Introduction
Assessment of the microcirculation of the heart has gained interest over recent years. This is partly due to the fact that up to 50% of patients with chest pain visiting the catheterisation laboratory do not present with significant epicardial stenosis (so-called angina with non-obstructive coronary artery disease [ANOCA])1.
Most knowledge regarding microvascular resistance has come from non-invasive imaging, from invasive index of microvascular resistance2, or from Doppler wires3, all of which are semi-quantitative and operator-dependent.
Recently, direct quantitative measurement of coronary blood flow and microvascular resistance has become possible by thermodilution with saline infusion, using a pressure-temperature guidewire and a multi-sidehole infusion catheter. Such measurements have been validated versus positron emission tomography (PET)4, have a high reproducibility and are operator-independent5. Procedural safety has been reported previously5; however, long-term safety and absence of late complications have not yet been described. The present study evaluates the safety of absolute flow measurements, both periprocedural, at 30 days and up to one-year follow-up.
Methods
STUDY DESIGN AND POPULATION
In a total of 100 patients, 213 coronary arteries were assessed, and 467 measurements of absolute blood flow and microvascular resistance were performed. The study was approved by the local independent review board (IRB) and informed consent was obtained from all patients to use their data for this post hoc safety analysis.
ABSOLUTE BLOOD FLOW AND RESISTANCE MEASUREMENT
Cardiac catheterisation, absolute blood flow and resistance measurements were performed according to routine, as previously described (Figure 1, Supplementary Appendix 1, Supplementary Figure 1) (complete methods in Supplementary Appendix 2)5,6.
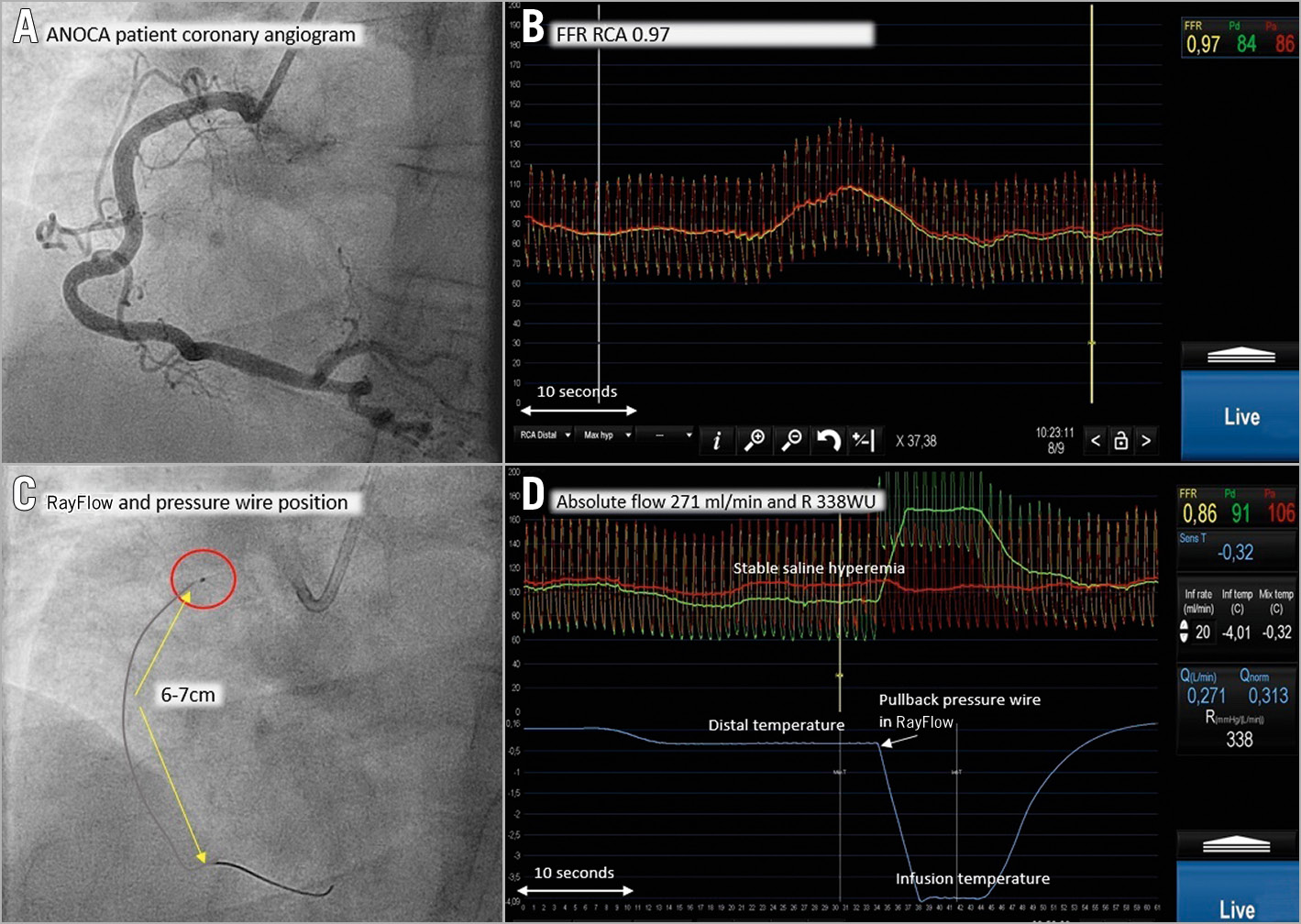
Figure 1. Procedural example. Normal RCA with FFR and absolute flow/resistance measurements. Extensive legend in Supplementary Appendix 1.
ASSESSMENT OF COMPLICATIONS AND SAFETY AT FOLLOW-UP
During the procedure, clinical, haemodynamic and electrocardiographic variables were recorded as usual. Special attention was paid to the occurrence of bradycardia/atrioventricular conduction abnormality. After removal of the RayFlow® catheter and guidewire (Hexacath, Rueil-Malmaison, France), an additional coronary angiogram was performed to document vessel integrity which was reviewed by an independent reviewer. Out-patient visits were scheduled at 30 days and at one year and follow-up data were obtained from the electronic patient files.
STATISTICAL ANALYSIS
Statistics are mainly descriptive. Continuous data are summarised as mean±standard deviation (SD) or median with interquartile range, as appropriate. Categorical data are presented as numbers with percentages. Analyses were performed using SPSS Version 25.0 (IBM Corp., Armonk, NY, USA).
Results
POPULATION CHARACTERISTICS
One hundred consecutive patients undergoing these measurements were included between August 2016 and October 2019. Patient characteristics are presented in Supplementary Table 1. All measurement details of the 467 absolute flow measurements are shown in Supplementary Table 2.
PERIPROCEDURAL SAFETY
Measurements were successful and well tolerated in all patients. Procedural complications are displayed per measurement and infusion rate in Table 1.
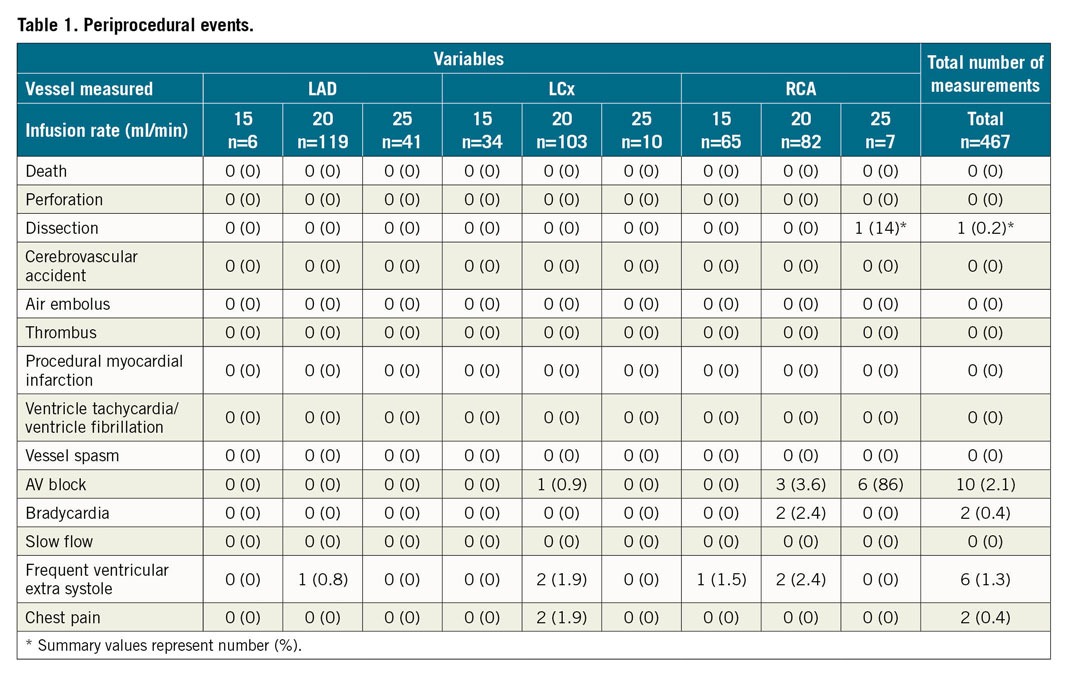
Only two patients experienced chest discomfort during infusion without electrocardiographic abnormalities. In 2.6% of all measurements (n=12), bradycardia or atrioventricular block occurred.
In one patient, dissection of the right coronary artery (RCA) was observed, more proximal than the tip of the infusion catheter. The dissection caused angina and transient ST-elevation and required stenting. After review by two independent interventionalists, this complication was adjudicated to be the result of Amplatz (Medtronic, Minneapolis, MN, USA) guiding damaging the coronary ostium.
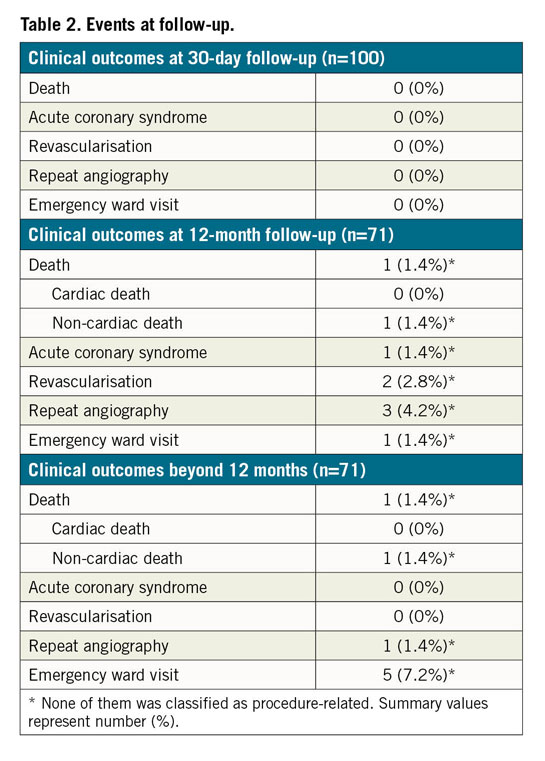
FOLLOW-UP AT 30 DAYS, 12 MONTHS AND BEYOND 12 MONTHS
Follow-up at 30 days was obtained in all patients and follow-up of at least one year in 71 patients. The median follow-up period was 511 days for all patients (range from 99 to 1,054 days). Events at follow-up are presented in Table 2. None of the events occurred in the index coronary artery. In 71 patients, follow-up beyond one year was obtained without any procedure-related adverse events (Supplementary Appendix 3 for complete patient-specific details).
Discussion
The present data demonstrate the safety of invasive measurement of coronary blood flow and microvascular resistance. Except for short, transient conduction disturbances in 2.6% of measurements, no noticeable periprocedural side effects were observed. No vessel-related events such as cardiac death, myocardial infarction (MI), or index vessel-related revascularisation had occurred at 30-day and one-year follow-up. Our study extends the experience of Xaplanteris et al5 and Everaars et al4 on the safety of this methodology.
HYPERAEMIA AND CHEST DISCOMFORT
In our study, mild chest discomfort was noted in only two patients. This is in contrast to the frequently observed chest pain observed during hyperaemia induced by intravenous (IV) adenosine7.
WIRE-RELATED COMPLICATIONS
As with every intracoronary technique, a small risk of 0.5-2%8 of vessel damage is associated with guidewire manipulations. In our study, only one case of coronary ostium dissection occurred and was most likely caused by the Amplatz #2 catheter. Therefore, the risk of the present technique does not seem to be different from the very small risk of any guidewire-based measurements.
INFUSION CATHETER-RELATED COMPLICATIONS
The RayFlow catheter has a diameter of 0.84 mm, comparable to optical coherence tomography (OCT) or intravascular ultrasound (IVUS) probes, with a risk for vessel damage of 0.5-2% in a general population9. In our study, no catheter-related complication could be attributed to the catheter, either periprocedurally or at follow-up.
Limitations
Post-procedure integrity of the coronary artery was assessed by angiography and not with IVUS/OCT. Therefore, minimal vessel injury undetected by the post-procedural angiogram cannot be excluded; however, its clinical relevance would be minimal given the complete absence of any vessel-related complications at follow-up. Finally, the results of this study should be confirmed in large multicentre registries.
Conclusion
Selective measurement of absolute coronary blood flow and microvascular resistance with thermodilution and using a specific multi-sidehole infusion catheter is safe and not related to noticeable adverse events either periprocedurally or at follow-up.
|
Impact on daily practice The results show that this method for invasive assessment of the coronary microcirculation can be used in catheterisation laboratory daily practice without noticeable risk or discomfort for the patient. |
Conflict of interest statement
N. Pijls reports institutional grants from Abbott and Hexacath, being a consultant for Abbott, Opsens, and GE, and holding minor equity in Philips, ASML and HeartFlow. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

