Abstract
Background: Treatment with proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors on top of statins leads to plaque regression and stabilisation. The effects of PCSK9 inhibitors on coronary physiology and angiographic diameter stenosis (DS%) are unknown.
Aims: This study aimed to investigate the effects of the PCSK9 inhibitor alirocumab on coronary haemodynamics as assessed by quantitative flow ratio (QFR) and DS% by three-dimensional quantitative coronary angiography (3D-QCA) in non-infarct-related arteries (non-IRA) among acute myocardial infarction (AMI) patients.
Methods: This was a prespecified substudy of the randomised controlled PACMAN-AMI trial, comparing alirocumab versus placebo on top of rosuvastatin. QFR and 3D-QCA were assessed at baseline and 1 year in any non-IRA ≥2.0 mm and 3D-QCA DS% >25%. The prespecified primary endpoint was the number of patients with a mean QFR increase at 1 year, and the secondary endpoint was the change in 3D-QCA DS%.
Results: Of 300 enrolled patients, 265 had serial follow-up, of which 193 underwent serial QFR/3D-QCA analysis in 282 non-IRA. At 1 year, QFR increased in 50/94 (53.2%) patients with alirocumab versus 40/99 (40.4%) with placebo (Δ12.8%; odds ratio 1.7, 95% confidence interval [CI]: 0.9 to 3.0; p=0.076). DS% decreased by 1.03±7.28% with alirocumab and increased by 1.70±8.27% with placebo (Δ–2.50%, 95% CI: –4.43 to –0.57; p=0.011).
Conclusions: Treatment of AMI patients with alirocumab versus placebo for 1 year resulted in a significant regression in angiographic DS%, whereas no overall improvement of coronary haemodynamics was observed. ClinicalTrials.gov: NCT03067844
Introduction
Hypercholesterolaemia negatively affects endothelial function, coronary vasodilator capacity1 and coronary flow2. Statins are the first-line therapy for hypercholesterolaemia34 and can counteract the unfavourable effects of elevated cholesterol blood levels on the coronary vasculature, by inducing stenosis regression5 and improving coronary microvascular function6.
Monoclonal antibodies inhibiting the proprotein convertase subtilisin/kexin type 9 (PCSK9) enzyme represent a valuable add-on therapy for patients who require additional low-density lipoprotein cholesterol (LDL-C)-lowering despite receiving statin therapy34. According to 3 randomised intracoronary imaging studies, PCSK9 inhibitors as compared to placebo induce plaque regression and stabilisation789. Whether atherosclerosis regression with PCSK9 inhibitors leads to an improvement of coronary physiology and changes in angiographic diameter stenosis (DS%) remains unknown to date.
Quantitative flow ratio (QFR) is a novel, non-hyperaemic method for assessing coronary haemodynamics from biplane coronary angiography using three-dimensional quantitative coronary analysis (3D-QCA) and Thrombolysis in Myocardial Infarction (TIMI) frame counting1011. Previous observational evidence suggests that lipid-lowering therapy with statins is associated with an improvement in QFR12 and regression in two-dimensional (2D)-QCA DS%13. However, the effects of PCSK9 inhibitors on QFR and 3D-QCA DS% have never been investigated.
Therefore, in a substudy of the randomised controlled double-blind PACMAN-AMI (Effects of the PCSK9 Antibody AliroCuMab on Coronary Atherosclerosis in PatieNts with Acute Myocardial Infarction. A Serial, Multivessel, Intravascular Ultrasound, Near-Infrared Spectroscopy And Optical Coherence Tomography Imaging Study) trial14, we aimed to assess the effects the PCSK9 inhibitor alirocumab added to high-intensity statin therapy with rosuvastatin on QFR and DS% by 3D-QCA in non-obstructive coronary lesions located in non-infarct related arteries (non-IRA), 1 year after acute myocardial infarction (AMI).
Methods
STUDY DESIGN AND MAIN RESULTS OF THE PACMAN-AMI TRIAL
The design and primary results of the PACMAN-AMI trial have been published previously714. Briefly, PACMAN-AMI was an investigator-initiated, randomised, double-blind, placebo-controlled, European multicentre study to evaluate the effect of intensive lipid-lowering therapy with alirocumab added to high-intensity statin therapy with rosuvastatin on coronary atherosclerosis by multimodality intracoronary imaging with intravascular ultrasound (IVUS), near-infrared spectroscopy (NIRS), and optical coherence tomography (OCT) in patients with AMI undergoing percutaneous coronary intervention (PCI). Detailed inclusion and exclusion criteria have been reported elsewhere14. In brief, patients were eligible if they had 1) 2 non-IRA suitable for intracoronary imaging with non-obstructive atherosclerotic disease (visual estimate >20 to <50% angiographic DS%); 2) LDL-C levels ≥125 mg/dl, if patients were statin-naïve or had not been on a stable (≥4 weeks) statin regimen at the time of screening; or 3) LDL-C ≥70 mg/dl, if patients were on an unchanged statin treatment for ≥4 weeks prior to study enrolment. The study was conducted at 9 academic centres in Switzerland (5), Austria (1), Denmark (1) and the Netherlands (2). A total of 300 patients were randomised in a 1:1 ratio to either alirocumab subcutaneous 150 mg biweekly or a matching placebo between 9 May 2017 and 7 October 2020. Web-based randomisation was performed using randomly varying block sizes of 2, 4, or 6 patients, stratified by study site; use of stable (≥4 weeks) statin treatment at presentation; and type of AMI (ST-elevation myocardial infarction vs non-ST-elevation myocardial infarction). All patients underwent serial coronary angiography and intravascular imaging at baseline and 1-year follow-up. The PACMAN-AMI main trial findings indicate that alirocumab added to rosuvastatin as compared to placebo leads to a 5% plaque regression, according to IVUS, plaque stabilisation as evidenced by a 59% increase in minimum fibrous cap thickness (minFCT), according to OCT, and a 30% decrease of lipid pool, according to NIRS7.
PACMAN-AMI QFR SUBSTUDY
This was a prespecified substudy of the PACMAN-AMI trial7 to assess QFR and 3D-QCA DS% in non-IRA with a proximal reference diameter ≥2.0 mm and >25% DS% as assessed by 3D-QCA (Central illustration). The 25% threshold was based on previous QCA studies51516. The sample size was determined by the main trial14. Analysis was per intention to treat. QFR-specific exclusion criteria included the absence of 2 projections with angles ≥25° apart, lack of isocentre calibration, substantial vessel overlap or vessel foreshortening, severe tortuosity, poor contrast, ostial left main or right coronary artery (RCA) stenosis, TIMI flow ≤2, tachycardia >100/min, and atrial or ventricular arrhythmia.
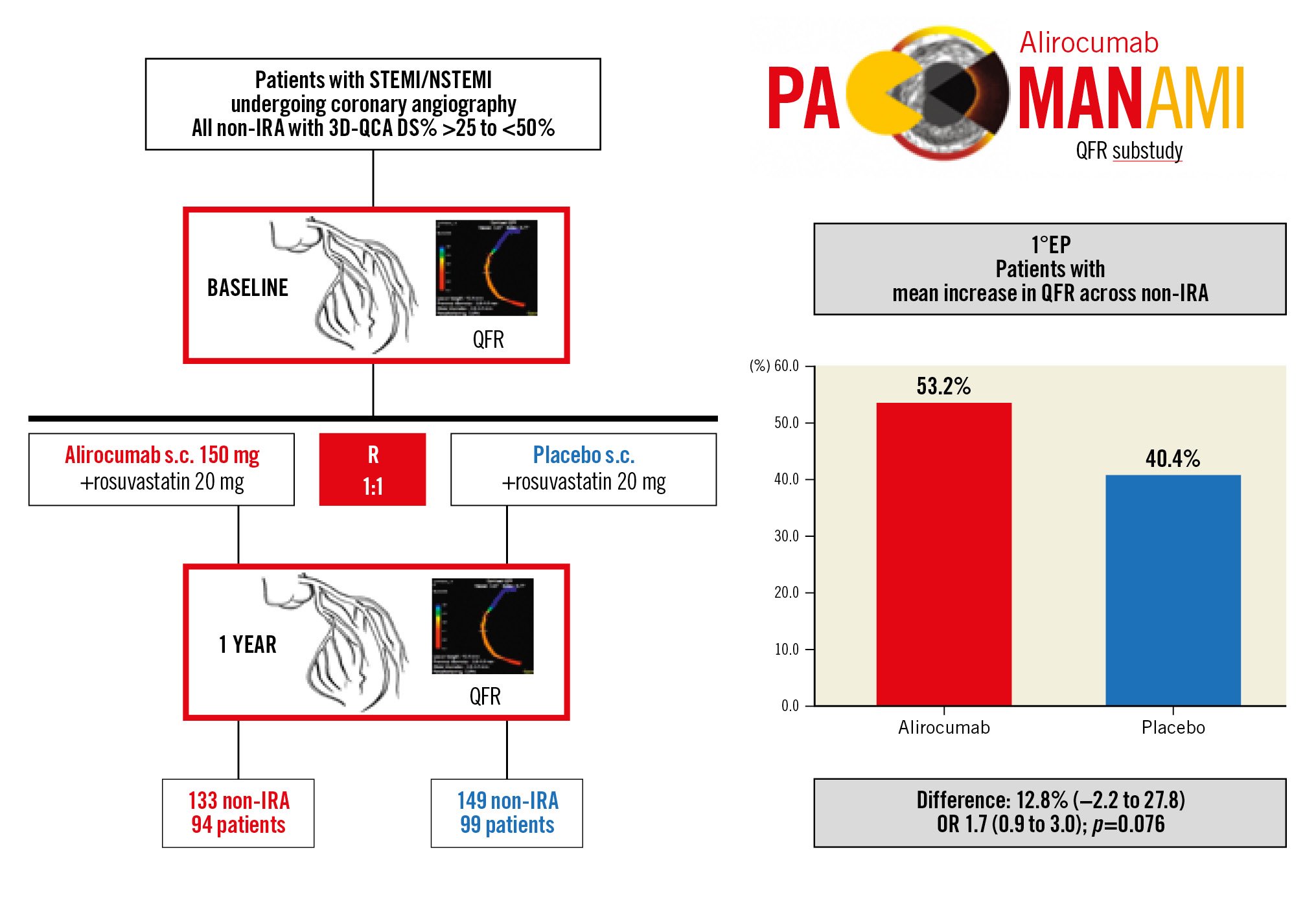
Central illustration. PACMAN-AMI QFR substudy design and primary endpoint result. PACMAN-AMI QFR substudy study design (left) and results of the primary endpoint (1°EP) (right). DS%: percent diameter stenosis; non-IRA: non-infarct-related artery; NSTEMI: non-ST-elevation myocardial infarction; OR: odds ratio; QFR: quantitative flow ratio; s.c.: subcutaneous; STEMI: ST-elevation myocardial infarction; 3D-QCA: three-dimensional quantitative coronary angiography
CORONARY ANGIOGRAPHY
Coronary angiographies at baseline and 1-year follow-up were performed after the administration of 100-200 μg intracoronary nitroglycerine using standard projections.
QFR AND 3D-QCA ANALYSIS
QFR and 3D-QCA were assessed at baseline and 1-year follow-up by experienced analysts blinded to treatment allocation, intracoronary imaging findings, and patient outcomes at the Bern University Hospital core lab, using a dedicated software (QAngio XA 3D, version 1.2; Medis Medical Imaging Systems). All non-IRA with ≥2.0 mm proximal reference diameter and DS% >25% according to 3D-QCA were eligible for QFR measurement. Whole-vessel contrast QFR using frame counting10 was measured from the ostium to a distal landmark at a minimum of 1.5 mm distal vessel reference diameter, as reported previously17. The conventional QFR cutoff of ≤0.80 was used to detect significant ischaemia1011.
INTRA- AND INTEROBSERVER RELIABILITY
For intra- and interobserver reliability analysis, repeated QFR and 3D-QCA analyses by 2 independent core lab analysts including 10 randomly assigned vessels were used.
ENDPOINTS
The prespecified primary endpoint was the number of patients with a mean increase in QFR across non-IRA from baseline to follow-up14. Secondary endpoints were the continuous change in DS% by 3D-QCA and the continuous change in QFR from baseline to follow-up14. Since many stenotic lesions have been reported to better respond to lipid-lowering therapy1819 and the potential effect of alirocumab can be blunted by an expectedly high baseline QFR related to the trial inclusion criteria of only non-obstructive non-IRA, we performed a non-prespecified sensitivity analysis that excluded vessels with the least flow limitation, using an empirical threshold of QFR>0.95 in the absence of prior data. Further, on the patient level, we quantified the relationship between QFR (lowest per patient) and DS% (highest per patient) and intracoronary imaging variables (i.e., IVUS: percentage atheroma volume [PAV; highest per patient]; NIRS: maximum lipid core burden index 4 mm [maxLCBI4 mm; highest per patient]; and OCT: minFCT [lowest per patient]).
STATISTICAL ANALYSIS
Continuous variables are presented as mean±standard deviation (SD) or median (interquartile range [IQR]) and categorical variables are presented as counts with percentages. Baseline characteristics, baseline medications and laboratory values were compared using the Student’s t-test or Fisher’s exact test, as appropriate. QFR variables at baseline were compared using Wilcoxon tests. For the primary endpoint (an increase or decrease in vessel QFR), we used a logistic model to compare the odds of a QFR increase between the alirocumab and the placebo groups. We report the corresponding odd ratios (OR) with 95% confidence intervals (95% CI) and associated p-values. The continuous change between groups for secondary QFR/3D-QCA variables were computed using mixed-effect models; we fitted the interaction between groups (alirocumab or placebo) and timepoints (baseline or follow-up) as fixed effects and patient identity as a random effect. These models account for repeated measures for a given vessel (baseline and follow-up) and for multiple vessels per patient. We report marginal differences between arms with robustified 95% CI. Intra- and interobserver agreement were assessed by intraclass correlation coefficients. All analyses were conducted in Stata 17 (Stata Corp) and R version 4.2.1 (R Foundation for Statistical Computing). Significance tests were 2-tailed with a significance level set to 0.05.
Results
PATIENT POPULATION
Of 300 enrolled patients, 265 underwent serial angiography. Among these, serial QFR was performed in 193 patients with a total of 282 non-IRA (64% of the trial population). The most frequent exclusion criteria were the absence of any non-IRA with >25% stenosis, the absence of 2 appropriate projections ≥25° apart, or a missing isocentre calibration (Figure 1). The mean age was 58 years, 19% were female, 9% had diabetes mellitus, and the baseline LDL-C level was 152.4±33.7 mg/dl. The non-IRA analysed were most frequently in the left anterior descending artery (LAD) territory (39%), followed by the left circumflex (LCx; 34%) and the RCA (27%) territory (Table 1). Patient baseline characteristics, non-IRA analysed, and laboratory values are reported in Table 1. The characteristics of included versus excluded patients from the PACMAN-AMI QFR substudy were comparable (Supplementary Table 1). At 1 year, LDL-C levels decreased to 23.1±21.0 mg/dl in the alirocumab arm compared to 78.3±32.7 mg/dl in the placebo arm (p<0.001). Treatment adherence at 1 year is shown in Supplementary Table 2.

Figure 1. CONSORT flow diagram. aSpecific reasons for other exclusions were not documented at the site of evaluation. See Table 1 in Supplement 4 in the main publication8 for additional inclusion and exclusion criteria. bThis patient fulfilled both exclusion criteria. BL: baseline; DS%: diameter stenosis; FUP: follow-up; LDL-C: low-density lipoprotein cholesterol; non-IRA: non-infarct related artery; PCI: percutaneous coronary intervention; PI: principal investigator; QFR: quantitative flow ratio; RCA: right coronary artery; 3D-QCA: three-dimensional quantitative coronary angiography
Table 1. Patient baseline characteristics.
| N | Alirocumab (N=94) | N | Placebo (N=99) | p-value | ||
|---|---|---|---|---|---|---|
| Age, years | 94 | 58.7±9.8 | 99 | 58.1±8.3 | 0.65 | |
| Sex, female | 94 | 14 (15) | 99 | 22 (22) | 0.20 | |
| Type of AMI | NSTEMI | 94 | 44 (47) | 99 | 45 (46) | 0.89 |
| STEMI | 50 (53) | 54 (54) | ||||
| Statin/LDL-C status at baseline | - On statin for at least 4 weeks prior to PCI and LDL-C ≥70 mg/dl | 94 | 10 (11) | 99 | 14 (14) | 0.52 |
| - No statin and LDL-C ≥125 mg/dl | 84 (89) | 85 (86) | ||||
| BMI, kg/m2 | 94 | 27.4±3.8 | 99 | 28.4±4.6 | 0.10 | |
| Systolic blood pressure, mmHg | 94 | 125.0±21.1 | 99 | 127.1±20.4 | 0.48 | |
| Diastolic blood pressure, mmHg | 94 | 74.4±12.5 | 99 | 74.8±14.7 | 0.84 | |
| Heart rate, beats/min | 94 | 80.2±15.5 | 99 | 76.2±12.7 | 0.05 | |
| Family history of CAD or CVD | 94 | 28 (30) | 99 | 35 (35) | 0.44 | |
| Peripheral arterial disease | 94 | 2 (2) | 99 | 3 (3) | 1.00 | |
| Diabetes mellitus | 94 | 8 (9) | 99 | 10 (10) | 0.81 | |
| Diabetes type | Type 1 | 94 | 2 (2) | 99 | 0 (0) | 0.18 |
| Type 2 | 6 (6) | 10 (10) | ||||
| Arterial hypertension | 94 | 40 (43) | 99 | 43 (43) | 1.00 | |
| Hypercholesterolaemia | 94 | 77 (82) | 99 | 80 (81) | 0.86 | |
| Active smoker | 94 | 52 (55) | 99 | 44 (44) | 0.15 | |
| Previous myocardial infarction | 94 | 1 (1) | 99 | 2 (2) | 1.00 | |
| Previous PCI | 94 | 1 (1) | 99 | 2 (2) | 1.00 | |
| Prematurea CAD, cerebral or peripheral vascular disease | 94 | 16 (17) | 99 | 17 (17) | 1.00 | |
| Non-IRA analysed by QFR/3D-QCA | LAD | 133 | 50 (38) | 149 | 59 (40) | 0.56 |
| LCx | 46 (35) | 51 (34) | 0.97 | |||
| RCA | 37 (28) | 39 (26) | 0.94 | |||
| Laboratory values | Baseline LDL-C, mg/dl | 94 | 155.6±30.3 | 99 | 149.2±36.6 | 0.19 |
| Haematocrit, l/l | 93 | 0.4±0.0 | 94 | 0.4±0.0 | 0.28 | |
| Haemoglobin, g/dl | 94 | 12.0±5.1 | 99 | 12.1±4.9 | 0.89 | |
| White blood cell count, G/l | 93 | 10.4±3.8 | 99 | 10.6±3.7 | 0.61 | |
| HbA1c, % | 87 | 5.8±0.9 | 91 | 5.8±0.9 | 0.72 | |
| Creatinine, µmol/l | 94 | 76.7±14.7 | 98 | 75.4±14.4 | 0.55 | |
| Peak CK, U/l | 91 | 7,560±1,190 | 94 | 491±641 | 0.06 | |
| Peak hs-troponin, ng/l | 92 | 1,354±3,269 | 94 | 726±1,494 | 0.09 | |
| Values are mean±standard deviation (SD) or number (%). p-values are from Student’s t-tests or Fisher’s exact tests. a<55 years in males and <60 years in females (first degree relatives). AMI: acute myocardial infarction; BMI: body mass index; CABG: coronary artery bypass grafting; CAD: coronary artery disease; CK: creatine kinase; CVD: cardiovascular disease; hs: high sensitivity; LAD: left anterior descending artery; LCx: left circumflex artery; LDL-C: low-density lipoprotein cholesterol; non-IRA: non-infarct-related artery; NSTEMI: non-ST-elevation myocardial infarction; PCI: percutaneous coronary intervention; QFR: quantitative flow ratio; RCA: right coronary artery; STEMI: ST-elevation myocardial infarction; TIA: transitory ischaemic attack; 3D-QCA: three-dimensional quantitative coronary angiography | ||||||
INTRA- AND INTEROBSERVER AGREEMENT
Intraobserver agreement analysis for continuous QFR yielded an intraclass correlation coefficient (ICC) of 0.96 (95% CI: 0.86-0.99) and R2(adjusted) of 0.92 (p<0.001). For 3D-QCA DS% ICC was 0.99 (95% CI: 0.96-1.00) and R2(adjusted) was 0.98 (p<0.001).
Interobserver agreement analysis for QFR yielded an ICC of 0.94 (0.79-0.99) and R2(adjusted) of 0.90 (p<0.001). For 3D-QCA DS% ICC was 0.95 (95% CI: 0.82-0.99) and R2(adjusted) was 0.90 (p<0.001).
QFR
At baseline, the median QFR was 0.96 (0.91-0.98) in the alirocumab arm and 0.96 (0.91-0.99) in the placebo arm (p=0.593) (Supplementary Figure 1A, Table 2). Overall, 96.8% (n=273) of vessels had QFR>0.80. The number of patients with a mean QFR increase across non-IRA from baseline to 1-year follow-up was 50 of 94 (53%, 95% CI: 43.2-63.0) in the alirocumab arm versus 40 of 99 (40%, 95% CI: 31.3-50.3) in the placebo arm (difference 13%, 95% CI: –2.2 to 27.8; OR 1.7, 95% CI: 0.9-3.0; p=0.076) (Central illustration, Figure 2A, Table 2). The between-group comparison of the change in continuous QFR from baseline to follow-up was not performed because of the small difference (Supplementary Figure 1A, Table 2). A sensitivity analysis considering only non-IRA with QFR ≤0.95 showed a significantly higher number of patients with a mean QFR increase on alirocumab (n=37 of 57; 65%, 95% CI: 51.9-76.0) compared to placebo (n=22 of 50; 44%, 95% CI: 31.2-57.7) (difference 21%, 95% CI: 0.5-41.3; OR 2.4, 95% CI: 1.1-5.2; p=0.031) (Table 2).
Table 2. Quantitative flow ratio and 3D-quantitative coronary angiography analysis.
| Primary endpoint All patients | Alirocumab N=94 | PlaceboN=99 | Difference % (95% CI) | OR (95% CI) | p-value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Patients with QFR increase | 50 (53% [43-63]) | 40 (40% [31-50]) | 13% (–2 to 28) | 1.7 (0.9-3.0) | 0.076 | ||||
| Primary endpoint Patients with QFR ≤0.95 | N=57 | N=50 | |||||||
| Patients with QFR increase | 37 (65% [52-76]) | 22 (44% [31-58]) | 20.9% (0.5-41.0) | 2.4 (1.1 to 5.2) | 0.031 | ||||
| Secondary endpoints [all patients (vessels)] | Baseline | 1-year follow-up | Change between baseline and 1-year follow-up | ||||||
| Alirocumab [N=94 (133)] | Placebo [N=99 (149)] | p-value | Alirocumab [N=94 (133)] | Placebo [N=99 (149)] | Alirocumab [N=94 (133)] | Placebo [N=99 (149)] | Difference in change | p-value | |
| QFR | 0.96 (0.91-0.98) | 0.96(0.91-0.99) | 0.593 | 0.96(0.92-0.98) | 0.95(0.89-0.98) | 0.00(–0.02 to 0.02) | –0.01(–0.03 to –0.01) | - | - |
| Diameter stenosis, % | 36.96±7.88 | 36.63±8.46 | 0.742 | 35.93±9.58 | 38.33±11.31 | –1.03±7.28 | 1.70±8.27 | −2.50(−4.43 to −0.57) | 0.011 |
| Area stenosis, % | 50.18±12.69 | 49.64±12.32 | 0.715 | 48.84±14.06 | 51.50±14.94 | –1.35±11.68 | 1.86±11.74 | −2.80(−5.73 to 0.14) | 0.062 |
| Lesion length, mm | 18.45±9.94 | 18.45±10.23 | 0.999 | 18.22±9.84 | 17.92±9.21 | –0.23±8.29 | –0.53±9.46 | 0.37(−1.83 to 2.58) | 0.739 |
| Proximal diameter, mm | 3.05±0.58 | 2.85±0.63 | 0.006 | 3.01±0.57 | 2.83±0.57 | –0.05±0.31 | –0.00±0.28 | −0.02(−0.10 to 0.05) | 0.520 |
| Minimum lumen diameter, mm | 2.00±0.53 | 1.82±0.52 | 0.004 | 1.99±0.53 | 1.76±0.56 | –0.01±0.26 | –0.06±0.28 | 0.05(−0.02 to 0.12) | 0.130 |
| Distal diameter, mm | 2.88±0.68 | 2.57±0.63 | <0.001 | 2.85±0.95 | 2.56±0.60 | –0.03±0.68 | –0.01±0.28 | −0.02(−0.10 to 0.05) | 0.520 |
| For the primary endpoint analyses, values are count (%). Patients with a QFR increase are those whose mean change in contrast vessel QFR across vessels is positive. The odds ratio (OR), associated 95% confidence interval (CI) and corresponding p-values were computed using a logistic model. For secondary endpoint analyses, p-values for continuous QFR at baseline are from Wilcoxon tests. For other variables, values are mean (SD) across vessels and differences in change are marginal differences with robustified 95% CIs and p-values computed using repeated mixed-effect models accounting for multiple vessels imaged per patient. Samples sizes correspond to number of patients (vessels). As per substudy design, vessels with diameter stenosis ≤25% were excluded. 3D: three-dimensional; QFR: quantitative flow ratio; SD: standard deviation | |||||||||
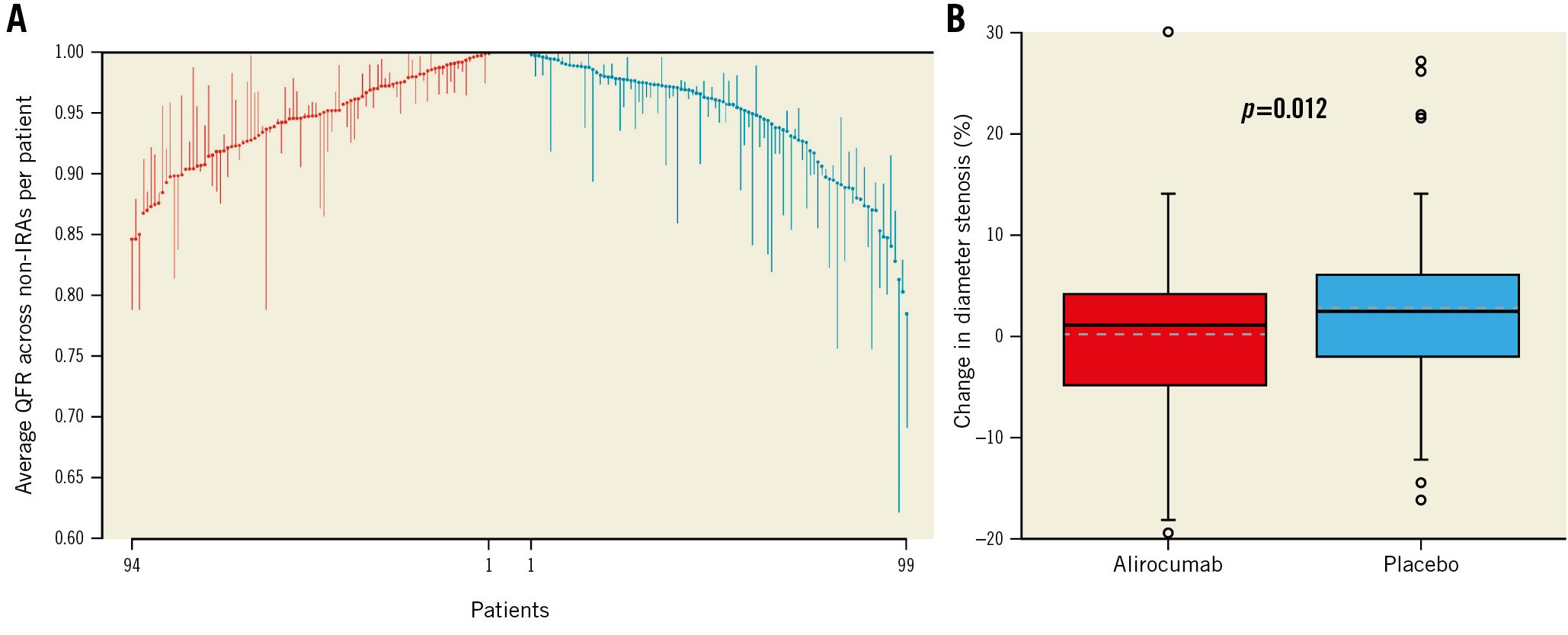
Figure 2. Waterfall plot illustrating quantitative flow ratio changes over time and boxplots with change in diameter stenosis (%). A) Each vertical line relates to 1 single patient and extends from the baseline quantitative flow ratio (QFR) value (averaged across non-infarct-related arteries [non-IRA] for each patient) to the QFR value at 1 year. Descending lines indicate a reduction in QFR over time, whereas ascending lines indicate an increase in QFR. Baseline values are placed in ascending order (red lines) for the alirocumab group and descending order for the placebo group (blue lines). B) Change in diameter stenosis (%) between baseline and follow-up. The ends of the boxes in the boxplots are located at the first and third quartiles, with the black line in the middle illustrating the median, and the dashed line indicating the mean. Whiskers extend to the upper and lower adjacent values, the location of the furthest point within a distance of 1.5 interquartile ranges from the first and third quartiles. Dots indicate more extreme values. Values are per vessel. The grey dashed line represents the mean for each group.
3D-QCA
At baseline, the mean DS% was 36.96±7.88% in the alirocumab arm and 36.63±8.46% in the placebo arm (p=0.742) (Supplementary Figure 1B, Table 2). In total, 92.9% (n=262) of vessels had a DS% <50%. Vessels in the alirocumab arm were larger (including larger minimum lumen diameter [MLD]) (p=0.004) than in the placebo arm, whereas area stenosis (p=0.715) and lesion length (p=0.999) were similar between the treatment groups (Table 2). The mean change in DS% was –1.03±7.28% in the alirocumab arm compared to +1.70±8.27% in the placebo arm (difference in change –2.50%, 95% CI: –4.43 to −0.57; p=0.011) (Figure 2B, Table 2). In the sensitivity analysis considering only non-IRA with QFR ≤0.95, this difference was more pronounced (alirocumab –1.53±7.63% vs placebo +2.74±9.67%, difference in change –4.11%, 95% CI: –7.41 to –0.80; p=0.015) (Supplementary Table 3).
In the exploratory analyses, we observed an association between change in DS% and on-treatment LDL-C (Figure 3, Supplementary Figure 2). Further, we observed a trend towards more pronounced DS% reduction with a higher degree of baseline stenosis (Figure 4) and increasing raw differences in the change in DS% between treatment groups favouring alirocumab, reaching −3.98% with baseline DS% >45% (Supplementary Figure 3).
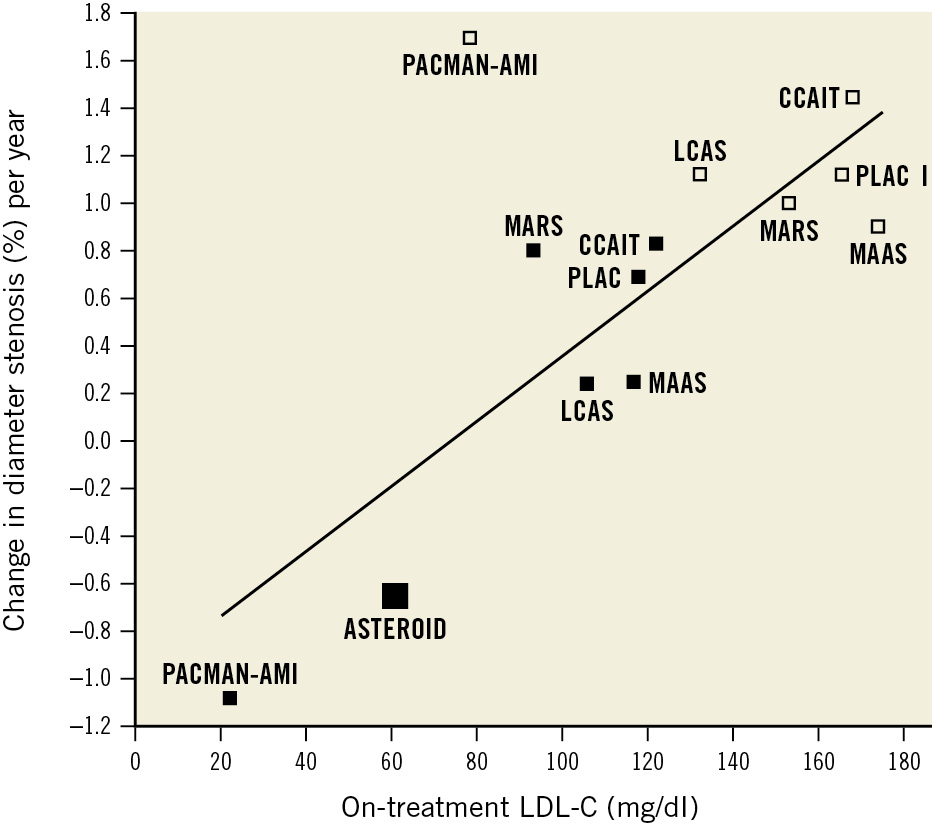
Figure 3. Relationship between change in diameter stenosis % and on-treatment low-density lipoprotein cholesterol. Relationship between change in diameter stenosis (DS%) and on-treatment low-density lipoprotein cholesterol (LDL-C) across 6 trials which investigated the effect of statins versus placebo: the Multicentre Anti Atheroma Study (MAAS)25, the Canadian Coronary Atherosclerosis Intervention Study (CCAIT)15, the Pravastatin Limitation of Atherosclerosis in the Coronary Arteries (PLAC I)24, the Lipoprotein and Coronary Atherosclerosis Study (LCAS)16, the Monitored Atherosclerosis Regression Study (MARS)18, and A Study to Evaluate the Effect of Rosuvastatin on Intravascular Ultrasound-Derived Coronary Atheroma Burden (ASTEROID)5 and the PACMAN-AMI QFR substudy which investigated the effects of alirocumab and rosuvastatin versus rosuvastatin alone. The previous trials assessed DS% by 2D-quantitative coronary angiography (QCA), whereas the PACMAN-AMI QFR substudy assessed DS% by 3D-QCA. Solid squares represent active treatment groups and empty squares represent placebo groups. The regression analysis based on 7 publications was weighted by the number of patients in each trial. Regression analysis: beta=0.013; p<0.001. QFR: quantitative flow ratio; 2D: two-dimensional; 3D: three-dimensional
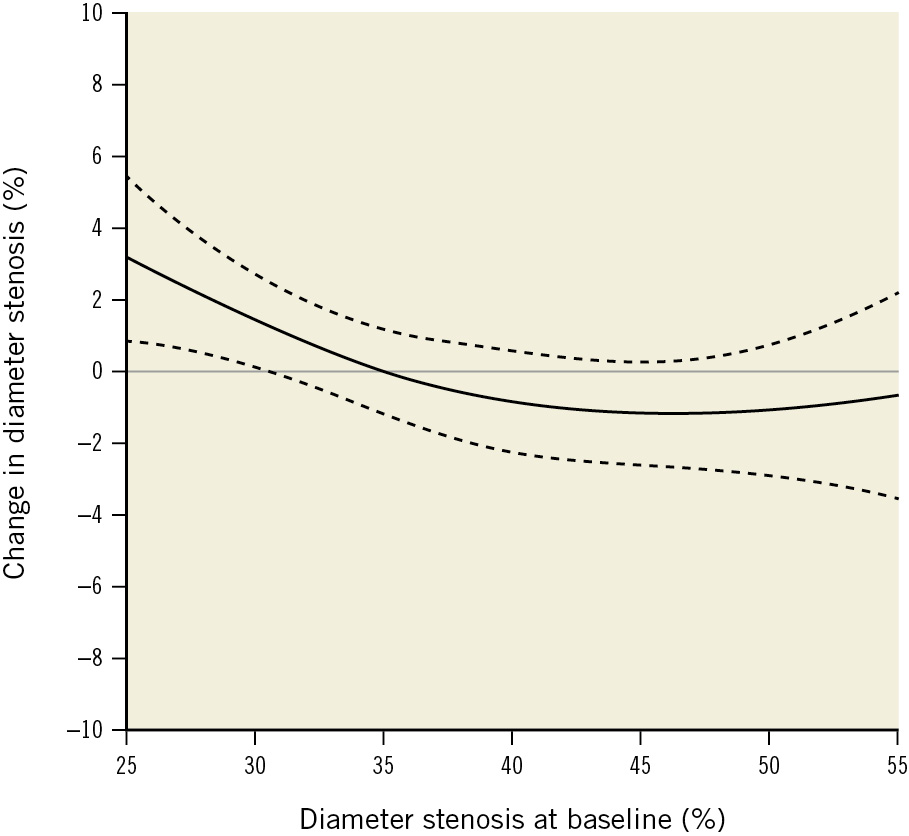
Figure 4. Change in diameter stenosis according to baseline diameter stenosis. Absolute change in diameter stenosis (DS%) between baseline and follow-up according to DS% at baseline. The solid line is the fitted line from a spline mixed-effect model and the dashed lines show the 95% confidence intervals. The x-axis was cropped at 55% due to the small number of values.
ASSOCIATION BETWEEN QFR AND DS% WITH INTRACORONARY IMAGING FINDINGS
For both QFR and DS%, there were significant associations with PAV and maxLCBI4 mm at baseline and at follow-up (Supplementary Figure 4, Supplementary Figure 5), and for QFR, there was a trend towards an association with minFCT at baseline (Supplementary Figure 6). There were no significant associations between the change in DS% and the change in intracoronary imaging variables (Supplementary Figure 7).
Discussion
PACMAN-AMI QFR represents the first prespecified, multicentre, randomised controlled trial substudy to assess the effects of intensive lipid-lowering therapy with the PCSK9 inhibitor alirocumab added to rosuvastatin on coronary haemodynamics as assessed by QFR and 3D-QCA DS% in non-obstructive non-IRA lesions of AMI patients. The salient findings of this study can be summarised as follows: 1) treatment with the PCSK9 inhibitor alirocumab for 1 year did not result in a statistically significant difference in the number of patients with a QFR increase compared to placebo. The results, after excluding vessels with the least flow limitation (i.e., QFR>0.95), suggest that alirocumab may exert a small but significant beneficial effect on coronary haemodynamics in more flow-limiting disease. 2) Patients on alirocumab showed statistically significant regression of DS% by 1.0% compared to a 1.7% increase with placebo.
EFFECTS OF LIPID-LOWERING ON CORONARY HAEMODYNAMICS
Several observational studies suggest an interplay between lipid levels and coronary haemodynamics. Coronary flow reserve (CFR) was shown to be reduced in patients with hypercholesterolaemia even in the absence of angiographically significant coronary artery disease2. Impaired endothelial function and coronary vasodilator capacity related to hypercholesterolaemia have been postulated as mechanistic explanations for this1. Lipid-lowering therapy can counteract the negative effects of hypercholesterolaemia on the coronary vasculature by inducing stenosis regression5, regression of diffuse disease without focal stenosis, and improving microvascular function, as evidenced by an increase in CFR (in the absence of epicardial stenosis) under statin therapy6 or LDL-C apheresis20. Further evidence exists from NIRS studies, where an association between LCBI and fractional flow reserve (FFR)2122 has been reported.
EFFECTS OF LIPID-LOWERING ON QFR
Lipid-lowering therapy may affect QFR by DS% regression5, improvement in microvascular function6, as well as changes in proximal or distal vessel diameters23.
Takenaka et al12 measured QFR in deferred lesions with >50% stenosis at baseline and at 6- to 18-month follow-up in a cohort of stable patients receiving standard lipid-lowering therapy with statins (and ezetimibe if clinically indicated). In patients with increased QFR, they observed lower LDL-C levels than in those with decreased QFR. On-treatment LDL-C was identified as an independent predictor of QFR improvement.
In line with this previous non-randomised investigation, the QFR changes in our study were very small. We only observed a trend towards a significantly higher number of patients with a QFR increase when there was more intensive lipid-lowering over the entire spectrum of baseline QFR. This is likely related to the small degree of flow limitation at baseline, which was substantially higher (median QFR 0.83) in the study by Takenaka et al12. Accordingly, the results of our sensitivity analysis excluding vessels with the least flow limitation (i.e.,>0.95) showed a significant difference in the primary endpoint favouring alirocumab. This may suggest that PCSK9 inhibition potentially induces a greater improvement in coronary haemodynamics across the flow-limiting disease spectrum. This warrants further investigation in the future, i.e., in patients with flow-limiting stenoses. Alternatively, microvascular compensation may blunt the effect of PCSK9 inhibition in lower grade stenoses.
EFFECTS OF LIPID-LOWERING ON DS%
Although previous QCA trials with statins have consistently shown more patients with stenosis regression on statins than on placebo, on average, patients continued to have a progression of atherosclerosis1516182425. ASTEROID was the first QCA study to show a reduction in DS% by 1.3% after 24 months of treatment with rosuvastatin 40 mg5. In general, comparisons of the PACMAN-AMI QFR substudy with previous QCA trials are limited by the inherent differences in 2D- versus 3D-QCA26. However, it is noteworthy, that alirocumab in combination with rosuvastatin reduced DS% after 1 year by almost the same amount as rosuvastatin 40 mg after 2 years in ASTEROID. We also observed an association between change in DS% and on-treatment LDL-C as shown in Supplementary Figure 2. These results are conceptually consistent with a previous summary of major QCA trials on statins, showing a positive linear relationship between change in DS% and on-treatment LDL-C5. The reproduced regression analysis updated by the current study findings is shown in Figure 3. Thus, in summary, the available evidence suggests, that the more intense the lipid-lowering, the higher the number of vessels with a decrease in DS% and the greater the magnitude of the effect.
Previous studies reported lesions with higher DS%1819 according to QCA, as well as plaque burden according to IVUS27, to be more responsive to lipid-lowering therapy. Our data support this concept, as shown in Figure 4. Furthermore, we observed a trend towards more pronounced DS% reduction with alirocumab versus placebo with a higher degree of baseline stenosis, i.e., the raw difference in DS% between treatment groups incrementally increased in favour of alirocumab with higher baseline stenosis, reaching –3.98% with DS% >45% at baseline (Supplementary Figure 3). This suggests, that alirocumab might induce a more pronounced stenosis regression in more obstructive lesions.
POTENTIAL CLINICAL IMPLICATIONS OF THE PACMAN-AMI QFR SUBSTUDY
PCSK9 inhibitors have been shown to reduce cardiovascular events2829. Although we observed significant associations between QFR and DS% with the extent of atherosclerosis in intracoronary imaging (PAV and maxLCBI4 mm), our trial suggests that, in patients with non-obstructive lesions, there seems to be no meaningful improvement of coronary haemodynamics or reduction in angiographic DS%, and thus, PCSK9 inhibitors may rather act through plaque stabilisation than clinically relevant improvement in coronary physiology. Accordingly, these were the PACMAN-AMI main trial findings, showing plaque stabilisation with a 59% increase in minimum fibrous cap thickness (i.e., baseline: 107.0 μm, follow-up: 169.6 μm), a decrease of lipid pool by 30% (i.e., baseline: 260.6 units, follow-up: 181.2 units) and a 5% relative reduction in plaque burden (i.e., baseline: 40.9%, follow-up 38.8%)7.
Limitations
The results of this study must be discussed in the light of several limitations. 1) The study population consisted of 64% of the enrolled patients. For the majority of patients (i.e., 44 of 70 [63%]), this was related to the absence of any non-IRA with 3D-QCA DS% >25%. Furthermore, to be able to follow small QFR changes over time, only the angiographic projections that met the highest quality standards at baseline and follow-up (e.g., 2 projections with optimal contrast and angulation, no overlap, no foreshortening) could be considered, and these quality criteria may have been applied more strictly in our analysis than in clinical settings or one-timepoint QFR studies for decision-making on ischaemia according to QFR (i.e., ≤0.80 vs>0.80). Furthermore, QFR only became available at Bern University Hospital in April 2019, whereas trial recruitment started in May 2017. Some of the technical issues with QFR (e.g., missing isocentre calibration, appropriate follow-up projections) were only detected (and addressed immediately) during dedicated data analysis from April 2019 onwards. However, we have compared the baseline characteristics of patients included versus those excluded from the QFR substudy and have found no differences in meaningful variables to affect the investigated outcomes. Further, among the included patients, baseline characteristics of the placebo and alirocumab groups were well balanced, so that, besides a limited sample size, the substudy as such may be regarded as valid. 2) By study design, patients with obstructive lesions with DS% ≥50% by visual estimation were excluded from the PACMAN-AMI trial. Since lesions with higher DS% seem to regress more with lipid-lowering therapy1819, it is possible that PCSK9 inhibitors would induce greater DS% regression in more stenotic lesions and consequently larger changes in coronary haemodynamics. Also, from a clinical perspective, the primary interest would have been to examine the effects of PCSK9 inhibitors on obstructive/flow-limiting stenoses; however, the feasibility of such a study would be limited in the presence of current guidelines mandating interventional treatment of these stenoses. For feasibility reasons, we therefore investigated non-obstructive lesions. Further, whereas interventional treatment of obstructive/flow-limiting stenoses is mandated by current guidelines, less is known about the detailed effects of medical therapy on non-obstructive non-culprit lesions of AMI patients. Therefore, aiming at mechanistic insights of PCSK9 inhibitors, an important class of lesions was the target of the current investigation. 3) The study was limited to 1 year. 4) Vessels in the alirocumab arm were larger than in the placebo arm, which must be interpreted as a chance finding, since QFR/3D-QCA analysts were blinded to treatment allocation and intracoronary imaging findings. Although our main endpoints were QFR/3D-QCA changes over time, the effect of vessel size at baseline on the overall study findings might therefore be minimal but cannot be excluded as confounding. 5) Since QFR is based on the angiographic lumenogram, we could not assess the impact of positive remodelling. 6) We did not investigate the direct metabolic effects of PCSK9 inhibition on the coronary endothelium, which warrants investigation in future studies.
Conclusions
In this prespecified randomised controlled trial substudy, treatment of AMI patients with the PCSK9 inhibitor alirocumab added to rosuvastatin for 1 year resulted in a significant 1.0% regression of 3D-QCA DS% compared to an increase of 1.7% with placebo. No difference in the change in coronary physiology was observed between treatment groups. However, alirocumab- as compared to placebo-treated patients with a baseline QFR ≤0.95 more frequently showed improvement in coronary haemodynamics.
Impact on daily practice
Treatment of non-obstructive lesions from acute myocardial infarction (AMI) patients with the proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor, alirocumab, as compared to placebo on top of rosuvastatin for 1 year leads to significant 1.0% angiographic stenosis regression. This does not translate into significant improvement in coronary physiology as assessed by quantitative flow ratio. Thus, PCSK9 inhibitors may rather act through plaque stabilisation than through improvement of coronary haemodynamics.
Funding
This study was conducted in an independent academic setting and funded by the Bern University Hospital.
Conflict of interest statement
S. Bär reports research grants to the institution from Medis Medical Imaging Systems, Abbott, and Bangerter-Rhyner Stiftung; and a personal research grant from the Swiss National Science Foundation, outside the submitted work. Y. Ueki reports personal fees from Infraredex, outside the submitted work. G.C.M. Siontis reports personal fees from Abbott Vascular, outside the submitted work. S. Stortecky reports research grants to the institution from Edwards Lifesciences, Medtronic, Abbott Vascular, and Boston Scientific; speaker fees from Boston Scientific; and consulting fees from BTG and Teleflex, outside the submitted work. J.F. Iglesias reports grants to the institution from Biotronik, AstraZeneca, Abbott Vascular, Philips/Volcano, Terumo Corp, Biosensors, and Medtronic; and personal fees from Biotronik, AstraZeneca, Philips/Volcano, Terumo Corp, Bristol-Meyers Squibb/Pfizer, Cardinal Health, Medtronic, and Novartis, outside the submitted work. R.J. van Geuns reports grants from Amgen, InfraRedx, AstraZeneca, and Sanofi; and personal fees from Abbott, outside the submitted work. J. Daemen reports institutional grant/research support from AstraZeneca, Abbott Vascular, Boston Scientific, ACIST Medical, Medtronic, MicroPort, Pie Medical, and ReCor Medical, outside the submitted work. T. Engstrøm reports speaker fees from Abbott, outside the submitted work. I. Lang reports grants and personal fees from Janssen and AOPOrphan; personal fees from MSD; and grants from Neutrolis, outside the submitted work. S. Windecker reports research, travel or educational grants to the institution from Abbott, Abiomed, Amgen, AstraZeneca, Bayer, Biotronik, Boehringer Ingelheim, Boston Scientific, Bristol-Myers Squibb, Cardinal Health, CardioValve, Corflow Therapeutics, CSL Behring, Daiichi Sankyo, Edwards Lifesciences, Guerbet, InfraRedx, Janssen-Cilag, Johnson & Johnson, Medicure, Medtronic, Merck Sharp & Dohm, Miracor Medical, Novartis, Novo Nordisk, Organon, OrPha Suisse, Pfizer, Polares, Regeneron, Sanofi-Aventis, Servier, Sinomed, Terumo, Vifor, and V-Wave. S. Windecker serves as advisory board member and/or member of the steering/executive group of trials funded by Abbott, Abiomed, Amgen, AstraZeneca, Bayer, Boston Scientific, Biotronik, Bristol-Myers Squibb, Edwards Lifesciences, Janssen, MedAlliance, Medtronic, Novartis, Polares, Recardio, Sinomed, Terumo, V-Wave, and Xeltis, with payments to the institution but no personal payments. He is also member of the steering/executive committee group of several investigator-initiated trials that receive funding by industry without impact on his personal remuneration. K.C. Koskinas reports grants from Sanofi, Regeneron, and Infraredx, during the conduct of the PACMAN-AMI main trial; and personal fees from Amgen and Daiichi Sankyo, outside the submitted work. D. Spirk reports personal fees from Sanofi-Aventis (Suisse), outside the submitted work. S. Losdat is employed by CTU Bern, University of Bern, which has a staff policy of not accepting honoraria or consultancy fees. However, CTU Bern is involved in the design, conduct, and/or analysis of clinical studies funded by not-for-profit and for-profit organisations. In particular, pharmaceutical and medical device companies provide direct funding to some of these studies. For an up-to-date list of CTU Bern’s conflicts of interest, refer to http://www.ctu.unibe.ch/research/declaration_of_interest/index_eng.html. L. Räber reports research grants to the institution from Abbott Vascular, Biotronik, Boston Scientific, Heartflow, Sanofi, Regeneron, Medis Medical Imaging Systems, and Bangerter-Rhyner Stiftung; and speaker or consultation fees from Abbott Vascular, Amgen, AstraZeneca, Canon, Occlutech, Sanofi, and Vifor, outside the submitted work. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

