Abstract
Aims: Nicorandil, an ATP sensitive potassium channel opener, may reduce the incidence of microvascular dysfunction after percutaneous coronary intervention (PCI) by dilating coronary resistance vessels. The aim of the study was evaluation of the impact of the administration of intravenous nicorandil on measuring the index of microcirculatory resistance (IMR) in PCI to patients with stable angina pectoris (SAP).
Methods and results: Intravascular ultrasound (IVUS), fractional flow reserve (FFR), IMR and blood examination (CK-MB), cardiac troponin I (cTnI) immediately post-PCI (and 24 hours later) were performed in 62 consecutive patients with SAP undergoing PCI. FFR and IMR were measured simultaneously with a single coronary pressure wire. IMR was defined as Pd/coronary flow (or Pd* mean transit time) at peak hyperaemia. Patients were randomised to the control (n=29), or nicorandil group (n=33). In the nicorandil group, nicorandil was intravenously administered as a 6 mg bolus injection just before PCI and as a constant infusion at 6 mg/hour for 24 hours thereafter. All volumetric IVUS parameters and FFR were similar between the two groups both pre- and post-PCI. However, IMR immediately post-PCI and cTnI 24 hours post-PCI were significantly higher in the control group compared to the nicorandil group (IMR: 25.4±12.1 vs. 17.9±9.1 units, and cTnI: 0.21±0.13 vs. 0.12±0.08 ng/mL, for control vs. nicorandil). The incidence for cTnI elevation more than fivefold the normal range (>0.20 ng/mL) was significantly larger in the control group than in the nicorandil group (41% vs. 12%, p<0.01). Additionally, the control group showed a closer correlation between plaque volume reduction during stenting as assessed by volumetric IVUS, and cTnI elevation than the nicorandil group (r=0.55 vs. 0.42, p<0.001 for control vs. nicorandil).
Conclusions: In patients undergoing successful coronary stenting for stable angina, administration of nicorandil is associated with reduced microvascular dysfunction induced by PCI.
Introduction
Previous studies suggest that the status of the coronary microvasculature is important in determining the long-term outcome after percutaneous coronary intervention (PCI), even in non-emergency settings1-5. The potential underlying mechanism is that microplaque disruption during PCI may increase microvascular dysfunction and myonecrosis, or change the coronary autoregulation system, resulting in subsequent cardiac troponin elevation or worse long-term outcomes1-7. Therefore, pharmacological management to mitigate the effects of embolisation and to decrease periprocedural myonecrosis has clinical significance7,8. Nicorandil, an ATP sensitive potassium channel opener, has been reported to reduce the incidence of microvascular dysfunction after PCI by dilating coronary resistance vessels9-12. Additionally, the index of microcirculatory resistance (IMR), acquired using a coronary pressure guidewire-based system, has been introduced for evaluating the microcirculatory resistance13-16. The advantages of IMR over current methods for assessing microvascular function include its relative ease of use, independence from epicardial vessel stenosis, reproducibility, and its quantitative evaluation13-18. While previous studies have suggested the relationship between myocardial damage and IMR value in patients with acute myocardial infarction18-20, recent studies have reported the usefulness of IMR for assessing microvascular dysfunction induced by PCI in patients with stable angina pectoris7,17,21. Thus, the purpose of this study was to evaluate the impact of nicorandil administration on the status of the coronary microvasculature by measuring IMR with the same coronary pressure wire, using the thermodilution technique.
Methods
STUDY PROTOCOL
This was a randomised, non-blinded, prospective study performed in a single centre. Patients with clinically stable angina pectoris scheduled for elective PCI, using conventional technique, of the left anterior descending coronary artery (LAD) were enrolled. All patients were administered aspirin (100-200 mg) and clopidogrel (75 mg) 24 hours prior to PCI, regardless of background therapy. During cardiac catheterisation, all patients received intravenous heparin bolus sufficient to attain an activated clotting time of 250 to 300 seconds. Patients were randomly assigned to either the control or to the treatment (nicorandil) group. In the treatment group, nicorandil was intravenously administered as a 6 mg bolus injection just before PCI and as a constant infusion at 6 mg/hour for 24 hours (total 150 mg) thereafter. All patients provided written informed consent and the study protocol was approved by the institution’s administrative panel on human subjects. Patients with complicated lesions, such as excessive tortuosity or calcified lesions, unable to cross with pressure wire or IVUS, chronic renal failure (Cr.>1.5), unstable patients, recent myocardial infarction within four weeks, and poor ejection fraction <25%, were excluded from the trial. Simultaneous FFR, coronary thermodilution coronary flow reserve (CFRThermo), and IMR were then measured and IVUS was performed in the LAD, as described in more detail below.
CORONARY PHYSIOLOGIC MEASUREMENTS
Coronary physiological indices (coronary flow reserve [CFR], IMR, and FFR) were measured in each patient before (FFR only) and after PCI (all indices) with previously described methods13-20. A 0.014 inch coronary pressure wire (PressureWire Certus; St. Jude Medical, Minneapolis, MN, USA) was calibrated outside of the body and then advanced so that the pressure sensor was positioned at the ostium of the guide catheter, where equal pressure readings by the guide catheter and the pressure wire were confirmed. The wire was then positioned in the mid to distal LAD (10 to 15 mm distal from the distal edge of the stented segment). The shaft of the pressure wire is able to act as a proximal thermistor and the pressure sensor acts as a distal thermistor. Room temperature saline was injected into the LAD in 3 ml aliquots, three times, and the resting mean transit time of the saline was recorded and averaged. Maximal hyperaemia was then induced by administering either intracoronary papaverine (15 mg) or intravenous adenosine (140 µg/kg/min) via a central venous line. The hyperaemic mean transit time was obtained by injecting 3 ml of saline, three times, and averaging the three individual hyperaemic transit times. FFR was measured by dividing the mean distal pressure by the mean aortic pressure during maximal hyperaemia. The mean transit time is inversely proportional to flow; CFRThermo was calculated by dividing the average resting mean transit time by the average hyperaemic mean transit time. IMR was calculated by dividing the distal pressure by the inverse of the hyperaemic mean transit time, or more simply, multiplying distal pressure by the hyperaemic mean transit time. FFR was measured in each patient before and after PCI. CFR and IMR were measured after PCI in this study.
ASSESSMENT OF CARDIAC MARKERS
Both CK-MB and troponin I levels were systematically measured before intervention, immediately post PCI, and at 24 hours after the procedure. All cardiac marker determinations were performed in the clinical chemistry laboratory by mass determination methods (normal range 5 to 19 IU/L for CK-MB, and 0 to 0.03 ng/ml for troponin I).
INTRAVASCULAR ULTRASOUND
IVUS studies were performed using a commercially available imaging system with a 40 MHz mechanical transducer ultrasound catheter (Atlantis Pro; Boston Scientific, Natick, MA, USA). Following intracoronary nitroglycerine administration, the imaging catheter was advanced >10 mm beyond the target lesion and imaging was acquired to a point >10 mm proximal to the lesion using automated pullback (0.5 mm/s). DVDs containing the intravascular ultrasound images for off-line quantitative analysis were analysed in a blinded fashion. After image acquisition, two-dimensional as well as three-dimensional volumetric analyses were performed using dedicated software (Echo Plaque; Indec systems, Santa Clara, CA, USA). Serial volumetric IVUS at the stented segment was performed pre-intervention and post-PCI. Measurements included the average vessel, lumen, plaque area, and plaque volume reduction (defined as subtraction of pre- and post-PCI plaque volume)22-24. All analyses were performed by the IVUS core laboratory in the Sakakibara Heart Institute of Okayama, blinded to clinical and angiographic information.
STATISTICS
Analyses were performed using SPSS v16 software (SPSS Inc., Chicago, IL, USA). Values are expressed as means±standard deviation. Parameters were compared with the unpaired Student’s t-test to test the differences between two sets of data with normal distributions. If normality tests failed, the Mann-Whitney U test was used. Linear regression was applied to determine correlations between plaque reduction or IMR and elevation of troponin I. A p-value <0.05 was considered significant.
Results
Between February 2011 and August 2011, 62 consecutive patients with stable angina pectoris undergoing elective PCI were enrolled. Patient characteristics, medications and procedural data are summarised in Table 1 and Table 2. All data are identical between the control and the nicorandil groups. At the time of enrolment, approximately 40 to 50 percent of patients were already being treated with antihypertensive agents, including ACE inhibitors or ARBs, and/or statins; however, there was no difference between the control and nicorandil groups. Glycoprotein IIb/IIIa inhibitors were not used because they are not approved in Japan. The incidence of angiographic complications during the procedure was similar between the two groups. Either first-generation drug-eluting stent (DES) CYPHER® (Cordis, Johnson & Johnson, Warren, NJ, USA) (53%) or TAXUS® (Boston Scientific, Natick MA, USA) (47%) was used in drug-eluting stent (DES) treatment and there was no difference between the two groups. The frequency of major side branch occlusions was 2% in the nicorandil group and 5% in the control group (p=0.8). There were no major arterial dissections or slow/no flow occurring in either group. Serial changes in IVUS parameters are presented in Table 3. At pre-intervention and post-PCI, all IVUS measurements, including average lumen and plaque area, were similar between the two groups. At pre-PCI, all cardiac markers were identical between the two groups. There was no change in CK-MB level (14.5±28.4 vs. 13.1±21.8, p=0.70), and troponin I value immediately after PCI (0.02±0.01 vs. 0.02±0.02, p=0.89, for control vs. nicorandil). However, 24 hour post-PCI elevations of the troponin I value tended to be lower in the nicorandil group than in the control group (p=0.06, median: 0.21 vs. 0.11 ng/dl). In addition, the incidence of troponin I elevation more than fivefold the normal range (>0.15 ng/mL) was significantly larger in the control group than in the nicorandil group (41% vs. 12%, p<0.01). Serial changes of FFR values, IMR and CFR after PCI are presented in Table 4. There was no difference in terms of FFR pre- and post-PCI, and CFR value at post-stenting between the two groups. However, IMR value immediately post-PCI was significantly higher in the control group compared to the nicorandil group (25.4 vs. 17.9 [unit], p<0.05). The distribution pattern of IMR values in both the control group and the nicorandil group according to post-PCI troponin I value is shown in Figure 1. Results of linear regression between delta-plaque volume, which is subtracted plaque volume from pre-intervention minus post-PCI, and IMR revealed a modest correlation between plaque volume reduction and IMR (r=0.43, p<0.01). Although there was a significant modest correlation between plaque volume reduction as assessed by volumetric IVUS and IMR in both groups, the control group showed a closer correlation than the nicorandil group (r=0.48, p<0.01 for control, and r=0.40, p<0.01 for nicorandil). In addition, there was a significant modest correlation between plaque volume reduction, as assessed by volumetric IVUS or IMR, and cardiac troponin I elevation in both groups, yet the control group showed a closer correlation than the nicorandil group (r=0.48 vs. 0.40 between IMR and troponin I elevation, r=0.55 vs. 0.42 between plaque volume reduction and troponin I elevation, control vs. nicorandil group, p<0.05 for all).
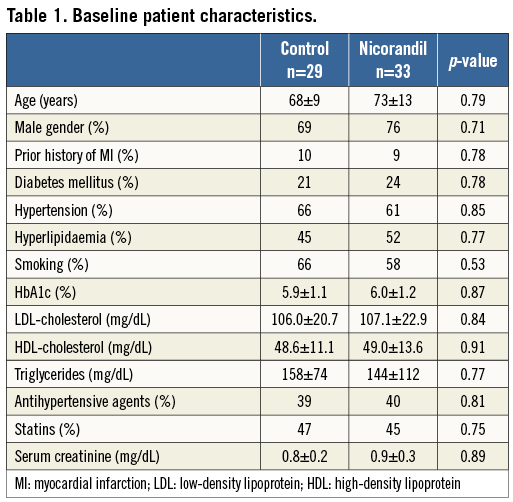
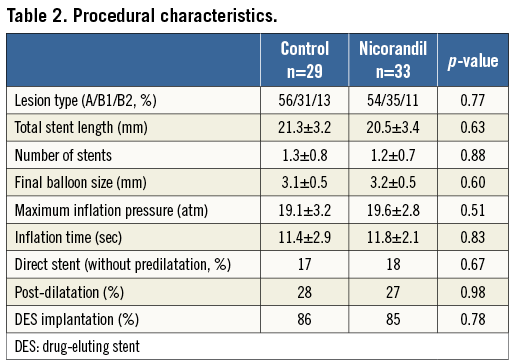
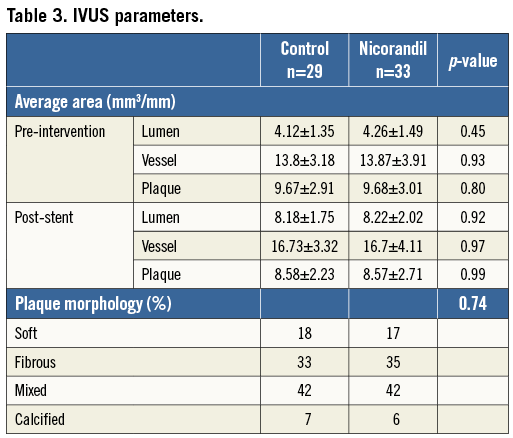
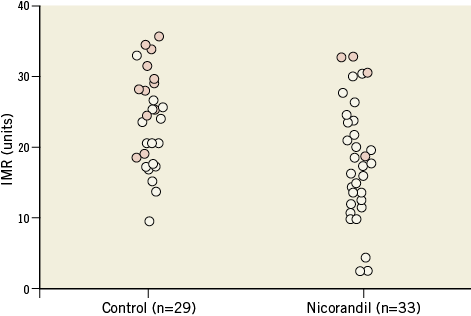
Figure 1. Distribution of IMR values among patients treated with and without nicorandil in post-PCI troponin I value. Solid circles show troponin I >fivefold the upper limit of normal level; open circles show troponin I
Discussion
In the present study, the major finding is that intravenous administration of nicorandil during PCI for patients with stable angina pectoris reduces microvascular dysfunction as assessed by IMR. Post-procedural cardiac marker elevations have been reported to be associated with poor long-term prognosis1-5. Previous pathophysiologic studies demonstrated that the potential mechanism of cardiac marker release resulted from distal embolisation of atherothrombotic material during the interventional procedure, and its occurrence may be entirely “silent”, during or after angiographically uneventful procedures. These studies imply that released atherothrombotic or platelet embolisation (debris) during PCI may affect the downstream microvasculature, resulting in limiting maximum achievable coronary blood flow1-8. A previous report suggested that individual cardiovascular risk factors, such as blood pressure, cholesterol or glucose level, were not independent determinants of IMR21. However, the presence or absence of atheroma was recruited as an independent determinant of IMR. Given that in the present study patients’ clinical and lesion characteristics, including plaque accumulation, were identical between the two groups, the difference in IMR value may be derived from the administration of nicorandil in the treatment group. A previous study reported that renal function and/or pretreatment with statins may affect the incidence of myocardial damage after PCI8,25,26. However, there was no difference in renal function or pretreatment with statins in the present study.
POSSIBLE MECHANISM OF NICORANDIL
Nicorandil is a unique hybrid pharmacologic agent of an ATP-sensitive potassium channel opener and nicotinamide nitrate. It has been reported to improve cardiac function and clinical outcomes in patients with acute myocardial infarction9-12,27-29. It has also been reported that the frequency of myocardial contrast echocardiography no reflow phenomenon was significantly lower in the nicorandil group than in the control group, suggesting improvement of microvascular function with acute myocardial infarction patients10. Furthermore, nicorandil administration is suggested to decrease the incidence of cardiac marker elevations after rotational atherectomy or conventional stenting in patients with stable angina pectoris30,31. Although these underlying mechanisms have yet to be clarified, several potential mechanisms have been reported10,12,25,26,30,31. First, suppression of Na+/Ca2+ exchange and the reduction of calcium in the myocardium by activation of ATP-sensitive K channels may minimise myocardial injury. Second, nicorandil may reduce inflammatory reactions by suppressing neutrophil activation, thereby reducing resistance in the microvasculature. Previous papers showed that neutrophil activation does occur in post-ischaemic coronary microcirculation, and that its inhibition reduces no-reflow34. This reduction may attenuate microvascular injury caused by neutrophils, leading to an increase in myocardial blood flow. Finally, nicorandil dilates microvessels less than 100 μm, resulting in decreased heart preload and/or improved myocardial microcirculation. These results, indicative of improved microcirculation, are attributed to suppression of both microvasculature spasm by vasopressors and inflammatory reactions, leading to the improved IMR value.
DOSE AND ADMINISTRATION PROCESS OF NICORANDIL
In a recent large-scale study, intravenous nicorandil did not affect the left ventricular ejection fraction32. However, another report suggests that higher doses of nicorandil administration reduced the rate of cardiovascular death or readmission to hospital for chronic heart failure9. Furthermore, several studies have demonstrated that microcirculatory disturbance may progress for several hours after coronary reperfusion6,9,10,17. The continuous infusion of nicorandil may restrain the progression of microvascular damage9-12,32,33. For these reasons, we adminstered nicorandil intravenously as a 6 mg bolus injection just before PCI and continued the intravenous administration of nicorandil 24 hours thereafter. While other investigators reported that intracoronary nicorandil administration after coronary revascularisation was safe and effective for restoring blood flow and functional improvement in patients with acute myocardial infarction33, the optimal dose of nicorandil and process for the administration of nicorandil are still under consideration. A recent clinical study showed that intracoronary nicorandil administration can induce hyperaemia in the FFR measurement. In that study, IMR was similar between the adenosine and nicorandil groups. This finding suggests microvasculature dilation by administrating nicorandil, thereby having a potent impact on the results of the present study. However, intracoronary administration of nicorandil has not been established as the “standard” hyperaemic agent measuring FFR. In addition, there was no difference in terms of FFR value in either group, possibly resulting from patients already having achieved a maximum hyperaemic state.
IMR MEASUREMENT AND INTERPRETATION
An increase in the blood flow through microvessels may improve myocardial dysfunction. IMR is recognised as a specific index of microvascular resistance in patients with absence of an epicardial stenosis13-18,21-24. Previous data reported that the index of microcirculatory resistance measured acutely predicts the extent and severity of myocardial infarction in patients with ST-segment elevation myocardial infarction19,20. In addition, coronary microvascular resistance index immediately after primary PCI served as a predictor of the transmural extent of infarction in patients with ST-segment elevation acute anterior myocardial infarction18,29. Most of the available evidence is in the acute setting of an acute myocardial infarction, which is a different clinical condition from stable angina. However, recent studies reported an emerging role for IMR in the interrogation of the status of the coronary microcirculation after successful PCI in patients with stable angina pectoris6,17,21. Therefore, one of the important findings in this study is that IMR is also useful in the validation of microvascular dysfunction in the chronic setting. Likewise, this result concurs with the previous studies using IMR in stable patients6,17,21.
It has been suggested that IMR value was influenced by myocardium mass21. Therefore, its value may reflect the regional change in coronary vessels or territory. Thus, in the present study, we measured the IMR value at the mid portion of the LAD to avoid potential variability. A combination of infusion of adenosine and bolus intracoronary injection of papaverine was used to achieve maximal hyperaemia in our patients. Ideally, a single agent should have been used to achieve hyperaemia to avoid potential variability in the extent of hyperaemia achieved. Furthermore, it has been suggested that there are no differences in the variability of maximal hyperaemic Tmn values within a set of three measurements between adenosine and papaverine21. In addition, there is still ongoing debate whether non-specific agonists such as adenosine/papaverine comprehensively interrogate both the endothelium-dependent and independent components of the coronary microcirculation. In the present study, the control group showed higher IMR than the nicorandil group after PCI. In addition, the control group showed a closer correlation between plaque volume reduction during stenting, as assessed by volumetric IVUS, and cardiac troponin elevation or IMR than the nicorandil group. These results may be helpful for understanding the mechanism of microcoronary circulatory remediation by nicorandil, and seem to concur with previous studies6,10,17,29,30. Further, IMR seems to be a reliable surrogate tool for assessing the degree of interruption of blood supply to and through the microvasculature.
Limitations
There are several limitations in our study. First, a small number of patients with stable angina pectoris were enrolled, and the study was non-blinded, therefore some selection bias may exist. Second, blood marker examinations may miss the peak of cardiac troponin I elevation. In addition, the length of time that these microvascular disturbances persist and their prognostic significance, if any, in the absence of troponin I increase are not known. Previous myocardial infarction in patients may affect IMR value. There are also contradictory data for the prognostic value of troponin I after elective PCI and long-term outcomes.
Conclusion
In this sample of 62 consecutive patients with stable angina undergoing PCI for an LAD lesion, our study results suggest that patients receiving intravenous administration of nicorandil have reduced microvascular dysfunction compared to patients not receiving nicorandil. Larger studies will be necessary to assess the true implications of this study’s findings more adequately.
| Impact on daily practice The status of the coronary microvasculature is important in determining long-term outcome after percutaneous coronary intervention (PCI), even in a non-emergency setting. The potential underlying mechanism is microplaque disruption during PCI, which may increase microvascular dysfunction and myonecrosis, or change the coronary autoregulation system, resulting in subsequent cardiac troponin elevation or worse long-term outcome. Therefore, pharmacological management to mitigate the effects of embolisation and to decrease periprocedural myonecrosis has clinical significance. Nicorandil has been reported to reduce the incidence of microvascular dysfunction after PCI by dilating coronary resistance vessels. In the current study, patients receiving intravenous administration of nicorandil had reduced microvascular dysfunction compared to patients not receiving nicorandil. In patients with stable angina, administration of nicorandil is associated with reduced microvascular dysfunction induced by PCI. |
Conflict of interest statement
The authors have no conflicts of interest to declare.

