Abstract
Background: Statins have been shown to prevent microvascular dysfunction that may cause periprocedural myocardial infarction after percutaneous coronary intervention (PCI). Evolocumab has more potent lipid-lowering properties than statins.
Aims: The aims of this study were to investigate whether evolocumab pretreatment on top of statin therapy could prevent periprocedural microvascular dysfunction.
Methods: This study included 100 patients with stable coronary artery disease who were scheduled to undergo PCI and had high low-density lipoprotein cholesterol (LDL-C) under statin therapy. Patients were randomised to receive evolocumab 140 mg every 2 weeks for 2 to 6 weeks before PCI (evolocumab group: N=54) or not (control group: N=46). The primary endpoint was the index of microvascular resistance (IMR) after PCI. Troponin T was measured before and 24 hours after PCI.
Results: Geometric mean LDL-C was 94.1 (95% confidence interval [CI]: 86.8-102.1) mg/dl and 89.4 (95% CI: 83.5-95.7) mg/dl at baseline, and 25.6 (95% CI: 21.9-30.0) mg/dl and 79.8 (95% CI: 73.9-86.3) mg/dl before PCI, in the evolocumab group and in the control group, respectively. PCI was performed 22.1±8.5 days after allocation. Geometric mean IMR was 20.6 (95% CI: 17.2-24.6) in the evolocumab group and 20.6 (95% CI: 17.0-25.0) in the control group (p=0.98). There was no significant difference in the geometric mean of post-PCI troponin T (0.054, 95% CI: 0.041-0.071 ng/ml vs 0.054, 95% CI: 0.038-0.077 ng/ml; p=0.99) and in the incidence of major periprocedural myocardial infarction between the 2 groups (44.4% vs 44.2%; p=1.00).
Conclusions: Evolocumab pretreatment did not prevent periprocedural microvascular dysfunction in patients on modern medical management with statins.
Introduction
In patients with stable coronary artery disease (CAD), percutaneous coronary intervention (PCI) not only eliminates myocardial ischaemia and improves quality of life but may also reduce cardiovascular events, including myocardial infarction (MI)12. However, periprocedural MI (PMI) attenuates such benefits of PCI3. Recently, prevention of PMI has become of great clinical interest4567. One of the mechanisms underlying PMI is microvascular dysfunction owing to distal embolisation of plaque materials8. Intravascular imaging studies have shown that large plaque volume and lipid-rich plaques are associated with PMI910. Statins, hydroxymethylglutaryl-CoA reductase inhibitors, are lipid-lowering agents which have plaque stabilising as well as anti-inflammatory properties. Several studies have reported that statins are protective against PMI111213. Although the prevention of periprocedural microvascular dysfunction can be attributed to the benefits of statins, procedure-related complications in the epicardial coronary artery, such as side branch occlusion, may cause myocardial injury. The index of microvascular resistance (IMR) is a novel method to assess coronary microvascular function independently of the epicardial artery1415. Using this technique, we have previously reported that statin pretreatment prevented periprocedural microvascular dysfunction in stable CAD patients undergoing PCI16. However, despite modern medical management with statins, PMI is frequently reported4567.
Evolocumab is a proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor that has strong lipid-lowering properties. A subcutaneous injection of evolocumab 140 mg lowers low-density lipoprotein cholesterol (LDL-C) by approximately 60% in patients on statin therapy1718. The Impact of evolocumab on periprocedural microvascular damage in patients undergoing PCI (EVOCATION) trial was designed to investigate whether pretreatment with evolocumab on the top of intensive statin therapy would reduce periprocedural microvascular dysfunction as assessed by IMR in stable CAD patients undergoing PCI.
Methods
Study design
The EVOCATION trial was a multicentre, randomised, open-label, active-controlled, parallel-group, exploratory, investigator-initiated clinical study (Japan Registry of Clinical Trials identifier: jRCTs051180022). The academic committee designed the protocol, identified the participating institutions, and is responsible for the conduct and oversight of the trial. This trial was funded by investigator-initiated research grants from Amgen K.K. The trial was coordinated by the Center for Clinical Research and Education of Hyogo College of Medicine. The authors are responsible for all study analyses and the final contents of the manuscript. This study was performed according to the Declaration of Helsinki, and in compliance with the Clinical Trials Act in Japan. The Certified Review Board at Hyogo College of Medicine approved this trial (CRB5180005). The design of the trial has been previously described19.
Patients 20 to 85 years old were eligible if they had stable CAD, were scheduled to undergo PCI and had high levels of LDL-C under maximally tolerated statin dose (Central illustration). The type and dose of statin were left to the physician's discretion. Because the guideline recommendation of LDL-C target for Japanese patients with CAD was expected to change during the study period, the definitive level of the high LDL-C was not shown and was set by the attending doctor according to the individual patient’s risk. Patients were required to have been treated with a stable statin dose for at least 2 weeks. Patients with prior MI in the territory of the coronary artery branch (left anterior descending artery, left circumflex artery or right coronary artery) where PCI would be targeted, recent MI in the past 6 weeks, 2 or more vessels or the left main tract as the target for the index PCI procedure, New York Heart Association Class III or IV heart failure, left ventricular ejection fraction <30%, estimated glomerular filtration rate <30 mL/min/1.73 m2, and/or other major comorbidities were excluded. All patients provided written informed consent.
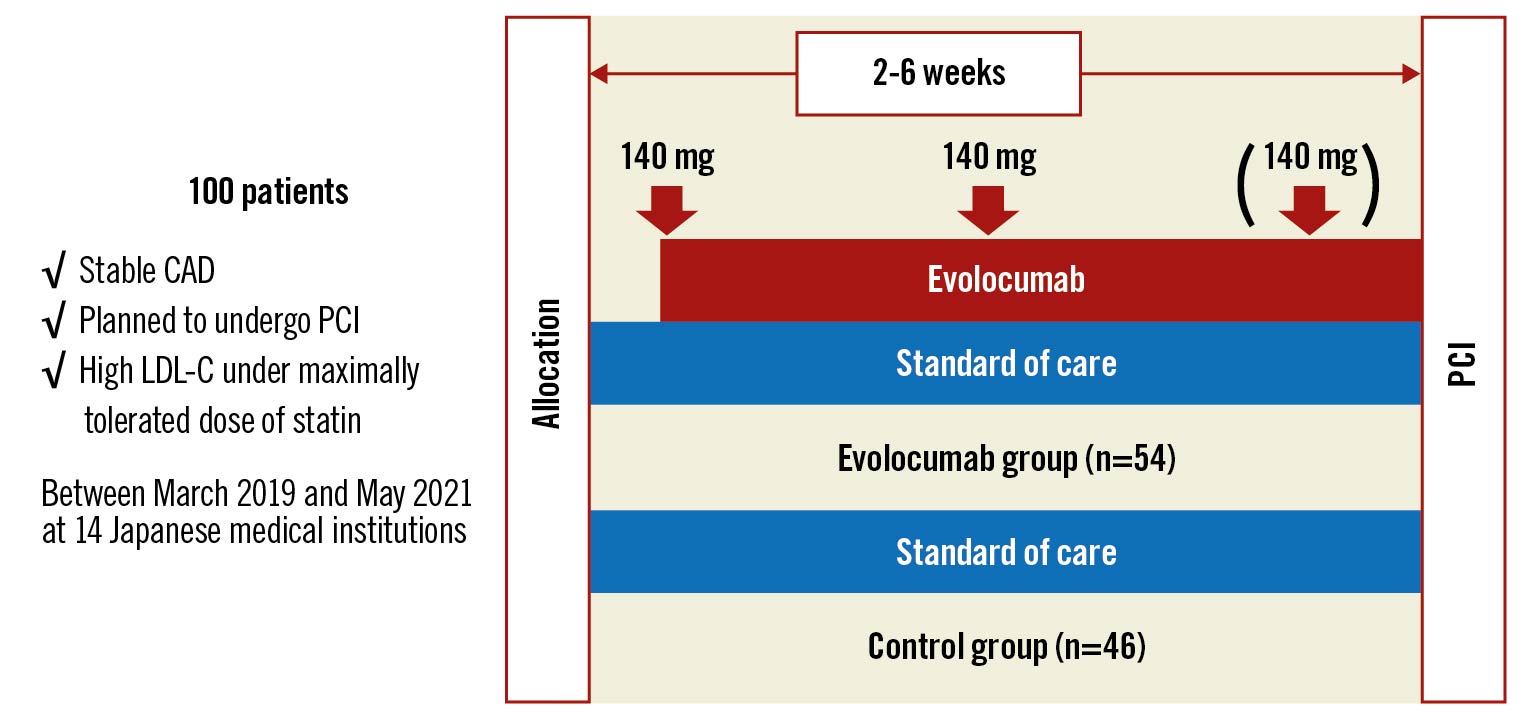
Central illustration. Study design of the EVOCATION trial. This study enrolled 100 patients with stable CAD who were scheduled to undergo PCI and had high LDL-C under a maximally tolerated statin dose. Patients randomised to the evolocumab group received subcutaneous injections of evolocumab 140 mg every 2 weeks before PCI for 2 to 6 weeks after allocation. The primary endpoint was IMR after PCI. CAD: coronary artery disease; IMR: index of microvascular resistance; LDL-C: low-density lipoprotein cholesterol; PCI: percutaneous coronary intervention
Patients underwent randomisation in a 1:1 allocation ratio to receive evolocumab (evolocumab group) or not (control group). Randomisation was performed with use of a central computerised system, with stratification according to the target lesion location (left anterior descending artery or other). Patients randomised to the evolocumab group received subcutaneous injections of evolocumab 140 mg every 2 weeks before PCI for 2 to 6 weeks after allocation. Patients randomised to the control group underwent PCI 2 to 6 weeks after allocation. All patients continued their baseline dose of statins.
Endpoints
The primary endpoint was IMR after PCI. PCI was performed according to standard techniques with coronary stents. Nitrates were administered during PCI, but the use of other vasodilators, including verapamil, papaverine, nicorandil and nitroprusside, were not allowed.
IMR was measured after the final balloon inflation. A coronary pressure-temperature wire (PressureWire Certus; St. Jude Medical/Abbott) was advanced until its sensor was positioned distal to the target lesion. Maximal hyperaemia was induced by intravenous administration of adenosine (120 µg/kg/min) or adenosine triphosphate (160 µg/kg/min). Under steady-state maximal hyperaemia, 3 ml of saline at room temperature was briskly injected through the guiding catheter. The hyperaemic mean transit time (Tmn) was automatically determined with commercially available software (Radi Analyzer; St. Jude Medical/Abbott) and the mean distal coronary pressure (Pd) was simultaneously measured. IMR was calculated as follows:
IMR=Pd x Tmn
The measurements were repeated 3 times. The average of the 3 measurements was used as the IMR value.
Blood samples were obtained at baseline, pre-PCI (within 1 week before PCI) and post-PCI (24±6 hours after PCI). The secondary endpoints for evaluation of myocardial damage after PCI were post-PCI troponin T values and the incidence of PMI. PMI was defined by post-PCI troponin T values >5 x 99th percentile upper reference limit (URL) in patients with normal pre-PCI troponin T values (≤99th percentile URL)6. In patients with elevated pre-PCI troponin T values (>99th percentile URL), troponin T must have risen by >20% and the post-PCI value must have been at least 5 times the 99th percentile URL.
Statistical analysis
Statistical analysis was performed according to the research plan19. Log-transformed IMR was analysed using the 2-way analysis of variance model with the treatment group (evolocumab or control) and lesion location (left anterior descending artery or other) as factors. The adjusted geometric mean of IMR and its confidence interval (CI) were calculated for each group based on the model by back transformation. Treatment effect was assessed by testing that the ratio of the geometric means (evolocumab/control) was equal to 1.
For troponin T, the geometric mean and its 95% CI were calculated for each group. The geometric mean ratio of the treatment groups (evolocumab/control) was also evaluated for troponin T at 24 hours after PCI. Relative changes before PCI from baseline LDL-C, high-density lipoprotein cholesterol, triglycerides, lipoprotein(a), high-sensitivity C-reactive protein, interleukin-6 and pentraxin-3 were analysed in the same manner as the troponin T. The proportions of PMI were compared between groups by Fisher’s exact test.
As post hoc analyses, triglycerides were analysed in the same manner as LDL-C. The log-transformed IMR was analysed using a 2-way analysis of variance model with the incidence of PMI (yes or no) and the target lesion location (left anterior descending artery or other) as factors. The geometric mean ratio of the incidence of PMI (yes/no) was evaluated for exploratory analysis.
A 2-sided p-value <0.05 was considered statistically significant without adjustment of multiplicity. All statistical analyses were conducted using SAS software version 9.4 (SAS Institute).
Results
Patients
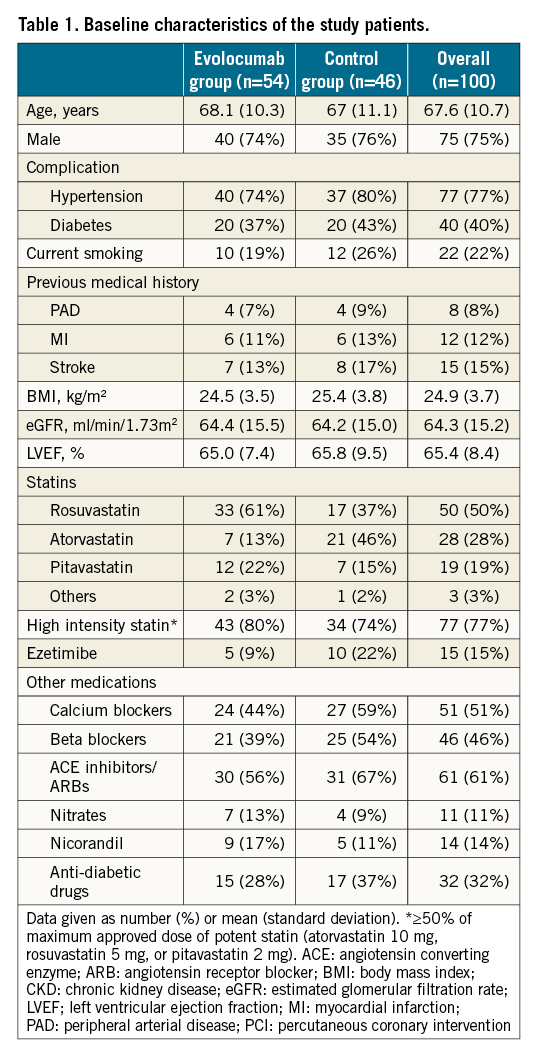
Between March 2019 and May 2021, 100 patients were enrolled and randomised to either the evolocumab group (n=54) or the control group (n=46). Baseline clinical and angiographical characteristics of the randomised patients are shown in Table 1. The 2 treatment groups were well balanced with respect to baseline characteristics. The mean age was 67.6±10.7 years, 75 patients were men, 40 patients had diabetes mellitus, 22 patients were current smokers, 15 patients had prior stroke, 8 patients had peripheral artery disease, and 12 patients had prior MI located in the region of the coronary artery branch other than the target of PCI. Mean left ventricular ejection fraction was 65.4±8.4%. All 100 patients received statin therapy and 15 patients received concomitant ezetimibe therapy; 77 patients received ≥50% of the maximum approved dose of a potent statin.
Laboratory data at baseline and before PCI
Laboratory data are shown in Table 2. A blood sample before PCI was obtained at a mean average of 21.7±8.5 days after allocation. Baseline lipid parameters were comparable between the evolocumab group and the control group. At baseline, 90 patients had LDL-C ≥70 mg/dl and 32 patients had LDL-C ≥100 mg/dl. Decreases in LDL-C, triglycerides and lipoprotein(a) were significantly larger in the evolocumab group than in the control group.
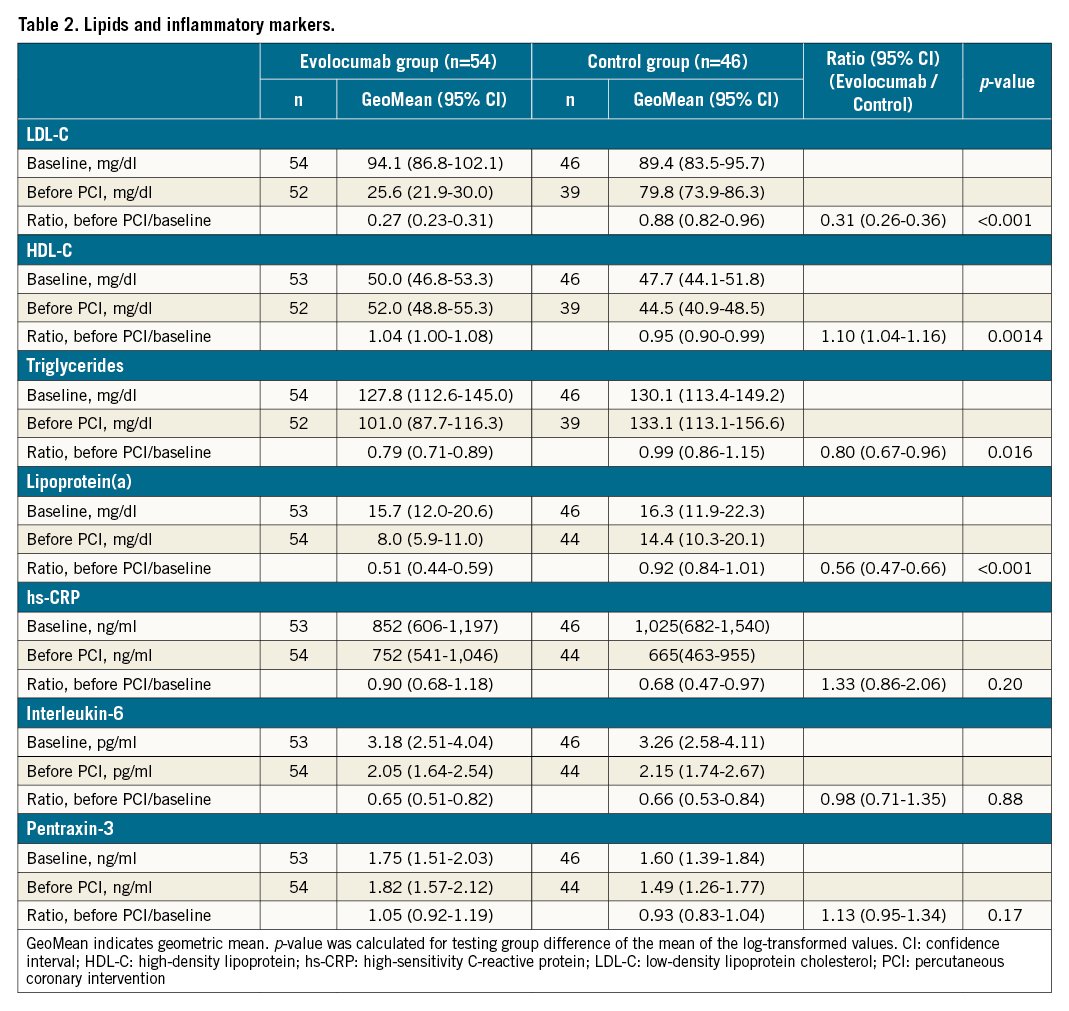
Inflammatory biomarkers, including high-sensitivity C-reactive protein, interleukin-6 and pentraxin-3, were measured. The geometric means of relative change before PCI from baseline for these 3 biomarkers were not different between the 2 groups.
PCI procedure
Angiographical and procedural characteristics are shown in Table 3. PCI was performed 22.1±8.5 days after allocation, 21.0±8.6 days after the first injection and 5.4±4.7 days after the final injection of evolocumab. PCI was cancelled for 1 patient in the control group. The target lesion was located in the left anterior descending artery in 60 patients. There was no clinically meaningful difference in the distribution of target vessels, the number of stents used, total stent length and stent size between the 2 groups. Transient no/slow flow occurred in 3 patients in the control group. Distal embolisation occurred in 1 patient in the control group. Transient ST-segment elevation and chest pain after balloon deflation occurred in 2 patients from the evolocumab group and 2 patients from the control group. No patient had persistent no/slow flow, ST-segment elevation in the electrocardiogram or chest pain at the end of the procedure. There were no other procedure-related complications, including major side branch occlusions. No patient developed new Q-waves.
Endpoints
After PCI, IMR was successfully measured in 97 patients. The geometric mean IMR was 20.6 (95% confidence interval [CI]: 17.2-24.6) in 52 patients allocated to the evolocumab group and 20.6 (95% CI: 17.0-25.0) in 45 patients allocated to the control group (Figure 1). There was no significant difference in IMR between the evolocumab group and the control group (geometric mean ratio, 1.00; 95% CI: 0.77-1.30; p=0.98).
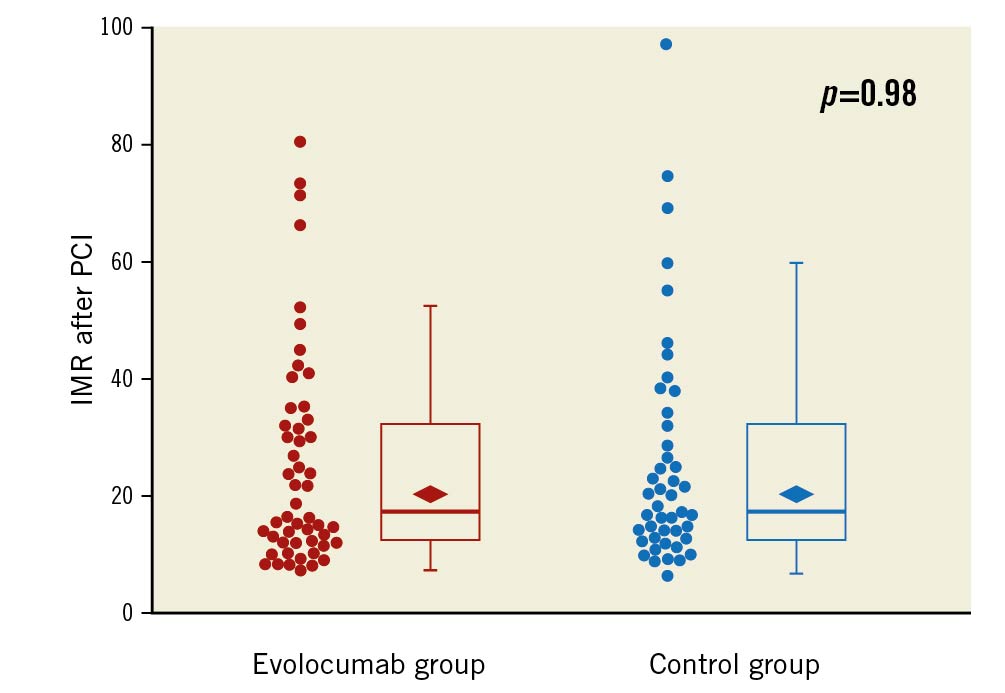
Figure 1. Box plot of IMR in the evolocumab group and the control group. Median IMR was 17.6 (interquartile range [IQR], 12.3-32.2) in the 52 patients allocated to the evolocumab group and 18.0 (IQR, 12.6-31.9) in the 45 patients allocated to the control group. The rhombus indicates geometric mean value: 20.6 (95% confidence interval [CI]: 17.2-24.6) in the evolocumab group and 20.6 (95% CI: 17.0-25.0) in the control group (p=0.98). IMR: index of microvascular resistance; PCI: percutaneous coronary intervention
Troponin T was measured before PCI and 24 hours after PCI in all 54 patients of the evolocumab group, but data were missing in one patient before PCI and two patients after PCI among the 45 patients in the control group. Geometric mean troponin T before PCI was comparable between the evolocumab group (0.009, 95% CI: 0.008-0.011 ng/ml) and the control group (0.010, 95% CI: 0.008-0.012 ng/ml). Geometric mean troponin T 24 hours after PCI was 0.054 (95% CI: 0.041-0.071) ng/ml in the evolocumab group and 0.054 (95% CI: 0.038-0.077) ng/ml in the control group (geometric mean ratio, 0.99; 95% CI: 0.65-1.53; p=0.99) (Figure 2). The incidence of PMI was similar between the evolocumab group and the control group (24/54 [44.4%] vs 19/43 [44.2%], difference, 0.3%; 95% CI: -2.0% to 2.1%; p=1.00). In a post hoc analysis, geometric mean IMR was 24.0 (95% CI: 19.7-29.2) in patients with PMI and 18.5 (95% CI: 15.5-22.2) in those without PMI (geometric mean ratio, 0.77; 95% CI: 0.60-1.01; p=0.056) (Figure 3).
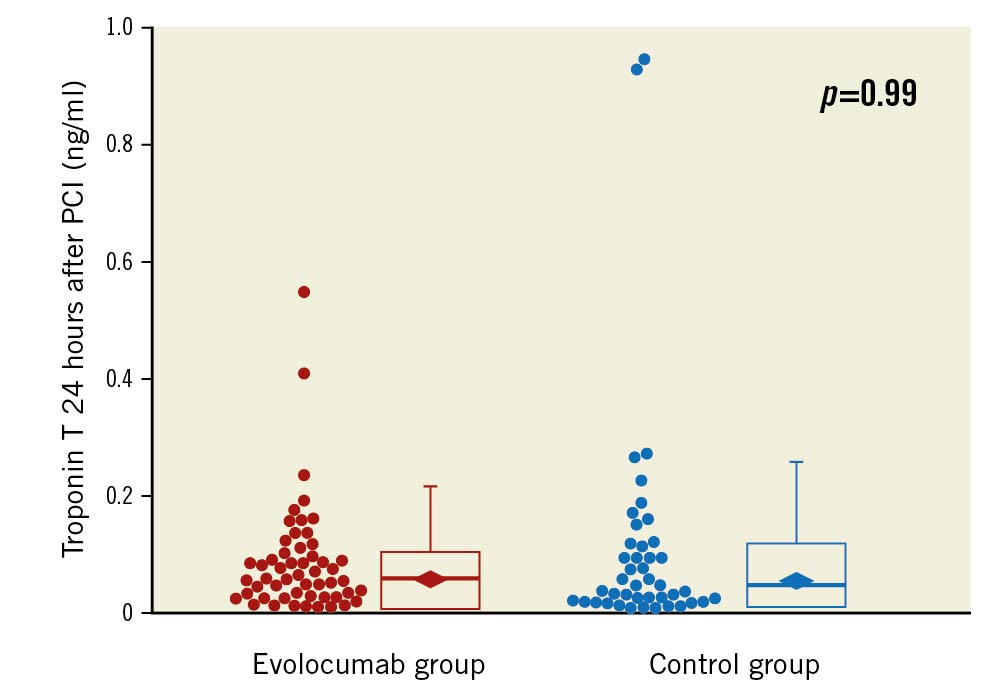
Figure 2. Box plot of cardiac troponin T 24 hours after PCI in the evolocumab group and the control group. Median troponin T was 0.057 (interquartile range [IQR], 0.027-0.102) ng/ml in the 54 patients allocated to the evolocumab group and 0.045 (IQR, 0.023-0.117) ng/ml in the 43 patients allocated to the control group. The rhombus indicates geometric mean value: 0.054 (95% confidence interval [CI]: 0.041-0.071) ng/ml in the evolocumab group and 0.054 (95% CI: 0.038-0.077) ng/ml in the control group (p=0.99). PCI: percutaneous coronary intervention
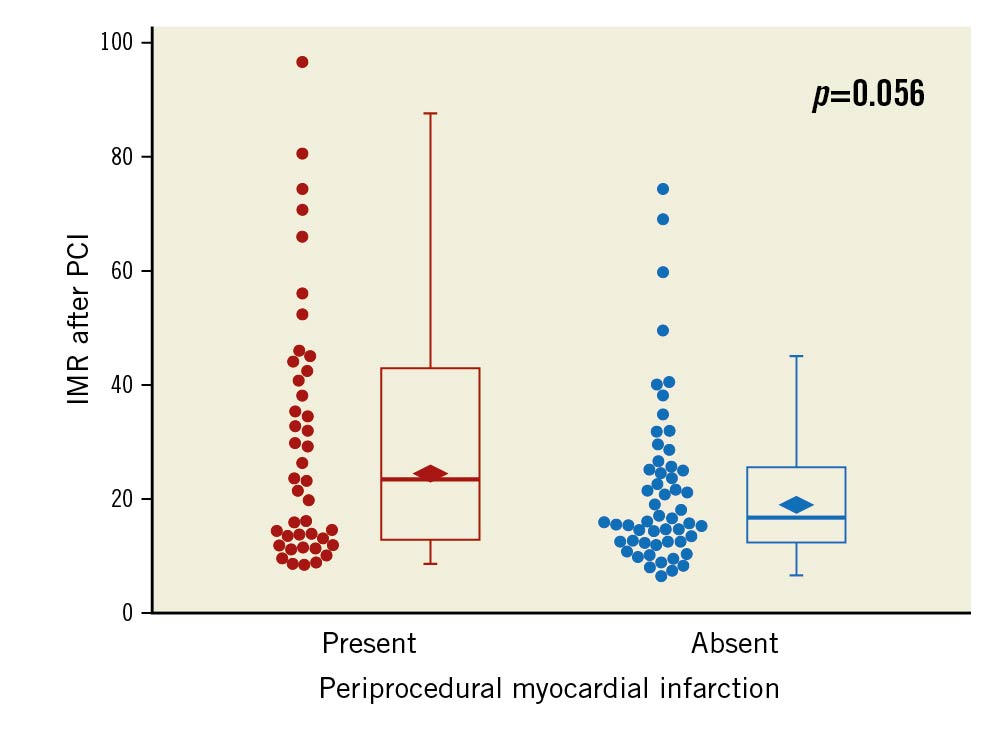
Figure 3. Box plot of IMR in patients with PMI and those without PMI. Median IMR was 23.3 (interquartile range [IQR], 12.9-42.8) in 42 patients with PMI and 16.3 (IQR, 12.4-25.5) in 54 patients without PMI. The rhombus indicates geometric mean value: 24.0 (95% confidence interval [CI]: 19.7-29.2) in patients with PMI and 18.5 (95% CI: 15.5-22.2) in those without PMI (p=0.056). IMR: index of microvascular resistance; PCI: percutaneous coronary intervention; PMI: periprocedural myocardial infarction
Discussion
Findings of this study
In this multicentre, randomised study, we found no difference in IMR after elective PCI in patients with stable CAD when evolocumab pretreatment was given on top of statin therapy compared to standard statin therapy. Troponin T levels 24 hours after PCI and the incidence of PMI were almost identical in the 2 study arms, despite a marked reduction of LDL-C in the evolocumab group.
Legitimacy of study plan
This study included patients with stable CAD who were scheduled to have elective PCI. Patients were randomised to receive either evolocumab or not, on top of statin therapy before PCI. Periprocedural microvascular dysfunction was assessed by IMR after PCI. Troponin T was measured before and 24 hours after PCI and PMI was diagnosed by post-PCI troponin T values >5 x 99th percentile URL. The definition of PMI has been different across studies, largely depending on the type of cardiac biomarkers, the magnitude of elevation of the biomarker and the requirement of angiographic, imaging or electrocardiographic evidence of new myocardial ischaemia. In this study, we defined post-PCI troponin T values >5 x 99th percentile URL without requirement of evidence of new myocardial ischaemia. The Consensus Document from the European Society of Cardiology (ESC) Working Group on Cellular Biology of the Heart and the European Association of Percutaneous Cardiovascular Interventions (EAPCI) was published recently6. They confirmed the prognostic relevance of the post-PCI cardiac troponin T elevation >5x 99th percentile URL threshold used to define type 4a MI. In the absence of periprocedural angiographic flow-limiting complications or electrocardiogram and imaging evidence of new myocardial ischaemia, they proposed the same post-PCI cardiac troponin T cut-off threshold be used to define prognostically relevant major periprocedural myocardial injury. “PMI” in our study corresponded to “major periprocedural myocardial injury” in the Consensus Document, without the requirement of evidence of new myocardial ischaemia. It has been well documented that both type 4a MI and major periprocedural myocardial injury are associated with increased mortality.
For patients undergoing PCI, several studies have shown that pretreatment with statins leads to a reduction in PMI. Although the incidence of PMI differs among studies, depending on the definition, it remains substantially high even among patients receiving modern medical management with statins4567. In this study, the incidence of PMI was 44% in the control group. Such a high incidence of PMI underlined the necessity for the development of a novel therapeutic intervention to prevent PMI. Evolocumab is a monoclonal antibody to PCSK9 that provides potent and rapid reduction of LDL-C. In this study, pretreatment with evolocumab markedly reduced LDL-C from a median 92 mg/dl at baseline to 26 mg/dl before PCI. The Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk (FOURIER) trial demonstrated that evolocumab reduced the risk of major cardiovascular events in patients with atherosclerotic cardiovascular disease on statin therapy17.
The primary endpoint of this study was IMR after PCI. The mechanisms underlying PMI are multifactorial. They were largely categorised into 2 groups according to distinct locations of flow reduction. One group had procedure-related complications in the epicardial coronary artery in proximity to the target lesion, including side branch occlusion, flow-limiting dissection and thrombus formation. Continuous efforts in improving devices, techniques and antithrombotic treatments have been made to avoid these complications of PCI. Another group had microcirculation dysfunction due to the microembolisation of atherosclerotic debris, which is the target for prevention of PMI by pretreatment with lipid-lowering agents. IMR is a unique measure to assess microvascular function independent of the epicardial artery and is suitable for investigating a potential therapeutic intervention for the prevention of periprocedural microvascular dysfunction that may cause myocardial injury. Post hoc analysis of this study showed that IMR was higher in patients with PMI than those without, although the difference was statistically marginally significant. It should be also pointed out that the median IMR in the control group was 18.0 (interquartile range [IQR], 12.6-31.9) which was in agreement with the value 18.0 (IQR, 13.0-30.0) that had been prespecified in the research plan19.
Interpretation of this study
Although not rejecting a null hypothesis does not mean equivalence, it is still noteworthy that post-PCI IMR, post-PCI troponin T and the incidence of PMI were almost identical between the evolocumab group and the control group. These findings suggest that pretreatment with evolocumab 140 mg every 2 weeks for 2 to 6 weeks did not prevent periprocedural microvascular dysfunction and PMI in patients with stable CAD undergoing elective PCI.
Many studies have demonstrated that pretreatment with statins reduces the incidence of PMI in both statin-naïve patients and patients receiving chronic statin therapy111213. Such beneficial effects could be observed before LDL-C decreased. ARMYDA-RECAPTURE (Atorvastatin for Reduction of Myocardial Damage During Angioplasty) reported that a high-dose atorvastatin reload 12 hours before PCI reduced PMI in patients on chronic statin therapy. The beneficial effects of statins are primarily driven by the lowering of LDL-C through inhibiting hepatic cholesterol biosynthesis and upregulating the hepatic LDL receptors. By inhibiting the production of isoprenoid intermediates in the cholesterol biosynthetic pathway, statins also exert LDL-independent (or pleiotropic) effects that alter the production of proinflammatory cytokines and reactive oxygen species, the expression of endothelial nitric oxides synthase, the reactivity of platelets, and the development of cardiac hypertrophy and fibrosis20. Because the degree of isoprenoid inhibition correlates to some extent with the amount of LDL-C reduction, it is difficult to quantify the relative contribution of a statin’s pleiotropic effect to clinical outcomes. However, recent studies reported that anti-inflammatory therapies such as canakinumab and colchicine reduced the risk of recurrent atherosclerotic events in patients with CAD2122. The protective effect against PMI seems to be, at least in part, based on the anti-inflammatory effect of statins. Experimental studies have reported that statins protect against coronary microembolisation-induced cardiac injury via inhibiting inflammation activation23.
Evolocumab decreases lipoprotein(a) which consists of apolipoprotein(a) covalently bound to an LDL-C core by a disulphide bridge, and possesses proatherogenic, prothrombotic and pro-inflammatory properties24. In addition, exposure to exogenous PCSK9 enhances oxidised LDL uptake and vascular cell adhesion protein-1 expression, which play important roles in inflammatory disease, such as atherosclerosis25. It has been reported that inhibition of PCSK9 by small interfering RNA suppresses the inflammatory response induced by oxidised LDL26. In this study, however, inflammatory biomarkers, including high-sensitivity C-reactive protein, interleukin-6 and pentraxin-3, did not change after treatment with evolocumab. Recent studies have reported that monoclonal antibodies to PCSK9 cause an extreme rise in total plasma PCSK9 levels by delayed clearance and increased hepatic secretion27. Although the clinical benefits of PCSK9 inhibitors for patients with atherosclerotic cardiovascular disease and elevated LDL-C levels are clear, large gaps remain in our understanding of PCSK9 biology, including how PCSK9 modulates inflammation28.
The cornerstone of lipid management to prevent atherosclerotic cardiovascular events is “the lower, the better”. Intensive lipid-lowering therapy by either a high-intensity statin or the addition of a PCSK9 inhibitor provides incremental lowering of LDL-C and more clinical benefits than standard therapy. The benefits of a high-intensity statin emerge as early as 30 days and are consistent over time29. However, in the FOURIER trial, the Kaplan-Meier curves of the primary endpoint began to diverge 6 months after randomisation and the magnitude of the risk reduction with evolocumab increased over time, from 12% in the first year to 19% beyond the first year23. Although the protective effect against PMI has been shown to appear very early, within days or weeks after the initiation of statins, pretreatment with evolocumab was not associated with a reduction in microvascular dysfunction and PMI. In this study, time from the first injection of evolocumab to PCI was 21.0±8.6 days, which might have been too short to provide any clinical benefit by intensive lipid-lowering therapy with evolocumab.
Recently, HUYGENS (High-Resolution Assessment of Coronary Plaques in a Global Evolocumab Randomized Study) investigated the effect of evolocumab on coronary plaque phenotype of non-culprit vessels in 161 patients with non-ST-elevation MI30. Patients were treated with evolocumab or a placebo for 52 weeks, and changes in coronary plaque phenotype were assessed by serial measurements with optical coherence tomography. At baseline, the majority had at least 1 image containing minimum fibrous cap thickness <65 μm and a lipid-rich plaque in a non-culprit artery. After 52 weeks of treatment, the evolocumab group had a greater increase in minimum fibrous cap thickness and a decrease in maximum lipid arc and macrophage index. Achieving very low LDL-C with evolocumab treatment for 52 weeks was associated with a stabilisation of lipid-rich, vulnerable plaque in patients with non-ST-elevation MI. In our study, however, the duration of evolocumab pretreatment was only 3 weeks. For patients with stable CAD, the major indication for PCI is the persistence of symptoms despite guideline-recommended medical treatment. Patients with stable CAD may have a longer period of time for evolocumab pretreatment before medical treatment optimisation is achieved, and this may produce a favourable change in coronary plaque phenotype before PCI. Further studies are warranted to investigate whether longer-term pretreatment with evolocumab could prevent PMI in patients with stable CAD.
Another major difference in methodology between HUYGENS and EVOCATION was patient selection. EVOCATION only enrolled patients with stable CAD. The selected patients were from a low-risk PCI population. Most patients had single-vessel disease, short lesions and received a single stent. No patient underwent debulking techniques. These factors were reflected by the low incidence of angiographical complications and clinically or electrocardiographically documented type 4 MI. Pretreatment with evolocumab might have provided some benefits to a high-risk PCI population, including PCI for complex lesions and staged PCI for non-culprit lesions in patients with acute MI.
Limitations
To our knowledge, this is the first study that evaluates the impact of evolocumab pretreatment on periprocedural coronary microvascular dysfunction after PCI in patients with stable CAD on the contemporary medical management with statins. However, our study has several limitations. First, the sample size of our study was small. We calculated the required sample size as 49 patients per treatment group (total of 98 patients) and enrolled 100 patients in this study19. However, due to the application of box randomisation, patients were not assigned in equal numbers to each group (46 patients in the control group and 54 in the treatment group). In addition, 1 patient in the control group did not undergo PCI and 2 patients in the evolocumab group failed to measure IMR after PCI. Thus, IMR was compared between 52 patients in the evolocumab group and 45 patients in the control group. However, it is still noteworthy that geometric mean IMR after PCI was comparable between the 2 treatment groups and was almost identical to the expected value of IMR prespecified in the protocol paper19. We must emphasise that our study was not powered to detect a treatment effect on troponin levels after PCI and the incidence of PMI. Second, this is not a placebo-controlled study. Third, to facilitate entry, measurement of IMR before PCI was not required. Microvascular dysfunction may be present before PCI in patients with stable CAD, even in the absence of prior MI. Measurement of IMR before PCI might have enabled better selection of patients who could potentially benefit from prevention of PMI. We did not collect data on intravascular imaging, including intravascular ultrasound and optical coherence tomography, which might have provided information to define high-risk plaque. Also, other coronary physiology data, including coronary flow reserve and fractional flow reserve, were not collected. Fourth, the statin dose was lower than in previous studies. The recommended statin dose is generally lower in Asian countries, including Japan. It has been reported that response to statin treatment is better and side effects related to high-dose statins are more common in Asians than Caucasians, presumably due to variations in drug metabolism. In Japan, the maximum approved dose is 20 mg for atorvastatin, 10 mg for rosuvastatin and 8 mg for pitavastatin, with the exception of atorvastatin 40 mg and rosuvastatin 20 mg for patients with familial hypercholesterolaemia. In this study, 77 patients received ≥50% of the maximum approved dose of potent statin. Finally, despite the low-risk PCI population of this study, the incidence of PMI was relatively high. This may be, in part, attributable to the lower dose of statins used in our patients. However, it should be noted that the use of statins was comparable between the 2 groups.
Conclusions
Pretreatment with evolocumab 140 mg every 2 weeks for 2 to 6 weeks did not prevent periprocedural microvascular dysfunction and major periprocedural myocardial injury after PCI in patients with stable CAD who were receiving modern medical management with statins. Further studies are warranted to investigate the potential benefits of evolocumab pretreatment to prevent PMI with a different methodology (e.g., longer-term pretreatment) and in other clinical settings (e.g., a high-risk PCI population).
Impact on daily practice
Despite modern management with statin therapy, troponin T elevation >5x 99th percentile of the URL threshold was observed in 44% of patients with stable CAD undergoing PCI. Additional evolocumab pretreatment for 2 to 6 weeks achieved significant reduction in LDL-C but did not prevent periprocedural microvascular dysfunction and troponin T elevation after PCI. Further studies with different methodologies are warranted to investigate the prevention of PMI.
Acknowledgements
We gratefully acknowledge K. Takashima, N. Fujita, N. Kinoshita and T. Ogawa for their secretarial work, M. Toyooka as a clinical research coordinator, and Y. Matsunaga as a data manager. The authors would like to acknowledge the members of the study group and the participating institutions19.
Funding
The EVOCATION trial was supported by Amgen K.K. (grant number, KEG-20192146). Amgen was not involved in the study design, data collection, data analyses or interpretation of the study data. The principal investigator and authors had complete scientific freedom.
Conflict of interest statement
K. Tsujita received remuneration for lectures from Amgen K.K. All other authors have reported that they have no conflicts of interest to declare relevant to the contents of this paper.
Supplementary data
To read the full content of this article, please download the PDF.

