Abstract
Background: The Second Primary Angioplasty in Myocardial Infarction (PAMI-II) risk score is recommended by guidelines to identify low-risk patients with ST-elevation myocardial infarction (STEMI) for an early discharge strategy.
Aims: We aimed to assess the safety of early discharge (≤2 days) for low-risk STEMI patients treated with primary percutaneous coronary intervention (PCI).
Methods: Using nationwide data from the SWEDEHEART registry, we identified patients with STEMI treated with primary PCI during the period 2009-2017, of whom 8,092 (26.4%) were identified as low risk with the PAMI-II score. Low-risk patients were stratified according to their length of hospital stay (≤2 days vs >2 days). The primary endpoint was major adverse cardiovascular events (MACE, including death, reinfarction treated with PCI, stroke or heart failure hospitalisation) at one year, assessed using a Cox proportional hazards model with propensity score as well as an inverse probability weighting propensity score of average treatment effect to adjust for confounders.
Results: A total of 1,449 (17.9%) patients were discharged ≤2 days from admission. After adjustment, the one-year MACE rate was not higher for patients discharged at >2 days from admission than for patients discharged ≤2 days (4.3% vs 3.2%; adjusted HR 1.31, 95% confidence interval [CI]: 0.92-1.87, p=0.14), and no difference was observed regarding any of the individual components of the main outcome. Results were consistent across all subgroups with no difference in MACE between early and late discharge patients.
Conclusions: Nationwide observational data suggest that early discharge of low-risk patients with STEMI treated with PCI is not associated with an increase in one-year MACE.
Introduction
An increasing length of hospital stay for ST-elevation myocardial infarction (STEMI) patients is associated with elevated hospital costs and increased morbidity and mortality12. The introduction of effective mechanical reperfusion and the improvements that have been made with regard to evidence-based treatment strategies have resulted in a decrease in early mortality3 and number of bed days456.
Small randomised clinical trials78910, as well as observational studies1112, have tried to assess the optimal length of hospital stay in patients with STEMI. The Second Primary Angioplasty in Myocardial Infarction (PAMI-II) risk score is a clinical tool that can be used to assess candidates for an early discharge strategy and is based on a clinical trial that randomised 471 low-risk STEMI patients (age <70 years, left ventricular ejection fraction [LVEF] >45%, one- or two-vessel disease undergoing successful percutaneous coronary intervention [PCI] with no persistent arrhythmias). The trial showed that early discharge is a safe alternative to traditional length of care1. Based on these studies, early discharge within 72 hours carries a Class IIa recommendation from the European Society of Cardiology13. Although the PAMI-II score is based on patients with STEMI who received PCI, it was introduced in the late 1990s and has only been validated in small observational studies1114.
The objective of this study was to assess whether low-risk STEMI patients treated with PCI may be safely discharged within two days from admission in a contemporary, nationwide setting using data from the Swedish quality registry of coronary care, SWEDEHEART (Swedish Web system for Enhancement and Development of Evidence–based care in Heart disease Evaluated According to Recommended Therapies).
Methods
Data source
All consecutive patients with symptoms suggestive of an acute coronary syndrome admitted to a coronary care unit or coronary catheterisation laboratory in Sweden are recorded prospectively in the SWEDEHEART registry15. The registry collects information on background characteristics, in-hospital examinations, coronary angiographic findings and PCI-related measures, complications, discharge medications and diagnoses. A personal identification number, unique to every Swedish citizen, allows merging with other nationwide registries. We linked the SWEDEHEART registry to the National Patient Registry and the National Population Registry to obtain data on comorbidities and date of death.
PAMI-II calculation
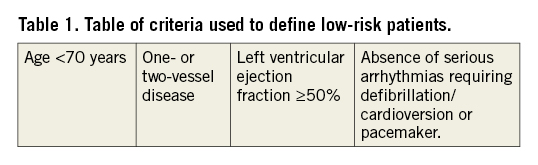
Data concerning age, revascularisation, arrhythmias and LVEF were available in the SWEDEHEART registry. LVEF is documented as ≥50%, 40-49%, 30-39% and ≤30% in the registry, barring us from using similar LVEF cut-offs to those in the PAMI-II risk score (>45%). Therefore, we used LVEF ≥50% as a low-risk criterion whereby we probably identified fewer patients in this group, but as a more robust low-risk feature. In the PAMI-II score, persistent arrhythmia is defined as arrhythmia that requires treatment with a pacemaker or lidocaine infusion. Today, lidocaine is not widely used as an antiarrhythmic drug. We therefore defined persistent arrhythmias as any serious arrhythmia requiring cardiopulmonary resuscitation, defibrillation, cardioversion or pacemaker. Arrhythmias treated medically with amiodarone or other antiarrhythmic drugs are not registered in SWEDEHEART. An overview of the criteria applied to define low-risk patients is shown in Table 1.
Study population and design
We included all patients with STEMI between January 2009 and April 2017 (Figure 1). Only initial admissions during the study period were included in this report; we excluded patients presenting with cardiogenic shock or resuscitated cardiac arrest. To adhere to the PAMI-II criteria, only STEMI patients who were treated with PCI and underwent transthoracic echocardiography were included in the analysis. The PAMI-II risk score was applied, categorising 8,092 patients as low risk and this comprised the final study population. Seven patients had at least one missing variable and in these the PAMI-II risk score could not be evaluated. These patients were excluded from this study. Patients categorised as low risk according to the PAMI-II risk score were stratified according to their length of hospital stay, as ≤2 days (early discharge group; left censored at 0 days) and ≥2 days (late discharge group; right censored at y time). The Regional Ethical Review Board in Lund approved the study (approval id: 2015/297). This study adheres to the STROBE criteria (Supplementary Appendix 1).
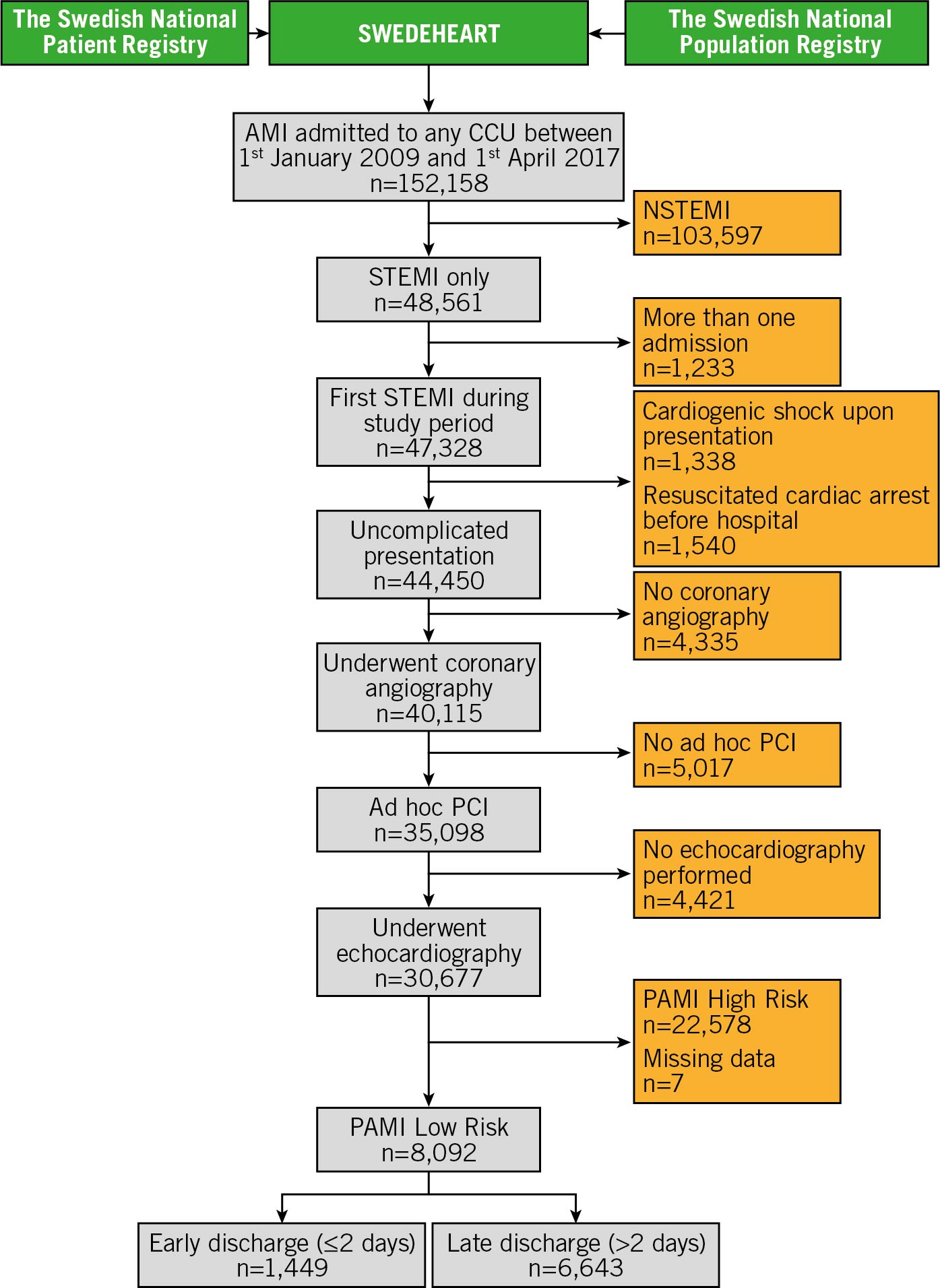
Figure 1. Study flow chart. Patients with ST-segment elevation myocardial infarction (STEMI) treated with percutaneous coronary intervention (PCI) were included in this study. All patients had to undergo echocardiography during hospitalisation. The PAMI-II risk score was used to identify low-risk patients as candidates for early discharge. Low-risk patients were subsequently stratified according to length of hospital stay as early discharge within 2 days from admission or late discharge with a hospital stay exceeding 2 days. Shown are the numbers of patients remaining after each step of the inclusion and exclusion criteria.
Outcomes
The main outcome was a major adverse cardiac event (MACE) at one year defined as the first occurrence of (1) all-cause mortality, (2) PCI-treated myocardial infarction (MI), (3) hospitalisation for decompensated heart failure (HF), or (4) stroke. Using each patient’s unique health record number, censorship dates and death status were ascertained by the National Board of Health and Welfare by deterministic linkage to the National Population Registry. Similar linkages were undertaken to enable estimation of rates of admission with stroke and HF from the National Patient Registry, whereas PCI-treated MI was extracted directly from the SWEDEHEART registry (without linkage) and was defined as a subsequent entry with a discharge diagnosis of MI treated with PCI. In secondary analyses, the association between early discharge and each component of the main outcome was explored. Follow-up was calculated from day of admission and all outcomes were ascertained up to April 2018 with complete follow-up of all patients.
Subgroup analyses
We explored the rates of the main endpoint in men versus women, age ≥60 versus <60 years, weights equal to and above versus below the cohort median of 84 kg, and the presence of known diabetes mellitus, hypertension, history of MI and number of diseased coronary arteries versus the absence of these factors. Two landmark analyses were conducted to test the robustness of the results: first, a landmark analysis assessing one-year MACE rates from discharge, and second, a 30-day landmark analysis from discharge to assess one-year MACE rates in patients discharged within two days compared to later discharge.
Statistical analyses
Baseline characteristics are presented as medians with interquartile range for continuous variables, counts with percentages for categorical variables. Differences between groups were calculated with the Mann-Whitney U test and the chi-squared test. The incidence of MACE together with the other outcome measures was calculated using Kaplan-Meier estimates. The association between early and late discharge and the hazards of MACE and the individual components of MACE was estimated using propensity score-adjusted Cox proportional hazards models with bootstrap resampling and are presented as hazard ratios (HR) with 95% CI. As the length of hospital stay was not randomly decided, we used two different methods to account for confounding and indication bias. The first method was a propensity score-adjusted Cox regression model. Propensity scores were computed from a logistic regression model predicting length of hospital stay. The covariates that were entered into this model were age, sex, creatinine, calendar year, diabetes, history of coronary artery disease (including any history of MI, PCI or coronary artery bypass grafting [CABG]) and in-hospital use of intravenous diuretics; the latter was added to the model due to differences observed between groups. All models had to pass the proportional hazards assumptions of Cox regression by assessment of Schoenfeld’s residuals. The second method was an inverse probability weighting propensity score analysis. This method estimates the average treatment effects (ATE) and the average treatment effect on the treated (ATET) by calculating the propensity score for hospital stay (using the same covariates as in model 1) and derives the inverse probabilities used to balance the covariate distribution. The only variable with missing values was creatinine, which had 245 (3.1%) missing values; due to the low missingness rate, no imputation was undertaken. Given the potential for type I error due to multiple testing, secondary analyses and subgroup analyses should be interpreted as exploratory and hypothesis-generating. All statistical analyses were performed using Stata version 14.1 for Mac (StataCorp). A two-sided p-value <0.05 was considered statistically significant.
Results
Patient characteristics
Among the 30,677 patients with uncomplicated STEMI treated with primary PCI and undergoing transthoracic echocardiography during hospital stay, the PAMI-II risk score identified 8,092 (26.4%) patients as low risk. Reasons for not being considered as having a low risk according to the PAMI-II score were age ≥70 years (58.3%), LVEF <50% (67.4%), persistent arrhythmia (7.6%), multivessel disease (26.8%), and successful PCI not achieved (<1%). Patients not considered low risk had a significantly higher risk of MACE within one year, 4,956 (22.0%) compared to 329 (4.1%) for individuals identified as low risk (unadjusted HR 6.00, 95% CI: 5.36-6.70, p<0.001) (Supplementary Figure 1).
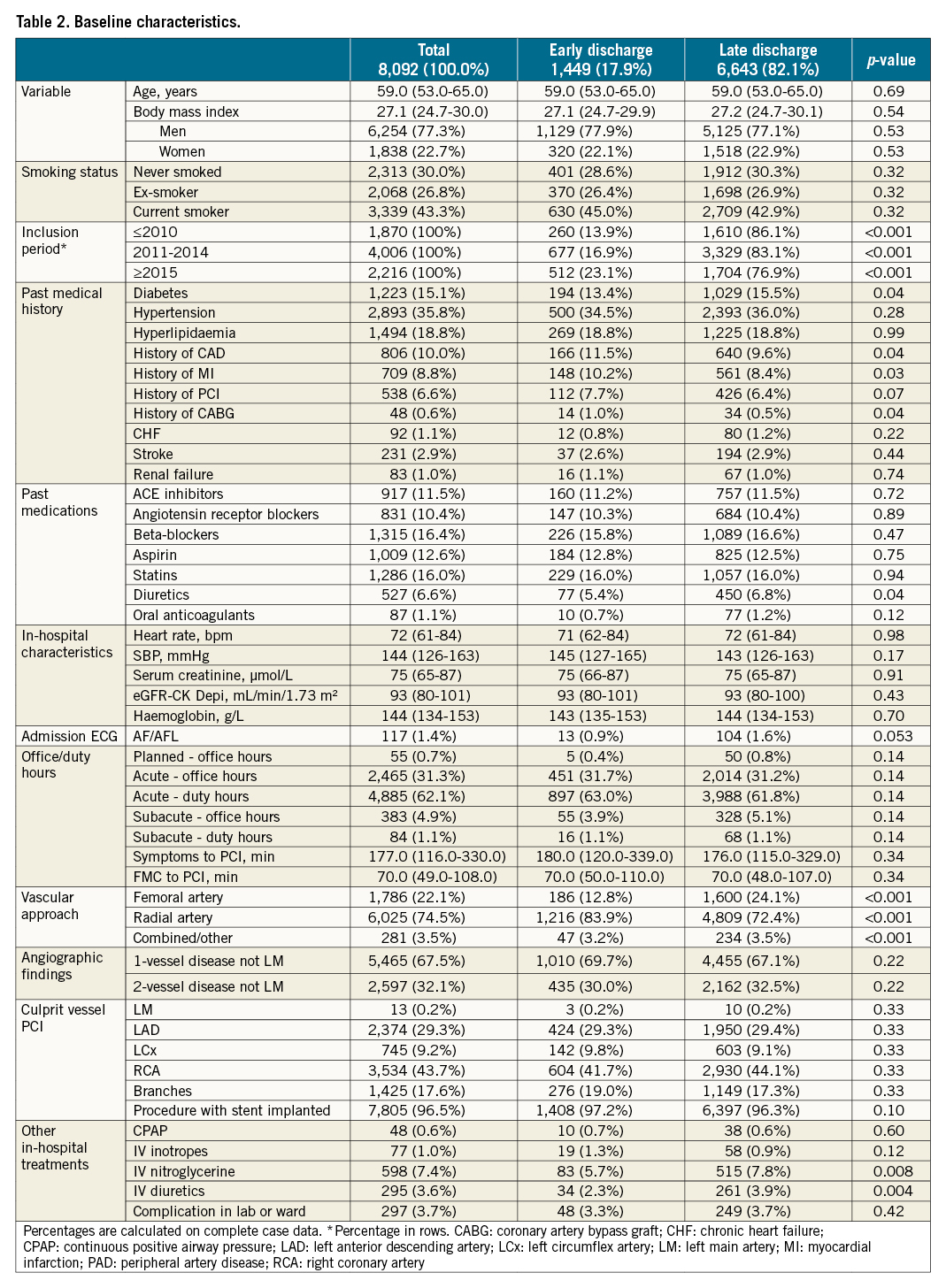
In total, 1,449 (17.9%) patients considered as low risk with the PAMI-II score were discharged within two days from admission (early discharge group) and 6,643 (82.1%) had a longer hospital stay (late discharge group). Differences in patient characteristics are shown in Table 2. Early discharge was more frequent during the last three years of the study period. Patients in the early discharge group were of similar age. Comorbidities and medications at presentation were also similar in the early and late discharge groups (Table 2). A radial vascular approach was more common in the early discharge group (83.9% vs 72.4%, p<0.001). Significant differences in length of hospital stay were observed among different centres (Supplementary Figure 2).
Comparison of early discharge and late discharge
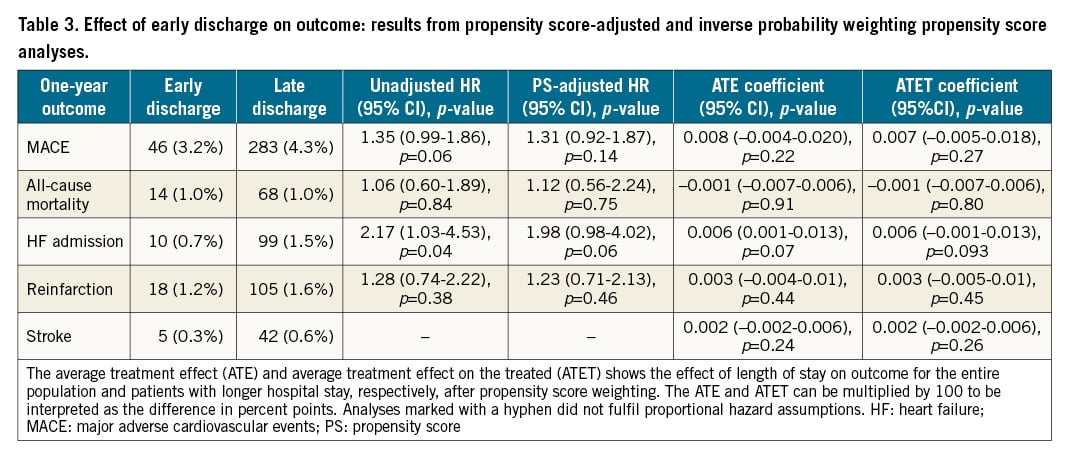
The two groups were well balanced in their baseline characteristics before propensity score weighting. After adjustment, the one-year MACE rate was not higher for patients discharged at >2 days from admission than for patients discharged ≤2 days from admission (4.3% vs 3.2%; adjusted HR 1.31, 95% CI: 0.92-1.87, p=0.14) (Figure 2, Central illustration). The one-year rate of all-cause mortality was not higher for patients discharged >2 days from admission than for patients discharged ≤2 days of admission (1.0% vs 1.0%; adjusted HR 1.12, 95% CI: 0.56-2.24, p=0.75), PCI-treated reinfarction (1.2% vs 1.6%; adjusted HR 1.23, 95% CI: 0.71-2.13, p=0.46), hospitalisation due to HF (1.5% vs 0.7%; adjusted HR 1.98, 95% CI: 0.98-4.02, p=0.06), or stroke (0.3% vs 0.6%), for which proportional hazard assumptions were not fulfilled (Table 3). Similar results were observed for unadjusted analyses as well as for short-term outcomes (Supplementary Table 1). After inverse probability weighting and adjustment, there were no outcome differences between patients with short hospital stay compared to longer stay regarding any outcome measure for both 30-day and one-year outcomes (Table 3).
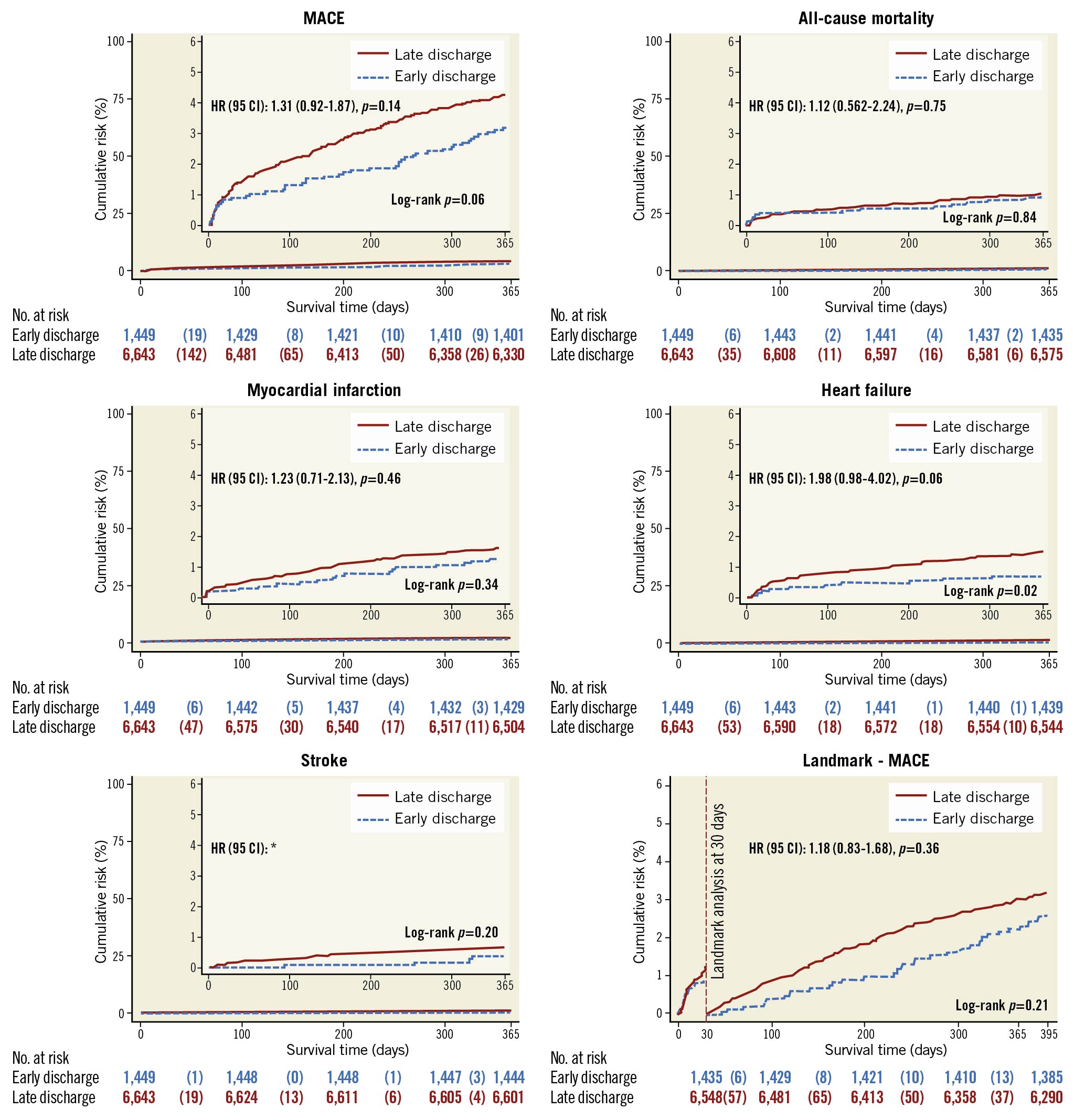
Figure 2. Kaplan-Meier failure estimate graphs for the primary endpoint, secondary endpoints and landmark analysis. A) Cumulative incidence and propensity score-adjusted hazard ratio of the composite primary endpoint of major adverse cardiovascular events (MACE) consisting of first event of death, reinfarction treated with PCI, stroke or hospitalisation due to heart failure in low-risk patients categorised according to length of hospital stay. No differences were observed between the early discharge and longer hospital stay groups. B- E) The cumulative incidence and propensity score-adjusted hazard ratio of each component of the primary endpoint in low-risk patients categorised according to length of hospital stay. F) The cumulative incidence and propensity score-adjusted hazard ratio of MACE after the landmark at 30 days from discharge for low-risk patients categorised according to length of hospital stay. * Proportional hazard assumptions were not fulfilled.
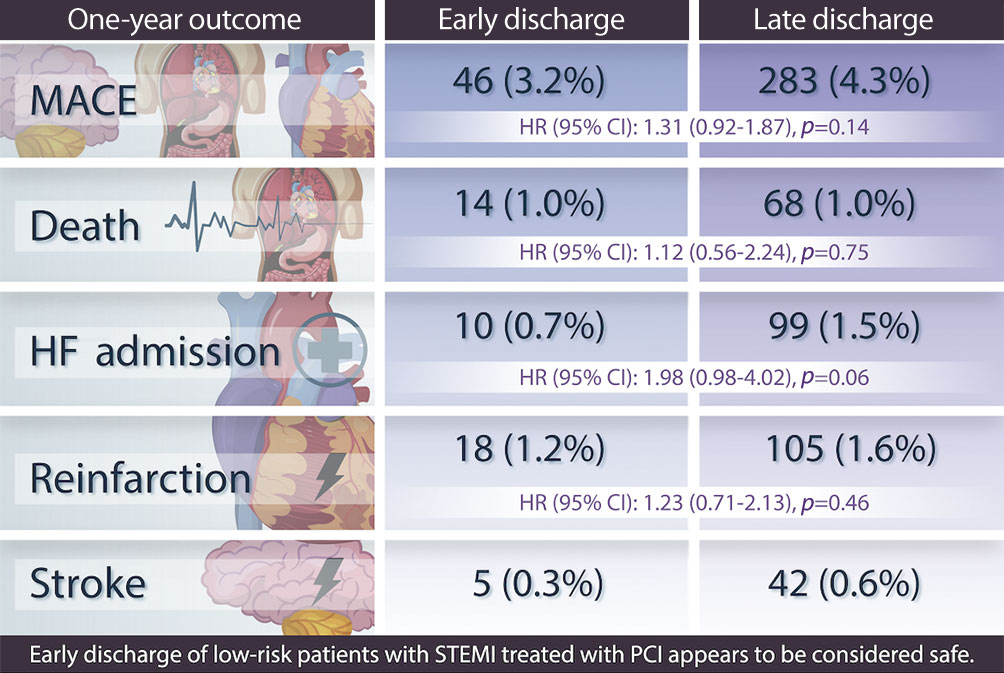
Central illustration. Early discharge of low-risk patients with STEMI treated with PCI appears to be safe.
Subgroup, sensitivity and landmark analyses
Results of the primary endpoint, MACE within 365 days, were consistent across all subgroups with no difference in MACE between early and late discharge patients (Figure 3). Early discharge was not associated with a higher incidence of one-year MACE in the landmark analyses at discharge or after 30 days from admission (Figure 2, Supplementary Figure 3). There were no differences in MACE rates in a separate sensitivity analysis stratified according to admission year into ≤2010, 2011-2014 and ≥2015.
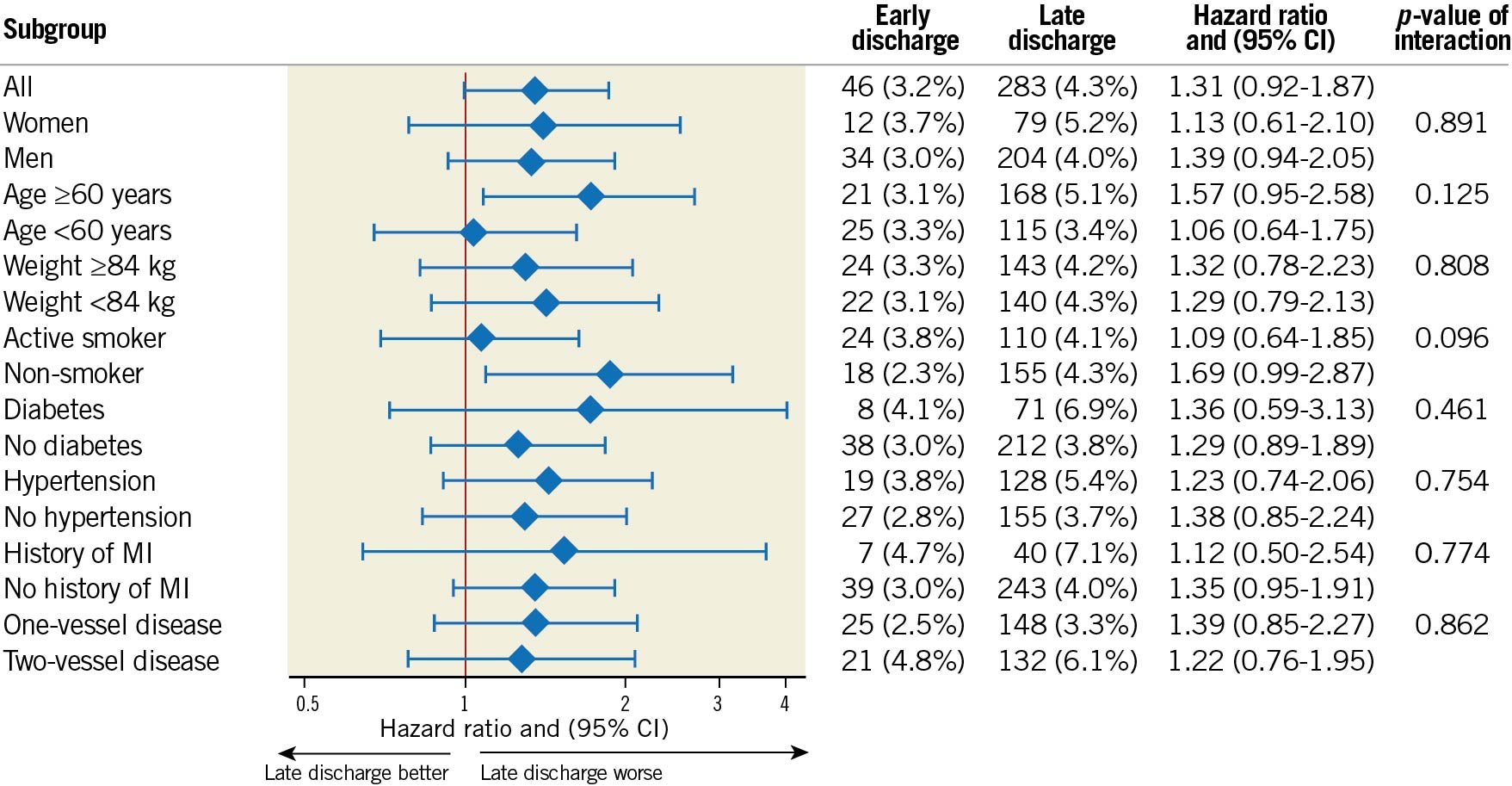
Figure 3. Forest plot visualising hazard ratios of MACE stratified by length of hospital stay. Subgroup analysis for the primary endpoint major adverse cardiovascular events (MACE) showing Kaplan-Meier event rates and propensity score-adjusted hazard ratio with interaction test performed for age, sex, weight, smoking status, diabetes, hypertension, history of myocardial infarction (MI) and number of diseased vessels. There were no significant interactions indicating a risk with early discharge in any subgroup.
Discussion
In this nationwide observational study of patients with STEMI treated with PCI, the PAMI-II risk score identified 1/4 of patients as low risk in whom early discharge was not associated with short- or long-term adverse outcome.
Our results serve as a real-world validation of the PAMI-II risk score1. To the best of our knowledge, this is the largest study assessing the safety of early discharge in low-risk STEMI patients. It supports the findings of smaller observational studies1112, as well as small randomised clinical trials78910, that early discharge of low-risk STEMI patients is safe. In our study, patients categorised as low risk had very low rates of death, reinfarction requiring PCI treatment, stroke or HF hospitalisation within the first year of admission, with no significant differences found between patients with short hospital stay compared to longer in any investigated outcome measure. Importantly, the low mortality rate of 1% (which did not differ between groups) is supportive of the safety of early discharge. To account for confounders and indication bias (i.e., sicker patients who need longer hospital stay will have a longer stay), we used propensity score analyses and inverse probability weighting propensity scores. The two landmark analyses that were undertaken were not associated with adverse outcome, suggesting a low rate of residual confounding.
Patients with longer hospital stay were more frequently treated with intravenous diuretics and discharged with diuretic prescriptions, probably signalling early symptomatic HF. In line with this finding, we observed a trend towards more frequent hospitalisations due to HF in this group. Although this trend was not statistically significant in any analyses except unadjusted analyses, a subgroup of low-risk patients, e.g., patients needing intravenous diuretics, heart rate regulation due to atrial fibrillation/flutter or patients in whom radial artery access was not possible, may naturally require a longer hospital stay. However, this relates to a small proportion of all patients identified as low-risk patients and does not explain the significant interhospital differences in length of hospital stay observed in Sweden (Supplementary Figure 1). We believe this to be a much larger variation than expected and is more reflective of local hospital traditions rather than different proportions of high-risk patients. The interhospital difference in hospital stay among low-risk patients inherently contributes to an uneven distribution in hospital cost for STEMI patients in Swedish hospitals. Adherence to guideline recommendations on the use of the PAMI-II risk score could reduce hospital costs. According to our findings, about 1,000 STEMI patients each year may be identified as candidates for early discharge in Sweden (10 million population), of whom ≈18% were discharged from the coronary care unit (CCU) within two days. Adherence to early discharge of low-risk STEMI patients would result in the saving of ≈1,700 care days per year (calculated as total care days among low-risk patients with >2 days minus care days if they were discharged within 2 days). Assuming that only patients with a length of stay of 3-5 days are candidates for early discharge would result in ≈1,200 care days saved per year. In countries with large populations, a higher incidence of STEMI, and with the increased proportion of patients treated with PCI, a more efficient use of hospital and CCU beds could result in substantial cost reduction and increased availability of cardiac health care to those in need. A major concern with early discharge is that patient education and information comprehension might be compromised. However, when early follow-up by coronary care staff is available, this is of lesser concern, and information on medication and lifestyle changes might even be better understood outside of the acute care setting.
There is a need for a more standardised hospitalisation strategy while continuing to secure patient safety. Ongoing small randomised clinical trials assessing the safety of early discharge, e.g., the EDAMI study7 (N=1,558, www.clinicaltrials.gov identifier: NCT01868256) will hopefully support the concept of a standardised hospitalisation strategy for low-risk patients. A large randomised clinical trial addressing this topic in the modern era is warranted to ascertain the safety of early discharge. However, a major difficulty with such a study is that it would require a large number of patients as outcome is infrequent in low-risk patients. For example, investigating the superiority of a prolonged hospital stay with a baseline mortality of 1% and an absolute risk reduction of 0.5% would require roughly 10,000 patients.
Limitations and strengths
Our definition of low-risk features differed somewhat from the original PAMI publication. This was due to the constraints of the available data. However, we believe that the definitions used in this study are more reflective of a low-risk patient in a contemporary clinical setting. We acknowledge the risk of residual confounding and indication bias inherent to observational studies. Although baseline differences were minor and unadjusted analyses were consistent with adjusted analyses, indicating low rates of confounding, residual confounding and indication bias cannot be ruled out completely. A proportion of patients identified as low risk may have needed a longer hospital stay, as indicated by the more frequent use of intravenous diuretics. Our study benefits from a number of strengths as well. It is a nationwide study using a validated high-quality registry including more than 8,000 low-risk patients with STEMI admitted over eight years and treated according to current guidelines15.
Conclusion
In a nationwide population of STEMI patients, the PAMI-II risk score adequately identified patients with a very low risk of short- and long-term adverse outcome in whom early discharge within two days may be considered safe.
Impact on daily practice
The safety of early discharge following ST-elevation myocardial infarction (STEMI) has been examined in observational studies and randomised clinical trials that are either small or were conducted prior to the widespread use of mechanical reperfusion with percutaneous coronary intervention. Using contemporary data from nationwide population-based registries, we show that early discharge within two days of admission is safe in low-risk patients with STEMI. A more standardised hospitalisation strategy for low-risk STEMI patients, that secures patient safety and reduces the length of hospital stay, may result in a more efficient use of coronary care unit beds and substantial cost reduction, and increase the availability of cardiac health care to those in need.
Acknowledgements
The authors thank the staff members of all coronary care units in Sweden for their continuous work collecting data for the SWEDEHEART registry.
Funding
This work was supported by the Bundy Academy, the Märta Winkler Foundation and the Anna-Lisa and Sven-Eric Lundgren Foundation for medical research. The sponsors were not involved in the study design, collection of data, analysis of data, interpretation of data, writing of the manuscript, approval of the manuscript or in the decision to submit the manuscript for publication.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

