Abstract
Background: In patients with ST-segment elevation myocardial infarction (STEMI), early initiation of high-intensity statin therapy, regardless of low-density lipoprotein (LDL) cholesterol levels, is the standard of practice worldwide.
Aims: We sought to determine the effect of a similar early initiation strategy, using a proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor added to the high-intensity statin, on LDL cholesterol in acute STEMI.
Methods: In a randomised, double-blind trial we assigned 68 patients with STEMI undergoing primary percutaneous coronary intervention (PCI) to early treatment with alirocumab 150 mg subcutaneously or to a matching sham control. The first injection was given before primary PCI regardless of the baseline LDL level, then at 2 and 4 weeks. The primary outcome was the percent reduction in direct LDL cholesterol up to 6 weeks, analysed using a linear mixed model.
Results: High-intensity statin use was 97% and 100% in the alirocumab and sham-control groups, respectively. At a median of 45 days, the primary outcome of LDL cholesterol decreased by 72.9% with alirocumab (2.97 mmol/L to 0.75 mmol/L) versus 48.1% with the sham control (2.87 mmol/L to 1.30 mmol/L), for a mean between-group difference of –22.3% (p<0.001). More patients achieved the European Society of Cardiology/European Atherosclerosis Society dyslipidaemia guideline target of LDL ≤1.4 mmol/L in the alirocumab group (92.1% vs 56.7%; p<0.001). Within the first 24 hours, LDL declined slightly more rapidly in the alirocumab group than in the sham-control group (–0.01 mmol/L/hour; p=0.03) with similar between-group mean values.
Conclusions: In this randomised trial of routine early initiation of PCSK9 inhibitors in patients undergoing primary PCI for STEMI, alirocumab reduced LDL cholesterol by 22% compared with sham control on a background of high-intensity statin therapy. A large trial is needed to determine if this simplified approach followed by long-term therapy improves cardiovascular outcomes in patients with acute STEMI. (ClinicalTrials.gov: NCT03718286)
Introduction
ST-segment elevation myocardial infarction (STEMI) is the most acute manifestation of ischaemic heart disease12. These patients often have multivessel coronary artery disease (CAD) with a high risk of long-term morbidity and mortality. Routine, early initiation of high-dose statin therapy, regardless of low-density lipoprotein (LDL) cholesterol levels, has become the standard of practice worldwide for patients with acute coronary syndromes (ACS)34. Data from randomised trials and meta-analyses show that routine early use of high-intensity statin therapy is associated with a rapid and sustained reduction in clinical events56789. Statin therapy proportionately reduces major adverse cardiovascular events and the greater the reduction in LDL, the lower the risk for events, with no apparent lower limit beyond which a benefit is not observed1011. The 2019 European Society of Cardiology (ESC)/European Atherosclerosis Society (EAS) guidelines for the management of dyslipidaemias and the 2021 ESC guidelines for cardiovascular disease prevention in clinical practice recommend an LDL cholesterol treatment goal of a 50% reduction or <1.4 mmol/L for patients with ACS (and <1.0 mmol/L in those with recurrent events)1213. The addition of more potent LDL-lowering therapy with proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors has shown a clinical benefit in patients with prior ACS or atherosclerotic cardiovascular disease who have persistently high LDL cholesterol despite the use of statins1415. In these landmark trials, a PCSK9 inhibitor was initiated after several months to years from the ACS event presentation and only in those patients with persistently elevated LDL despite high-dose statin therapy. This approach has not been widely adopted in clinical practice, with <1% of eligible patients being treated with these agents16. While cost and access remain barriers to the use of PCSK9 inhibitors, it is unknown whether a simplified regimen of initiating PCSK9 inhibitors routinely in the acute setting of STEMI, analogous to how statins are administered, would add additional benefit by further reducing LDL cholesterol levels in a much wider population of patients. Such an approach would be expected to substantially reduce the number of major cardiovascular events in this high-risk population. We designed a randomised trial to evaluate the routine, early administration of a PCSK9 inhibitor, regardless of baseline LDL levels or prior statin use, in order to quantitate the degree of LDL reduction that might be expected with this approach, as well as the time course of LDL lowering during the acute period after STEMI, and its overall feasibility.
Methods
EPIC STEMI (Effects of Acute, Rapid Lowering of Low Density Lipoprotein Cholesterol with Alirocumab in Patients with ST Segment Elevation Myocardial Infarction Undergoing Primary PCI) is a randomised, double-blind, parallel-group clinical trial of alirocumab or sham control in patients undergoing primary percutaneous coronary intervention (PCI) for acute STEMI. The primary aim was to determine the effect of routine early treatment with alirocumab added to high-intensity statin therapy irrespective of the baseline LDL level. The trial was conducted independently by the Population Health Research Institute and overseen by a Steering Committee (Supplementary Appendix 1). The trial was an investigator-initiated study that was funded by the Population Health Research Institute and by an unrestricted grant to the STEMI primary PCI program at Hamilton Health Sciences from Sanofi (who had no role in the design, execution, or interpretation of the study).
Patients were eligible if they presented with STEMI, defined as symptoms of myocardial ischaemia lasting for ≥30 minutes, and definite electrocardiogram (ECG) changes indicating STEMI (ST-segment elevation of greater than 0.1 mV in 2 contiguous limb leads or 0.2 mV in 2 contiguous precordial leads) and were referred within 12 hours of symptom onset for primary PCI for the presenting symptoms. The main exclusion criteria were allergy/contraindication to a PCSK9 inhibitor, Killip class ≥2, known creatinine clearance <30mL/min, suspected takotsubo cardiomyopathy or acute pericarditis. Written informed consent was obtained in all patients and the study was approved by the research ethics board.
Patients were randomised after diagnostic angiography was performed and prior to the start of the primary PCI procedure. Randomisation was performed centrally using a web-based interface, and treatment allocation was assigned according to a computer-generated randomisation list stratified by prior use of statins within 7 days of randomisation. Randomisation and study drug administration were performed by a predesignated unblinded study coordinator who did not participate in any of the study assessments.
Blood samples were collected at baseline (pre-PCI), during hospitalisation (4, 8, 12 and 24 hours post-PCI), at hospital discharge, and at each follow-up visit (2, 4, 6 and 12 weeks post-randomisation). The central laboratory was blinded to treatment assignment.
Administration of study treatments
For patients randomised to receive alirocumab, a commercially acquired alirocumab 150 mg prefilled pen was administered subcutaneously by the study coordinator at baseline (prior to primary PCI), 2 weeks and 4 weeks. For patients in the sham-control group, an alirocumab training pen, identical in appearance to an active alirocumab pen but without an internal needle, was used in an identical fashion as an active alirocumab pen. In both groups, firm pressure was applied to the skin injection site, such that the yellow safety needle cover was not visible prior to the deployment of the needle plunger. To maintain the study blind, a predesignated, unblinded study team member, who had no role in any other trial-related procedures, performed randomisation and administered the alirocumab/sham-control injections. Following administration of the first dose of the study drug, PCI was performed as per standard practice.
The primary outcome was the between-group percent change in direct LDL cholesterol concentration from baseline to week 6. The secondary outcome included the percent change in levels of apolipoprotein B (Apo B), non-high-density lipoprotein (HDL) cholesterol and total cholesterol. The rate of decrease in direct LDL cholesterol was also evaluated within the first 24 hours. Other outcomes included C-reactive protein (CRP), lipoprotein(a) (Lp[a]), infarct size as measured using creatine kinase myocardial band (CKMB), area under the curve and NT-proBNP levels (Supplementary Appendix 2, Supplementary Table 1). Safety outcomes included local injection-site reactions, general allergic reactions and intracranial haemorrhage.
Statistical analysis
We estimated that a sample size of 58 participants would have 95% power to detect a mean percent difference of 25% in LDL cholesterol levels between the treatment groups from baseline to week 6 at a significance level of 0.05 for a 2-sided test, assuming a common standard deviation of 25%, and 5% non-compliance or lost to follow-up. We estimated a slightly more modest LDL reduction of 25% with PCSK9 inhibitor therapy than previously shown in trials of stable CAD because we intended to enrol patients regardless of baseline LDL levels or prior statin use1415. A sample size of up to 100 patients was planned to collect additional data on safety events. Baseline characteristics were compared using a t-test for continuous variables and the chi-square or Fisher’s exact test for categorical variables. Mean levels of LDL and other biomarkers were presented at baseline and follow-up. A modified intention-to-treat analysis consisting of patients who received at least 1 measurement of LDL cholesterol between 2 and 6 weeks and at least 1 injection of alirocumab 2 weeks prior to the blood draw was used for the primary analysis. The primary and the secondary outcomes were analysed using a mixed model with repeated measures (MMRM) with baseline values, prior statin use, treatment group, timepoints, and interaction between the treatment and timepoints as fixed effects, and patients as a random effect. For biomarker outcomes (collected only at 6-week follow-up), comparisons were made using a linear regression model. The mean difference with the 95% confidence interval (CI) was reported for continuous outcomes and the odds ratio with the 95% CI for categorical outcomes. The non-normally distributed outcomes were summarised using median and interquartile range (IQR) and compared using a Wilcoxon rank-sum test.
All analyses used SAS, version 9.4 or higher (SAS Institute). A 2-tailed p-value <0.05 was considered statistically significant.
Results
Between May 2019 and April 2021, a total of 97 patients were randomised into the trial. Sixty-eight patients received at least 1 dose of the study drug and had at least 1 blood draw for LDL cholesterol between 2 and 6 weeks. A total of 29 patients were excluded, mainly because of hospital research clinic closure due to the COVID-19 pandemic (n=23), and the remaining patients due to consent withdrawal (n=5) and in-hospital death (n=1) (Figure 1). Baseline characteristics are shown in Table 1. The mean age was 62 years, 19% were women, and approximately 24% were on prior statin therapy at the time of enrolment. There were no significant differences in baseline characteristics and lipid values among patients included and excluded from the trial (Supplementary Table 2, Supplementary Table 3). Compliance with the study drug in the alirocumab and sham-control groups was 100% at baseline in both groups, 97% and 93% at 2 weeks, 97% and 93% at 4 weeks, and 89% and 87% at 6 weeks, respectively. After randomisation, 97.4% of patients in the alirocumab group and 100% in the sham-control group received background high-intensity statin therapy (atorvastatin 40-80 mg or rosuvastatin 40 mg daily). Ezetimibe was used in 2 patients in the alirocumab group and 1 patient in the sham-control group. The median follow-up was 45 days in each group.
Figure 1. CONSORT diagram. LDL: low-density lipoprotein
Table 1. Baseline characteristics.
| Alirocumab N=38 | Sham control N=30 | p-value* | ||
|---|---|---|---|---|
| Age, years, mean (SD)* | 61.37 (11.04) | 63.63 (10.38) |
0.39 | |
| Sex (male), n (%) | 27 (71.05) | 28 (93.33) | 0.03 | |
| Systolic blood pressure, (mmHg) – mean (SD) | 140.8 (23.80) |
148.3 (23.51) |
0.20 | |
| Diastolic blood pressure, (mmHg) – mean (SD) | 88.89 (15.79) |
89.63 (16.08) |
0.85 | |
| Medical history | Diabetes, n (%) | 5 (13.16) | 1 (3.33) | 0.16 |
| Prior myocardial infarction, n (%) | 3 (7.89) | 3 (10.00) | 1.00 | |
| Current smoker, n (%) | 16 (42.11) | 7 (23.33) | 0.10 | |
| Hypertension, n (%) | 17 (44.74) | 13 (43.33) | 0.91 | |
| Dyslipidaemia, n (%) | 13 (34.21) | 12 (40.00) | 0.62 | |
| Prior stroke, n (%) | 1 (2.63) | 0 (0.00) | 1.00 | |
| Time from symptom onset to primary PCI, hours – mean (SD) | 3.40 (2.23) | 3.96 (2.26) | 0.31 | |
| Statin use within 7 days of randomisation, n (%) | 8 (21.05) | 8 (26.67) | 0.59 | |
| Statin use after randomisation,n (%) | Atorvastatin 40-80 mg or rosuvastatin 40 mg daily† |
37 (97.37) | 30 (100) | 0.37 |
| Atorvastatin 80 mg or rosuvastatin 40 mg daily |
37 (97.37) | 27 (90.00) | 0.20 | |
| Ezetimibe, n (%) | 2 (5.26) | 1 (3.33) | 0.70 | |
| *A t-test was used for continuous variables. A chi-square or Fisher’s exact test when a cell had less than 5 counts were used for categorical variables. †1 patient in the alirocumab group received atorvastatin 20 mg daily. PCI: percutaneous coronary intervention; SD: standard deviation | ||||
Primary outcome
At a median of 45 days of follow-up, the primary outcome of direct LDL cholesterol level was reduced from 2.97 mmol/L at baseline to 0.75 mmol/L (72.9% reduction; p<0.001) in the alirocumab group and from 2.87 mmol/L at baseline to 1.30 mmol/L (48.1% reduction; p<0.001) in the sham-control group. The difference in LDL with alirocumab as compared with the sham control was –22.3% (95% CI: –31.1 to –13.5; p<0.001) (Table 2, Central illustration). A sensitivity analysis adjusted for the baseline gender difference demonstrated similar results, with a between-group difference of –23.7% (95% CI: –32.8% to –14.7%; p<0.001) (Supplementary Table 4). LDL reductions at 2, 4, 6 and 12 weeks are shown in Figure 2. A greater proportion of patients in the alirocumab group achieved the ESC/EAS guideline LDL target of ≤1.4 mmol/L (92.1% alirocumab vs 56.7% sham control; p=0.002) and >50% reduction from baseline (89.5% vs 60%; p=0.007) (Figure 3). At 12 weeks (8 weeks after the last injection of the study drug), LDL was 1.64 mmol/L in the alirocumab group and 1.41 mmol/L in the sham-control group (between-group difference 6.2%, 95% CI: –18.0 to –30.3; p=1.0).
Table 2. Main outcomes at baseline and up to 6-week follow-up.
| Alirocumab N=38 |
Sham control N=30 |
Between-group difference (95% CI) | p-value | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Follow-up | Percent change | Baseline | Follow-up | Percent change | |||
| LDL cholesterol (mean [SD], mmol/L) | 2.97 (1.09) |
0.75 (0.46) |
−72.9% (17.5) |
2.87 (0.90) |
1.30 (0.45) |
−48.1% (29.5) |
−22.3% (−31.1 to −13.5) |
<0.001 |
| Apo B (mean [SD], g/L) | 0.88 (0.33) |
0.40 (0.11) |
−51.4 (17.2) |
0.83 (0.25) |
0.49 (0.13) |
−36.3 (24.4) |
−11.5% (−17.8 to −5.3)* |
<0.001 |
| Non-HDL cholesterol (mean [SD], mmol/L) | 3.68 (1.37) |
1.13 (0.62) |
−67.3% (18.8) |
3.46 (1.01) |
1.79 (0.50) |
−48.2% (20.0) |
−19.1% (−29.4 to −8.7) |
0.001 |
| Total cholesterol (mean [SD], mmol/L) | 4.73 (1.35) |
2.22 (0.65) |
−50.9% (18.0) |
4.51 (1.01) |
2.84(0.65) | −38.0% (18.4) |
−13.0% (−22.7 to −3.3) |
0.010 |
| Triglycerides (median [IQR], mmol/L) | 1.20 (0.68, 1.89) |
1.01 (0.82, 1.40) |
−8.9% (−41.1, 50.6) |
0.85 (0.55, 1.32) |
1.19 (0.88, 1.62) |
25.9% (−8.9, 61.0)† |
– | 0.044† |
| HDL cholesterol (median [IQR], mmol/L) | 0.97(0.82, 1.28) | 1.09 (0.92, 1.23) |
5.8% (−12.0, 21.1) |
1.06 (0.83, 1.26) |
0.98 (0.86, 1.23) |
−1.7% (−15.6, 8.3) |
– | 0.29† |
| Lp(a) (median [IQR], mg/L) | 68 (34, 431) |
87 (34, 489) |
5.3% (0, 40.2) | 101 (50, 414) |
171 (43, 482) |
34.8% (14.7, 68.6) |
– | 0.023† |
| NT-proBNP (mean [SD], pmol/L) | – | 83.33 (99.43) |
− | − | 75.02 (79.47) |
− | 8.31 pmol/L (−40.50 to 57.13) |
0.71† |
| CRP (median [IQR], mg/L) | 2.70 (1.14, 5.53) |
1.84 (0.69, 5.97) |
−34.5% (−67.7, 17.86) |
1.86 (0.43, 4.88) |
0.99 (0.56, 2.54) |
−33.3% (−57.9, 30.0) |
1.02% (0.57 to 1.60) |
0.93‡ |
| CKMB area under the curve (mean [SD], units) | – | 4,436 (3,512) |
− | − | 4,416 (3,079) |
− | −180 units (−1,803 to 1,443) |
0.67† |
| *68.4% of patients in the alirocumab group versus 26.7% in the sham-control group had Apo B levels ≤the assay lower detection limit of 0.35 mmol/L (p=0.001). All of these patients were assigned an Apo B level of 0.35 mmol/L. †Wilcoxon rank-sum test. ‡Comparison made on log-transformed data. CKMB: creatine kinase myocardial band; CRP: C-reactive protein; HDL: high-density lipoprotein; IQR: interquartile range; LDL: low-density lipoprotein; Lp(a): lipoprotein(a); NT-proBNP: N-terminal pro-brain natriuretic peptide; SD: standard deviation | ||||||||
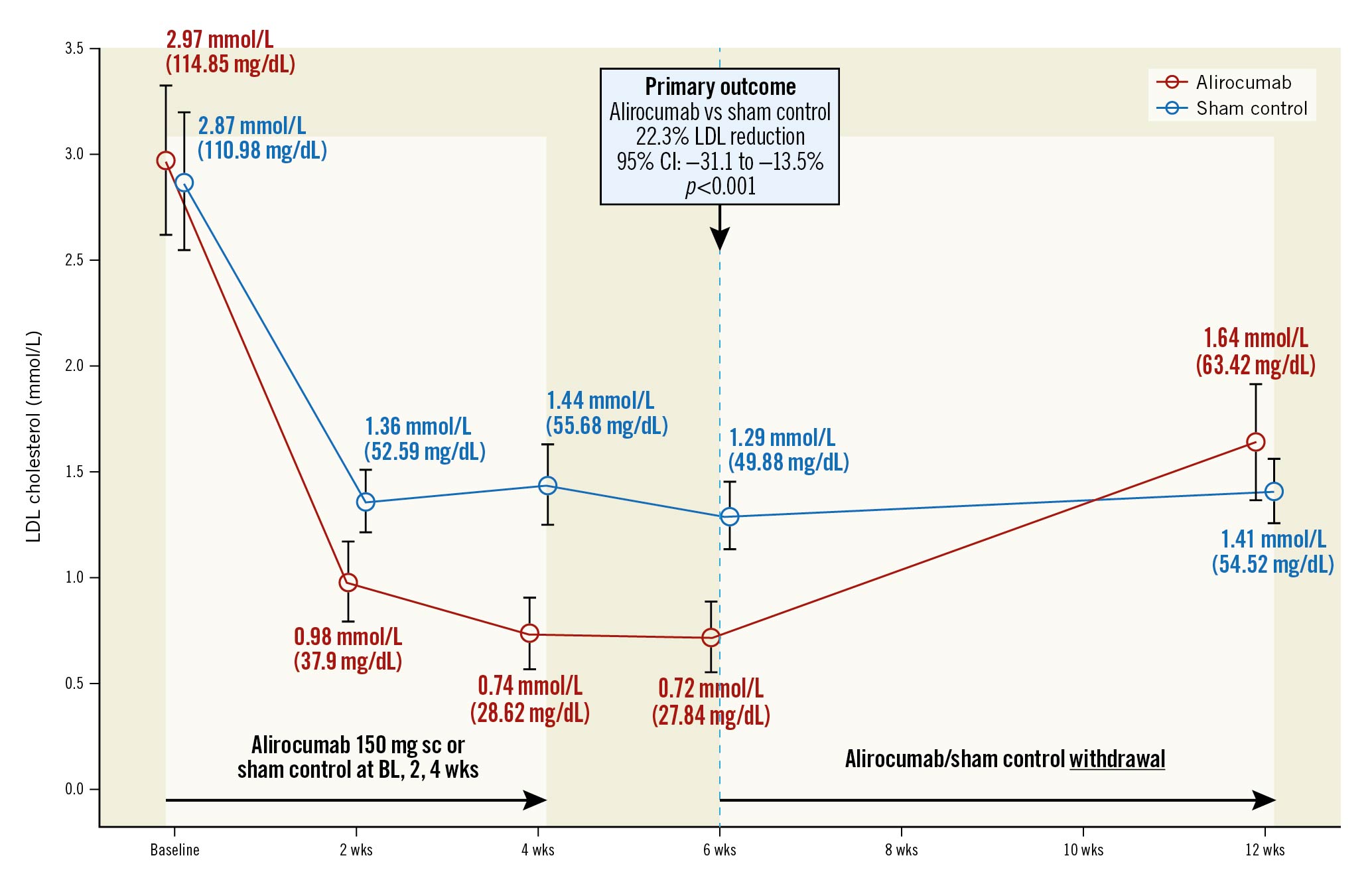
Central illustration. Direct LDL levels at follow-up. BL: baseline; CI: confidence interval; LDL: low-density lipoprotein; sc: subcutaneous
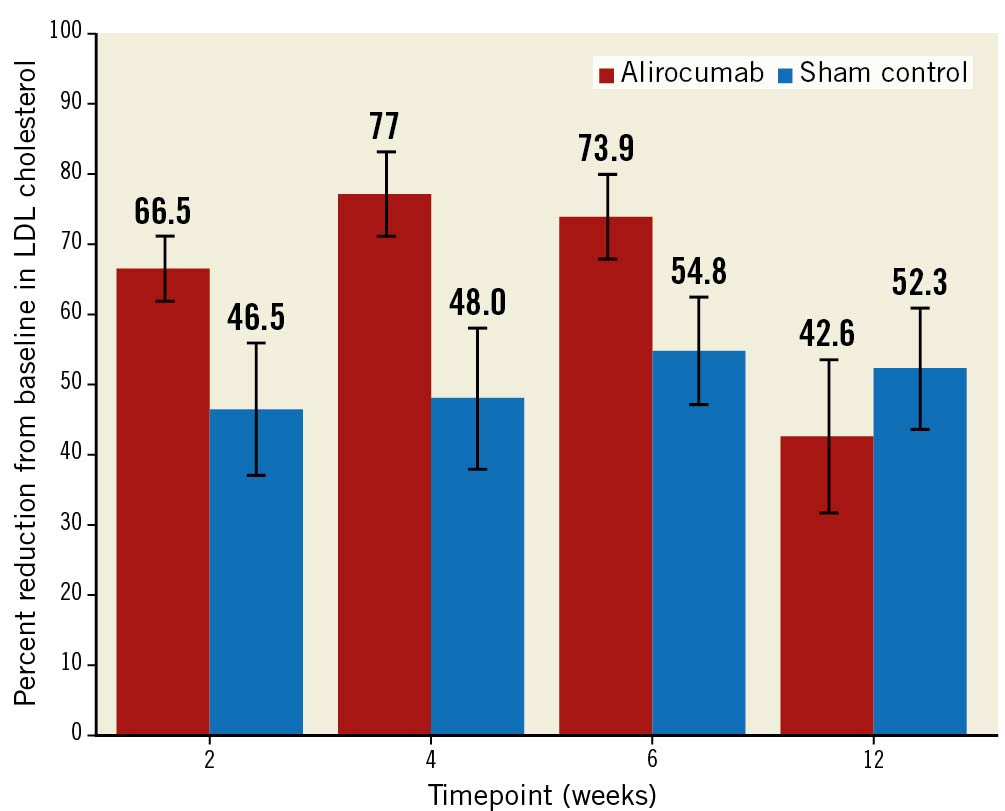
Figure 2. Reduction in LDL at each timepoint after discharge. CI: confidence interval; LDL: low-density lipoprotein
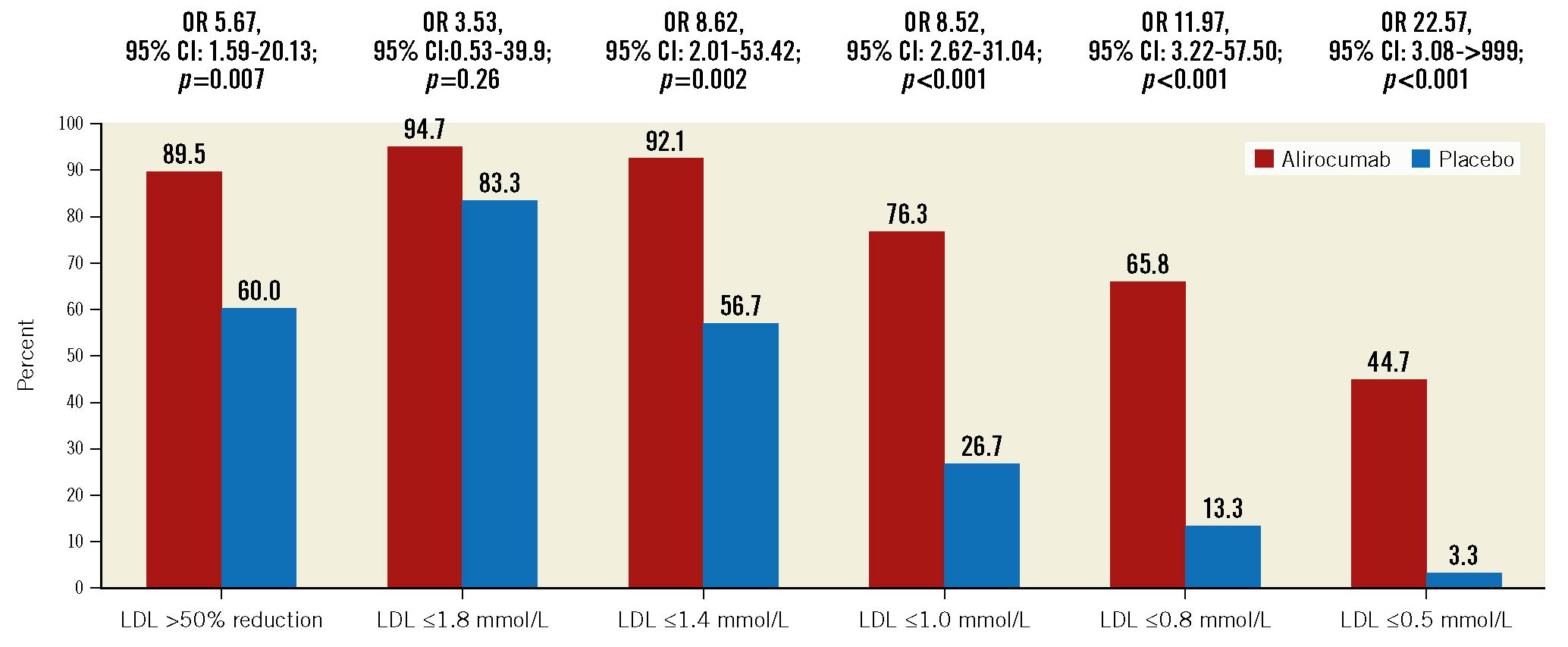
Figure 3. Proportion in the alirocumab and sham-control groups achieving various LDL cholesterol targets at a median follow-up of 45 days. CI: confidence interval; LDL: low-density lipoprotein; OR: odds ratio
Secondary and other outcomes
The Apo B level was reduced from 0.88 g/L at baseline to 0.40 g/L at a median of 45 days of follow-up (50.6% reduction; p<0.001) in the alirocumab group and from 0.83 g/L at baseline to 0.49 g/L at follow-up in the sham-control group (36.3% reduction; p<0.001). The mean between-group difference in Apo B with alirocumab as compared with sham control was 11.5% (95% CI: 4.83 to 5.3; p<0.001) (Table 2). Of note, 68.4% of patients in the alirocumab group versus 26.7% in the sham-control group had Apo B levels less than or equal to the assay lower detection limit of 0.35 g/L (p=0.001). All of these patients were assigned a value of 0.35 g/L. Non-HDL cholesterol was reduced from baseline by 67.3% in the alirocumab group and by 48.2% in the sham-control group, for a between-group difference of 19.1% (p=0.001). There was no significant difference between the groups in the mean Lp(a) levels (change +18.1 alirocumab vs +75.0% sham control; p=0.058), but there was a significant difference for median Lp(a) levels where alirocumab blunted the rise in Lp(a) from baseline to a median of 45 days (alirocumab +5.3%, IQR 0 to 40.2 vs sham control +34.8%, IQR 14.7 to 68.6; p=0.023).
Acute LDL lowering, infarct size and other biomarkers
Within the first 24 hours, the rate of change in LDL cholesterol was –0.02 mmol/L/hour in the alirocumab group versus –0.01 mmol/L/hour in the sham-control group for a between-group difference of 0.01 mmol/L/hour (p=0.032) (Figure 4). There were no differences in the LDL levels between the groups at 4 hours (mean LDL in alirocumab group 3.16 mmol/L vs sham-control group 2.97 mmol/L), 8 hours (3.07 mmol/L vs 2.88 mmol/L), 12 hours (3.05 mmol/L vs 2.78 mmol/L) and 24 hours (2.75 mmol/L vs 2.80 mmol/L). There was no difference between the groups in infarct size, as measured by CKMB area under the curve in NT-proBNP levels (Table 2). The change in high-sensitivity CRP from baseline to 6 weeks did not differ between the groups (between-group difference 1.02%; p=0.93) (Table 2).
Clinical events were infrequent. In the overall population of patients recruited (N=97), there was 1 death in the sham-control group and none in the alirocumab group (Supplementary Table 5). There were no myocardial infarctions (MI) or strokes in either group. There were 4 heart failure events in the alirocumab group and none in the sham-control group. There were no reported local injection-site reactions, allergic reactions or intracranial haemorrhages. There were no major bleeds, with 1 minor bleed in the sham-control group.
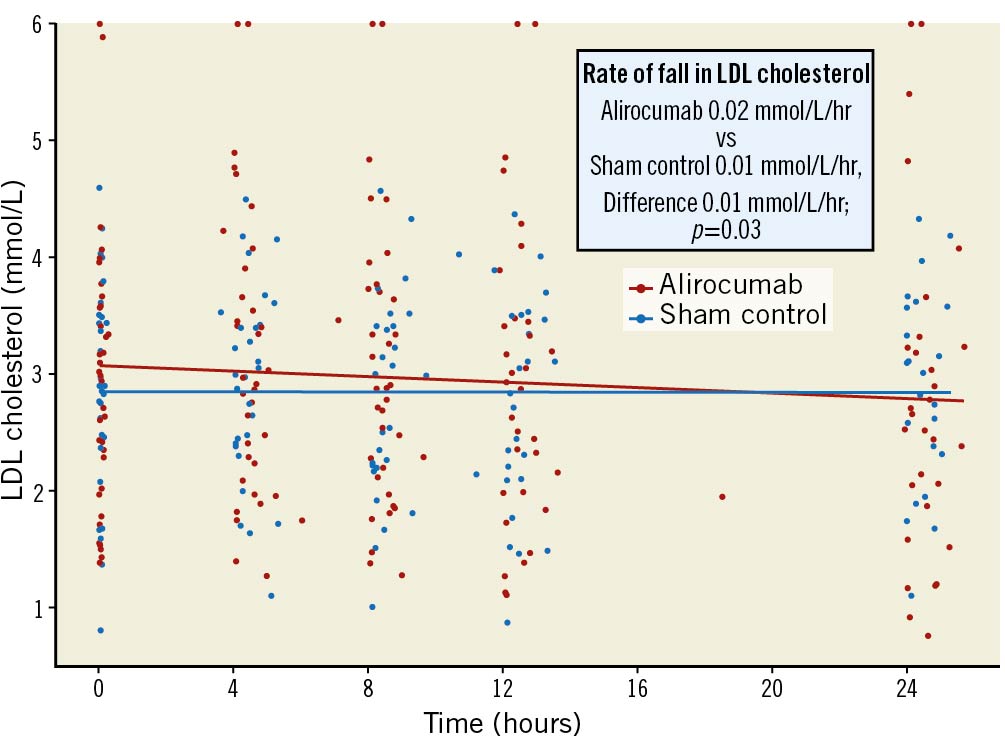
Figure 4. Scatter plot of very early effect of alirocumab vs sham control on direct LDL levels. LDL: low-density lipoprotein
Prior statin therapy vs statin naÏve patients
Prior to randomisation, 16 patients were on statin therapy (8 alirocumab and 8 sham control) and 52 patients were statin naïve (30 alirocumab and 22 sham control). In patients on prior statin therapy, LDL was reduced from 1.81 mmol/L at baseline to 0.63 mmol/L at follow-up in the alirocumab group (64.2% reduction) and from 1.87 mmol/L at baseline to 1.52 mmol/L at follow-up in the sham-control group (10.5% reduction), with a mean between-group difference of –53.4% (95% CI: –78.2 to –28.7). In patients who were statin naïve, LDL was reduced from 3.28 mmol/L at baseline to 0.78 mmol/L at follow-up in the alirocumab group (74.9% reduction) and from 3.23 mmol/L at baseline to 1.22 mmol/L at follow-up in the sham-control group (61.8% reduction), with a mean between-group difference of –13.1% (95% CI: –21.1 to –5.0) and an interaction p-value<0.001.
Discussion
In the EPIC STEMI trial, we evaluated the effects of routine, early administration of a PCSK9 inhibitor on top of high-intensity statin therapy in patients presenting with STEMI undergoing primary PCI. We administered the first dose of alirocumab upon patient presentation to the catheterisation laboratory, irrespective of baseline LDL levels or prior statin use, with subsequent injections given at 2 weeks and 4 weeks. We found that at a median of 45 days after randomisation, direct LDL cholesterol was reduced by 73% in the alirocumab group and by 48% in the sham-control group, a 22% difference favouring alirocumab. This difference translated into 92% of patients achieving the ESC/EAC guideline-recommended secondary prevention LDL target of 1.4 mmol/L in the alirocumab group compared with 57% in the sham-control group. In the acute period after primary PCI for STEMI, there was a slightly more rapid rate of fall in LDL cholesterol with alirocumab compared with sham control; however the between-group difference in LDL within the first 24 hours did not differ, nor was infarct size reduced. Finally, 6 weeks after withdrawal of the study drug, LDL levels were no different between alirocumab and the sham control, emphasising the fact that any potential benefit of LDL reduction with alirocumab would be lost after treatment discontinuation.
Our study builds on the results of randomised trials demonstrating the clinical benefit of PCSK9 inhibitors added to statin therapy in patients with stable CAD or prior ACS1415. Two recent trials have evaluated earlier initiation of PSCK9 inhibitors in ACS. In the EVOPACS trial, evolocumab reduced LDL cholesterol by 40.7% in ACS patients with elevated LDL cholesterol17. In the EVACS trial, a single dose of evolocumab was given to non-ST-segment elevation acute coronary syndrome patients, and LDL cholesterol was lower in this group at 1 month18. Intracoronary imaging trials of patients with ACS have demonstrated evidence of plaque stabilisation and regression with both evolocumab and alirocumab when started early after ACS1920. In the PACMAN-AMI trial, alirocumab resulted in greater coronary plaque regression and stabilisation compared with placebo on a background of rosuvastatin 20 mg daily in non-infarct-related arteries at 52 weeks20. EPIC STEMI adds to the results of these trials, as it evaluates the initiation of PCSK9 inhibitors routinely in STEMI patients before primary PCI, irrespective of baseline LDL cholesterol levels or prior statin use. Moreover, as we had expected, the 22% reduction in LDL was slightly lower than in prior trials. We included patients who were statin naïve and introduced high-intensity statin therapy to these patients, resulting in a much greater reduction in LDL levels in the comparator (sham-control) group (48%) than in the placebo group of prior trials where there was little change in the LDL level1415. This large reduction in LDL in the sham-control group reduced the magnitude of the between-group difference. Also, because we did not have an LDL threshold for entry, the baseline LDL levels were lower than in prior trials. In spite of this, we still found a substantial between-group difference of 23% overall, and a much larger proportion of patients achieved ESC/EAS guideline-recommended targets of 1.4 mmol/L or a 50% reduction from baseline.
The subgroup analysis of patients on prior statin therapy versus those who were statin naïve suggested that patients who were on prior statin therapy may have had a greater LDL reduction. Given that this is a subgroup analysis with few patients (only 16 in the prior statin group), it should be interpreted cautiously. At baseline, prior statin users had lower LDL levels than statin-naïve patients. In the sham-control group the reduction in LDL was small in prior statin users, and this may have exaggerated the between-group difference. Importantly, the actual achieved LDL level in the alirocumab group was similar in prior statin users (0.63 mmol/L) and statin naïve patients (0.78 mmol/L).
One of the objectives of EPIC STEMI was to evaluate the time course of LDL reduction after PCSK9 inhibitor initiation after STEMI. Within the acute phase, we did find a slightly greater rate of fall in LDL cholesterol with alirocumab at 24 hours; however, the mean differences in LDL levels at 4, 8, 12 and 24 hours did not differ between the groups. Moreover, we also did not find a difference in STEMI infarct size (as determined by CKMB area under the curve) nor a difference in NT-proBNP levels between the groups, suggesting that the very early initiation of alirocumab may not modify the size or severity of the index STEMI event. By 2 weeks after randomisation, a clear reduction in LDL cholesterol had emerged with alirocumab treatment. This implies that early administration of alirocumab could be given after the primary PCI but during the index hospitalisation.
By 6 weeks, a greater proportion of patients in the alirocumab group had achieved the ESC/EAC target LDL level of ≤1.4 mmol/L for very high-risk patients. More alirocumab patients than sham-control patients had also achieved an LDL level reduction of 50% from baseline and substantially lower targets, including levels ≤1.0 mmol/L, ≤0.8 mmol/L and ≤0.5 mmol/L (Figure 3). Whether this would translate into a benefit in terms of reduced long-term major cardiovascular events compared with current practice is uncertain. Over the longer term, non-compliance with statin therapy has been reported to be as high as 25-30%, and these patients may have increased mortality21. The EVOLVE-MI trial is an open-label, randomised trial that is planned to evaluate the impact of early- and long-term treatment with evolocumab every 2 weeks on major cardiovascular events in patients with ACS (ClinicalTrials.gov: NCT05284747). The short-chain single interfering RNA PCSK9 inhibitor inclisiran22 might be ideal for this simplified regimen of early initiation in the acute setting of ACS followed by long-term treatment, given that it is administered only twice yearly.
Limitations
There are some limitations of our trial that deserve consideration. First, the COVID-19 pandemic necessitated closure of hospital clinics which impacted 23 patients in whom the primary outcome could not be assessed. However, because the initial sample size calculation of 58 patients gave 95% power to detect a 25% reduction in LDL cholesterol at 6 weeks, with 68 patients in the primary analysis we still had high statistical power to evaluate the main hypothesis. In addition, there were no significant differences in baseline characteristics and lipid values among patients included and excluded. Second, the sham control consisted of an alirocumab training syringe that was identical in external appearance to the active alirocumab syringe but without an internal needle. It is possible that some patients could have been unblinded as they may not have felt the needle puncture after the plunger was depressed, and this could potentially have affected follow-up. However, all primary and secondary outcomes in the trial were laboratory based and unlikely to be influenced by patient knowledge of treatment allocation. Third, although there was a clear reduction in LDL levels with early PCSK9 inhibitor administration in this high-risk population, our trial was not designed to evaluate clinical outcomes, which requires an adequately powered outcomes trial. The results of EPIC STEMI are informative for the design of such a trial.
Conclusions
In conclusion, routine early initiation of the PCSK9 inhibitor alirocumab in patients undergoing primary PCI for acute STEMI reduced LDL cholesterol by 22% compared with the sham control on a background of high-dose statin therapy. This strategy appears to be both safe and feasible. A large outcomes trial evaluating routine, early initiation of a PCSK9 inhibitor followed by long-term treatment in patients with STEMI is needed to determine if this simplified approach improves major cardiovascular outcomes.
Impact on daily practice
In this double-blind, randomised trial of patients presenting with acute STEMI undergoing primary PCI, routine early initiation of a PCSK9 inhibitor reduced LDL cholesterol by 22% compared with sham control on a background of high-intensity statin therapy. This simplified strategy increased the proportion of patients achieving the ESC/EAS LDL cholesterol target of 1.4 mmol/L. A large outcomes trial is needed to determine if this simplified strategy of PCSK9 initiation followed by long-term therapy translates into a reduction in major cardiovascular events.
Acknowledgements
We wish to thank Tara Bryce of the Hamilton Regional Laboratory Medicine Program for supervising the laboratory analyses.
Funding
EPIC STEMI was an investigator-initiated study that was funded by an unrestricted grant from Sanofi and by the Population Health Research Institute.
Conflict of interest statement
S.R. Mehta reports an unrestricted grant from Sanofi to Hamilton Health Sciences for the present study and consulting fees from Amgen, Sanofi, and Novartis. E.M. Lonn reports grants from Amgen, Novartis, Boehringer Ingelheim, the Montreal Heart Institute, and Vifor Pharma; consulting fees from Amgen, Sanofi, HLS Therapeutics, Novartis, Alnylam Pharmaceuticals, Novo Nordisk, and Bayer; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Amgen, HLS Therapeutics, Servier, Novartis, and Bayer; participation on a DSMB or advisory board for Novartis, Resvirologix, and LIB Therapeutics. G. Pare reports grants from Bayer and Lilly; consulting fees from Amgen, Bayer, and Sanofi; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Amgen, Sanofi, and Novartis. N. Pinilla-Echeverri reports consulting fees from Abbott Vascular and Conavi; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Abbott Vascular, Conavi, Novartis, and Amgen; payment for expert testimony from Conavi; support for attending meetings and/or travel from Abbott Vascular; and participation on an advisory board for Abbott and Philips. J.D. Schwalm reports consulting fees from Novartis and payment for an educational event from Novartis. M. Sibbald reports grants from Abbott Vascular and Philips. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

