
Introduction
The potential of β-blockers to limit myocardial necrosis was proposed long ago, but their cardioprotective capacity during STEMI has been disputed.
The EARLY-BAMI (Early Intravenous Beta-Blockers in Patients With ST-Segment Elevation Myocardial Infarction Before Primary Percutaneous Coronary Intervention) trial randomised 683 STEMI patients to either i.v. metoprolol or placebo before primary PCI (PPCI). It was previously reported that metoprolol did not reduce infarct size1. In the current paper, we report the one-year clinical outcome of the EARLY-BAMI trial (EudraCT no: 2010-023394-19).
Methods
The study design has been published previously2. The EARLY-BAMI trial was a double-blind, placebo-controlled randomised clinical trial.
Patients >18 years of age with symptoms of acute STEMI for >30 minutes but <12 hours, plus ST-segment elevation >1 mV in two adjacent electrocardiogram leads or new left bundle branch block were eligible for enrolment. Exclusion criteria were Killip class III and IV, systolic blood pressure (BP) <100 mmHg, heart rate <60 beats/min, type II and III atrioventricular block.
The protocol included one-year follow-up for all patients regarding major adverse cardiac events (MACE), defined as the pre-specified composite of either cardiac death, non-fatal myocardial infarction, or revascularisation.
Results
Between February 2012 and November 2015, a total of 683 patients were randomised to metoprolol (n=336) or placebo (n=347). Mean age was 62±12 years and 75% were male. The median time from first study dose to reperfusion was 54 min (32-72 min). One-year follow-up was obtained in 629 patients (92%). Mean follow-up time was 374±20 days in the metoprolol group vs. 373±23 days in the placebo group. Metoprolol did not cause an increased risk of either shock/heart failure or severe bradycardia on admission. The incidence of MACE at one year was 7.7% in the metoprolol group vs. 7.3% in the placebo group, p=0.835, CI: 1.07 (0.59-1.93).
Kaplan-Meier curves concerning MACE are shown in Figure 1, demonstrating no differences between the treatment groups.
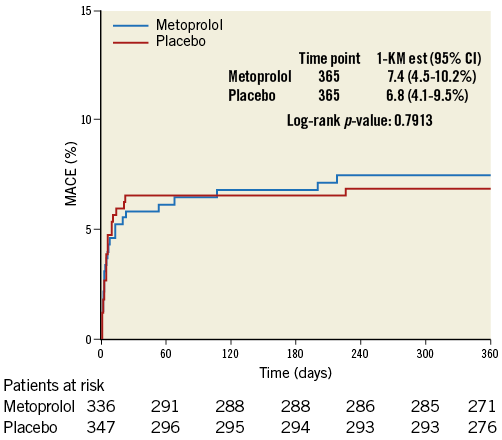
Figure 1. Kaplan-Meier curve for MACE by treatment group.
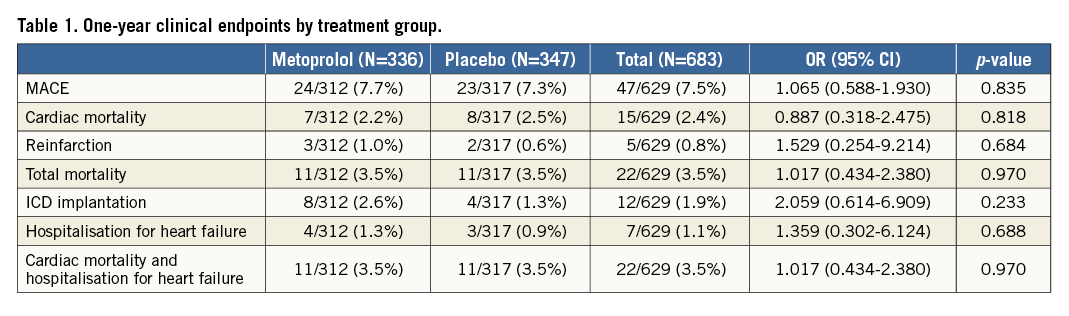
The rate of MACE and other endpoints (hospitalisation for heart failure and indication for internal cardiac defibrillator [ICD]) are listed in Table 1.
Subgroup analyses concerning MACE in pre-specified subgroups also showed no difference between the metoprolol and placebo groups (Figure 2). The MACE rate between very early presenters (<3 hours) compared to patients presenting later (>3 hours) was also comparable: 20/225 (8.9%) in the metoprolol group vs. 17/218 (7.8%) in the placebo group in very early presenters (p=0.68), compared to 1/63 (1.6%) and 5/65 (7.7%) in late presenters (p=0.21).
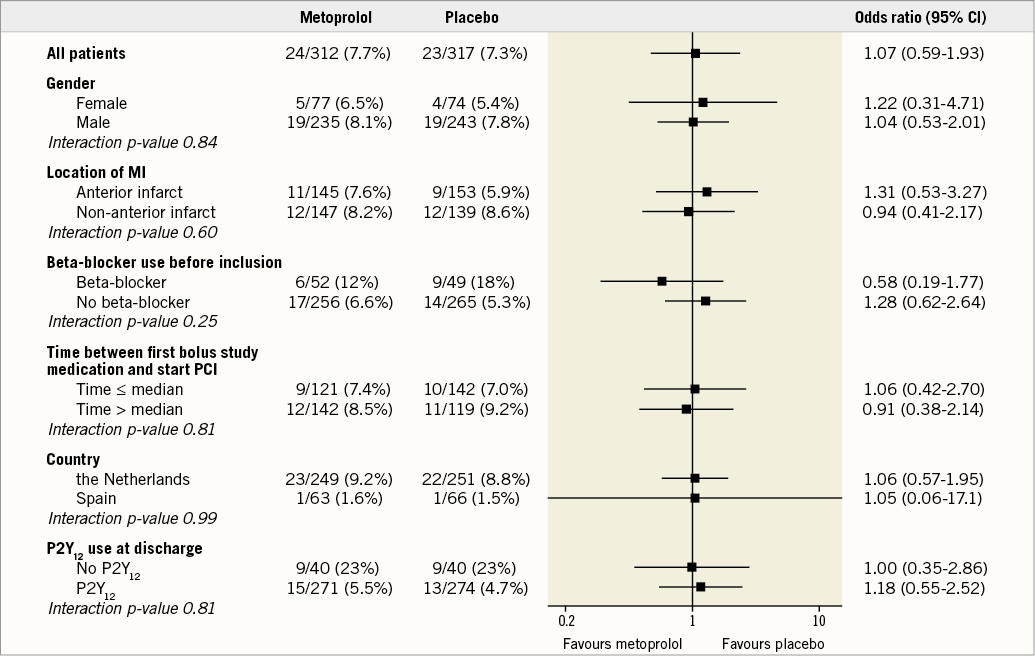
Figure 2. MACE in pre-specified subgroups.
Discussion
This planned secondary analysis of the EARLY-BAMI trial presenting the one-year clinical follow-up demonstrated no difference in MACE, re-admission for heart failure or ICD implantation. These results are in line with the previously reported lack of difference in the primary endpoint (infarct size) and suggest that metoprolol administered at the time and the dose used has neither a beneficial nor a harmful effect on long-term prognosis.
Early administration of a beta-blocker before reperfusion is still controversial because of conflicting results of the COMMIT (Efficacy and Safety of Adding Clopidogrel to Aspirin or Use of Metoprolol in Myocardial Infarction) trial, the METOCARD-CNIC (Effect of METOprolol in CARDioproteCtioN During an Acute Myocardial InfarCtion) trial and our EARLY-BAMI trial1,3,4.
In the COMMIT CCS-2 trial (n=45,852), metoprolol 3×5 mg intravenously followed by oral administration up to four weeks did not improve survival in STEMI patients4. However, this was mainly caused by a higher incidence of cardiogenic shock in patients treated by early beta-blocker, possibly due to inclusion of patients with heart failure. In the current era of PPCI, the METOCARD-CNIC trial showed reduced infarct size and increased LV ejection fraction in STEMI patients treated with early intravenous metoprolol3. However, this study had a relatively small sample size (N=270), was neither blinded nor placebo-controlled, and included a selected patient group (anterior STEMI presenting <6 hours from symptom onset). The EARLY-BAMI trial included all patients with STEMI, with a double-blind, placebo-controlled design, and could not confirm the results of METOCARD-CNIC. One possible explanation could be the difference in infarct size between the two trials: the smaller the infarction size, the less likely an additional treatment effect can be demonstrated. The average infarct size (infarcted myocardium, % LV) in the METOCARD-CNIC trial was 21.2% in patients treated with i.v. metoprolol, compared to 15.3% in the EARLY-BAMI trial. Another explanation could be the dose of metoprolol. In METOCARD-CNIC, the dose was higher: up to three times 5 mg (15 mg target dose), compared to two times 5 mg (10 mg target dose) in EARLY-BAMI. A third explanation could be that 18.8% of patients in the EARLY-BAMI trial were on long-term beta-blocker treatment before admission, whereas long-term beta-blocker treatment was an exclusion criterion in the METOCARD trial. A fourth explanation could be that early beta-blocker treatment may only be beneficial in patients with anterior infarction location, and less beneficial or even harmful in patients with an inferior location, although this is not confirmed by the sub-analysis of EARLY-BAMI1. A final potential reason is the timing of metoprolol administration: a sub-analysis of the METOCARD-CNIC trial5 showed that only patients receiving i.v. metoprolol long before reperfusion had a reduction in infarct size. In the EARLY-BAMI trial, the second 5 mg bolus was administered per protocol immediately before catheterisation in the cathlab.
Limitations
The most important limitation of the EARLY-BAMI trial is that the study was not powered with respect to clinical outcome, particularly with the smaller than expected infarct size and the small number of event rates. Second, there is a wide CI for the treatment comparison in terms of the MACE rate: 1.07 (0.59-1.93). This rules out neither important benefit nor harm and makes it difficult to conclude whether i.v. metoprolol in STEMI patients is completely ineffective or not. Despite these limitations, the EARLY-BAMI trial is still the largest trial of its kind, with pre-hospital administration of i.v. beta-blockade in STEMI patients with a double-blind, placebo-controlled design.
Based on our data and the conflicting results with regard to previous trials, additional randomised trials are needed to clarify whether early pre-reperfusion beta-blocker treatment has any effect in these patients. The safety profile and low cost encourage the performance of additional larger trials in this regard.
Conclusions
In a non-restricted STEMI population undergoing PPCI, early pre-hospital i.v. metoprolol before reperfusion caused no harm, but did not result in a reduction in clinical events at one year. The study was not powered with respect to clinical outcome. With a wide CI for the treatment comparison in terms of the MACE rate, we can rule out neither small benefits nor harm, especially in the subgroups.
| Impact on daily practice Early diagnosis and treatment have improved outcome in patients with STEMI. However, additional treatment strategies might improve outcome further. Beta-blockers have been proposed to limit myocardial necrosis and might have a cardioprotective capacity during STEMI. We have demonstrated that early beta-blocker administration in STEMI does not reduce infarct size, MACE or rehospitalisation for heart failure. However, early beta-blocker administration in the dose used in EARLY-BAMI is not harmful. Beta-blockers after reperfusion therapy still have a class IIa recommendation in the STEMI guidelines. |
Funding
Trial funding came from a research grant from the Dutch Heart Foundation (Utrecht, the Netherlands, no. 2010B125) and an unrestricted grant from Medtronic (Heerlen, the Netherlands), which was used for additional analyses.
Conflict of interest statement
The authors have no conflicts of interest to declare.

