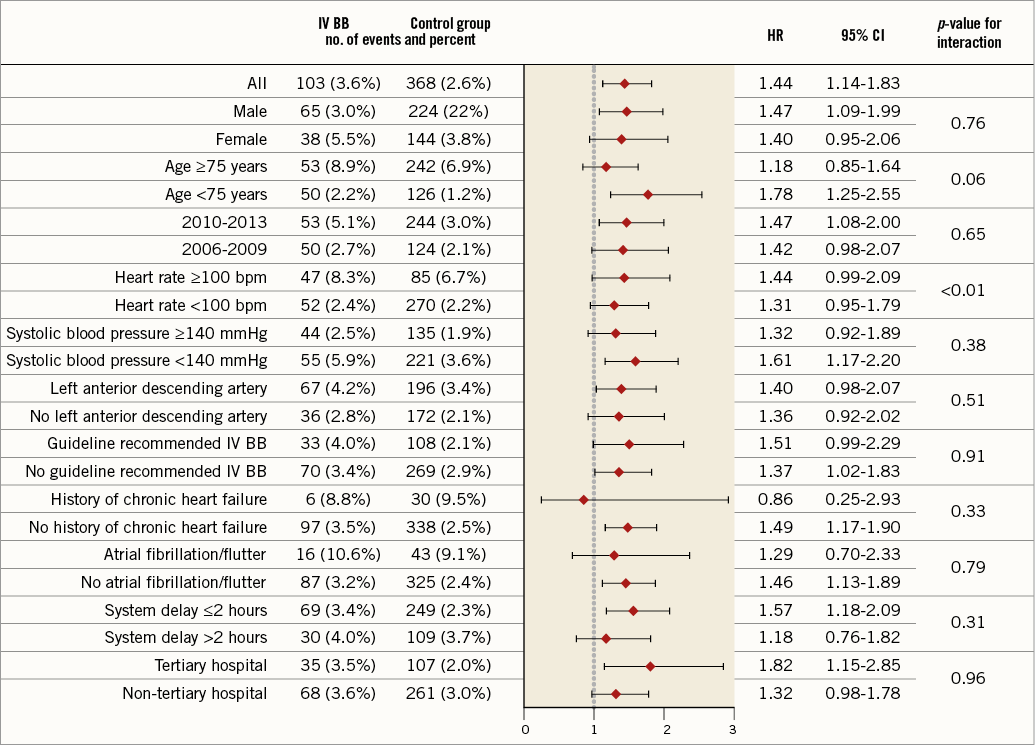
Figure 4. Forest plot. Adjusted hazard ratios are shown for the primary endpoint. All the subgroups were pre-specified.
SENSITIVITY ANALYSIS
A landmark analysis was performed for patients alive 30 days after the initial event, showing no additional association with mortality between days 31-60 in patients receiving IV beta-blocker therapy (unadjusted HR: 1.02, 95% CI: 0.66-1.56, p=0.94) (Figure 3C). Including patients with previous regular beta-blocker treatment in a sensitivity analysis resulted in an associated higher all-cause mortality within 30 days with the use of an IV beta-blocker (unadjusted HR: 1.22, 95% CI: 1.05-1.45, p=0.01). A sensitivity analysis addressing the DAPT inclusion criteria was carried out evaluating the primary endpoint of 30-day mortality in patients who were not pre-treated with DAPT and compared to the current study population. The 30-day mortality was higher in the group of patients that was not pre-treated with DAPT (3.7% vs. 2.8%, p=0.001), indicating a sicker group at baseline. The association of higher mortality with IV beta-blockers remained when including this subpopulation, (3.6% vs. 2.9%, p=0.022 [unadjusted HR 1.241, 95% CI: 1.03-1.49]).
Discussion
In this Swedish nationwide observational study, we found no evidence of benefit with the use of IV beta-blocker therapy in patients with STEMI treated with primary PCI in terms of short-term patient survival or other in-hospital clinical outcomes. After adjustment for confounders with a multivariable model and propensity score matching, the all-cause mortality at 30 days after admission was higher in patients treated with IV beta-blockers compared to the control group. The risk of in-hospital death, cardiogenic shock and LVEF <40% at discharge was statistically significantly higher in the IV beta-blocker group. Results were consistent throughout a number of subgroups.
Current guidelines issued by the European Society of Cardiology as well as the American College of Cardiology Foundation/American Heart Association recommend the consideration of IV beta-blocker treatment as a class II recommendation (level of evidence B) at the time of presentation in patients without contraindications, with high blood pressure, tachycardia and no signs of heart failure2,17. Furthermore, the European Society of Cardiology STEMI guidelines emphasise that it is prudent to wait for the patient to stabilise before starting beta-blocker therapy and preferably to use oral administration, rather than IV. Oral treatment with beta-blockers should be considered during hospitalisation and continued thereafter in all STEMI patients without a contraindication2,17.
Our aim was to investigate the impact of IV beta-blockers in patients with STEMI, treated according to current guidelines. To omit high-risk patients, a core set of exclusion criteria was adopted. Patients already treated with beta-blockers, patients presenting with cardiac arrest or cardiogenic shock and patients who did not receive upstream DAPT were excluded. The rationale behind excluding patients with no upstream DAPT was to exclude patients initially considered unsuitable for invasive intervention. Results were consistent in a sensitivity analysis including patients not pre-treated with DAPT. Our study did not cover the topic of long-term treatment with oral beta-blockers post MI. However, in Sweden oral beta-blockers are usually started within 24-48 hours and up-titrated in-hospital before being prescribed at discharge. In our population, nearly 90% of patients were discharged with a prescription of an oral beta-blocker with a slightly higher rate in the IV beta-blocker group.
STUDY ENDPOINTS
We could not demonstrate any benefit of IV beta-blockers and our results even suggest potential harm. In contrast to the ClOpidogrel and Metoprolol in Myocardial Infarction Trial (COMMIT), our IV beta-blocker group had higher mortality. In accordance with the COMMIT trial, patients treated with IV beta-blockers in our study had a higher incidence of cardiogenic shock than did patients not treated with IV beta-blockers9. We also observed a higher incidence of LVEF <40% on discharge echocardiograms in patients treated with IV beta-blockers, perhaps contributing to the higher 30-day mortality in this group. We believe that the observed higher mortality in our study is most likely due to beta-blocker-induced cardiogenic shock, as suggested by the higher rates of cardiogenic shock and the higher usage of IV inotropic drugs during hospitalisation in the IV beta-blocker group. This could possibly explain the delayed curve diversion in our study, as patients who received IV beta-blocker therapy developed cardiogenic shock with subsequent intensive care treatment to a higher extent, resulting in higher mortality at a later stage of hospitalisation. A similar mortality curve was observed in the MIAMI trial in the subgroup of patients considered a low-risk group6. It is possible that the overrepresentation of LAD infarctions in the IV beta-blocker group manifested in reduced LVEF at discharge and contributed to the observed higher mortality. However, our results remained consistent after adjusting for culprit vessel and, although hidden confounding cannot be ruled out, results were consistent in the PS-matched population, in which rates of LAD infarction were equal between both groups. Furthermore, no difference in outcome was observed in the subgroup analysis of LAD infarctions vs. non-LAD infarctions. In the sensitivity analysis of LVEF <40% at discharge in LAD vs. non-LAD infarctions, IV beta-blocker use was associated with lower LVEF at discharge in both subgroups with no interaction indicating that the association of reduced EF was independent of culprit vessel (Figure 5). Interestingly, the association of decreased ejection fraction at discharge with IV beta-blocker therapy was observed in the COMMIT trial as well as in the GUSTO-1 experience as an increase in patients developing heart failure in the IV beta-blocker group, whereas the opposite was noted in the METOCARD-CNIC trial; patients allocated IV metoprolol had a higher EF at discharge and fewer heart failure hospitalisations9,12,13.
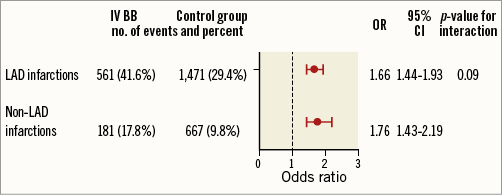
Figure 5. Sensitivity analysis. Adjusted odds ratios are shown for the endpoint LVEF <40% in LAD vs. non-LAD infarctions, showing an associated higher OR for LVEF <40% at discharge with IV beta-blockers in both subgroups with no interaction, indicating that the association of reduced EF was independent of the culprit vessel.
In our study, patients treated with IV beta-blockers were younger, had a comparable comorbidity pattern compared to controls and were equally well treated at discharge. Our results are therefore not likely to be biased by the IV beta-blocker group being frailer, although this cannot be excluded. The higher mortality was not observed in the landmark analyses after 30 days from admission, further suggesting a low rate of residual confounding, although it cannot definitely be ruled out.
In an observational study with data from the Global Registry of Acute Coronary Events (GRACE), Park et al presented data on patients treated between 2000-2007. In this study, only 40% were treated with PCI, and results were in accordance with our study with cardiogenic shock being more prevalent in patients treated with an IV beta-blocker therapy18. In a substudy from the CADILLAC (Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications) trial, the 30-day mortality was significantly reduced with IV beta-blockers. In this study, the IV beta-blocker group was four years younger at baseline, had lower rates of previous myocardial infarction and renal failure and higher rates of LAD infarction. Furthermore, there were significant differences in discharge medications, i.e., oral beta-blocker, ACE-inhibitor and statin prescription rates were significantly higher in the IV beta-blocker group11.
TRENDS IN THE USE OF IV BETA-BLOCKERS
During the study period we observed a steady decline in the use of IV beta-blockers in STEMI patients and an increase in patients treated with PCI and DAPT. This could indicate that clinicians have become more restrictive with IV beta-blocker therapy in favour of watchful waiting for patients to stabilise and slowly up-titrating oral beta-blockers to minimise the risk of cardiogenic shock. This trend was also seen in the GRACE study18.
CURRENT PERSPECTIVES
METOCARD-CNIC and EARLY-BAMI are the most recent trials assessing the effect of early IV beta-blocker administration on myocardial infarct size19. Although the METOCARD-CNIC trial did show reduced infarct size in patients who received an IV beta-blocker, only patients with anterior infarctions were included and a trend towards larger myocardium at risk was also observed in the control group. The EARLY-BAMI trial on the other hand failed to show any superiority with preprocedural IV beta-blocker therapy and with no association of any adverse events14.
The limited available data from contemporary trials thus show conflicting results. Our study shows no clinical improvement with IV beta-blocker therapy in STEMI patients undergoing primary PCI, results in accordance with the EARLY-BAMI trial. In addition, we observed potential harm from IV beta-blocker use, most likely due to beta-blocker-induced cardiogenic shock. IV beta-blocker therapy in STEMI patients undergoing primary PCI should perhaps be reserved for selected patients only, although this would require a larger randomised trial to confirm.
Limitations
Because of this study’s observational design, residual confounding and indication bias cannot be ruled out. Despite neutralising most of the baseline differences with PS matching, remaining baseline differences were still observed. As with all observational studies, hidden confounding may have affected the results. Since IV beta-blocker treatment decreased over time, time bias may have affected our results in favour of the control group. This is illustrated with the radial puncture approach and switching to ticagrelor, which were both less common in the IV beta-blocker group. Although we adjusted for both inclusion year and vascular approach, there could remain unmeasured time-dependent period effects biasing the results. Therefore, no definite conclusion of causality can be drawn and the results should be seen as hypothesis-generating. The exclusion of patients on previous regular beta-blocker treatment has been used in other beta-blocker studies, i.e., the METOCARD-CNIC trial; however, it cannot be excluded that this might have resulted in increased rates of patients with relative or absolute contraindication to beta-blockers in our population, as supported by the slightly lower HR in the sensitivity analysis. A more specific limitation is the lack of information regarding the exact time and dose of IV beta-blocker administered.
Our study has a number of strengths. It is a nationwide study, including nearly 17,000 patients treated according to up-to-date guidelines with PCI and upstream DAPT, and was retrieved from a validated large population-based registry.
Conclusions
In this large nationwide Swedish observational study, the use of IV beta-blockers in patients with STEMI treated with primary PCI was associated with higher short-term mortality, lower LVEF at discharge as well as a higher risk of in-hospital cardiogenic shock. A clinical trial investigating these questions, as relevant today as they were almost 40 years ago, is therefore needed. Until then, routine IV beta-blocker administration is questionable and should perhaps be reserved to carefully selected patients.
| Impact on daily practice Intravenous beta-blockers are still commonly used for STEMI patients treated with PCI despite the lack of solid evidence of benefit. Our registry results indicate a lack of benefit and even a potential risk of harm. Randomised studies regarding the use of IV beta-blockers in patients with STEMI revascularised with PCI are warranted. Until then, routine IV beta-blocker administration should perhaps be reserved to carefully selected patients. |
Acknowledgements
The authors would like to thank the staff members at all coronary care units in Sweden for their help and cooperation in contributing data to the Swedish Web-system for Enhancement and Development of Evidence-based care in Heart disease Evaluated According to Recommended Therapies (SWEDEHEART) system.
Funding
This work was supported by The Swedish Heart and Lung Foundation, Swedish Scientific Research Council, SSF (TOTAL-AMI), Knut and Alice Wallenbergs Foundation, ALF and Skane University Hospital funds.
Conflict of interest statement
P. Andell has received speaker honoraria from AstraZeneca. D. Erlinge has received speaker honoraria from AstraZeneca. T. Jernberg has received lecture and consultant fees from AstraZeneca and Aspen. S. Koul has received speaker honoraria from Pfizer, BMS and AstraZeneca. H. Persson has received lecture fees from Novartis. J. Spaak has received speaker honoraria from MSD, Hospira Nordic, ResMed, Baxter, Astellas Pharma and Medtronic. The other authors have no conflicts of interest to declare.

