Abstract
Background: Quantification of microvascular function requires the measurement of flow and resistance at rest and during hyperaemia. Continuous intracoronary thermodilution accurately measures coronary flow during hyperaemia.
Aims: The aim of this study was to investigate whether continuous coronary thermodilution using lower infusion rates also enables volumetric coronary blood flow measurements (in mL/min) at rest.
Methods: In 59 patients (88 arteries), the ratio of distal to proximal coronary pressure (Pd/Pa), as well as absolute blood flow (in mL/min) by continuous thermodilution, was recorded using a pressure/temperature guidewire. Saline was infused at rates of 10 and 20 mL/min. In 27 arteries, Doppler average peak velocity (APV) was measured simultaneously. Pd/Pa, APV, thermodilution-derived coronary flow reserve (CFRthermo) and coronary flow velocity reserve (CFVR) were assessed. In 10 arteries, simultaneous recordings were obtained at saline infusion rates of 6, 8, 10 and 20 mL/min.
Results: Compared to baseline, saline infusion at 10 mL/min did not change Pd/Pa (0.95±0.05 versus 0.94±0.05, p=0.49) or APV (22±8 versus 23±8 cm/s, p=0.60); conversely, an infusion rate of 20 mL/min induced a decrease in Pd/Pa and an increase in APV. Stable thermodilution tracings were obtained during saline infusion at 8 and 10 mL/min, but not at 6 mL/min. Mean values of CFRthermo and CFVR were similar (2.78±0.91 versus 2.76±1.06, p=0.935) and their individual values correlated closely (r=0.89, 95% CI: 0.78-0.95, p<0.001).
Conclusions: In addition to hyperaemic flow, continuous thermodilution can quantify absolute resting coronary blood flow; therefore, it can be used to calculate coronary flow reserve and microvascular resistance reserve.
Introduction
To understand the mechanisms of myocardial ischaemia and, more specifically, to quantify microvascular function, it is important to measure volumetric coronary flow (expressed in mL/min). Until recently, only positron emission tomography (PET) was able to measure myocardial perfusion (expressed in mL/min/g of myocardial tissue)1. However, PET for coronary flow is not widely available, and cannot be performed simultaneously with invasive assessment of the coronary circulation.
More than 10 years ago, a method to assess volumetric coronary flow was described2,3. The method is based on the principle of continuous coronary thermodilution. Recent improvements in hardware4 and software5 have enabled the technique to be introduced into routine clinical practice. The method has been shown to be accurate6, reproducible5 and safe7. Moreover, since both hyperaemic absolute flow and pressure in the distal part of the epicardial coronary artery are assessed simultaneously, it is possible to calculate minimal absolute microvascular resistance8.
The infusion of saline at room temperature at a rate of 20 mL/min through the lateral holes of a dedicated infusion catheter induces stable maximal hyperaemia9. The mechanisms of this hyperaemic response are not fully understood10. Consequently, only maximal hyperaemic flow and minimal microvascular resistance could be assessed. Since flow and resistance – in contrast to pressure – are directly proportional to myocardial mass, the clinical significance of maximal flow or minimal microvascular resistance remains elusive, as long as the hyperaemic value is not “normalised” for mass or, alternatively, for the corresponding resting value of flow and resistance.
Accordingly, we aimed to measure resting coronary flow, using continuous thermodilution at lower infusion rates of saline. Practically, by using both Doppler flow velocity wires and wires equipped with pressure/temperature sensors, we investigated whether lower infusion rates of saline at room temperature were able to provide reliable coronary thermodilution tracings under true resting conditions without inducing hyperaemia. For the first time, this would enable the calculation of absolute volumetric coronary flow reserve (CFR) in humans.
Methods
PATIENTS
Patients were selected because they had either stable angina, a strong suspicion of epicardial stenosis on coronary computed tomography, preoperative patent foramen ovale assessment, or new-onset heart failure, or because of a post-percutaneous coronary intervention (post-PCI) coronary angiogram control. Coronaries were considered elegible for flow measurements if they were free from stenosis of more than 30% by visual estimation.
INSTRUMENTATION
Simultaneous pressure and thermodilution tracings were obtained. A 6 Fr guiding catheter was advanced into the coronary ostium of the vessel to be studied, and 0.2 mg of nitroglycerine was administered by intracoronary injection. A guidewire equipped with a pressure/temperature sensor (PressureWire™ X; Abbott Vascular, Santa Clara, CA, USA) was connected to a dedicated software for tracings analysis (CoroFlow™ software system; Coroventis Research AB, Uppsala, Sweden) and properly zeroed. The pressures recorded by the pressure/temperature wire and by the fluid-filled guide catheter were equalised close to the ostium of the coronary artery. Then, this wire was advanced into the distal part of the artery. A dedicated infusion catheter with four lateral side holes (RayFlow®; Hexacath, Rueil-Malmaison, France)4 was connected to the 200 cc syringe of an injector (Medrad® Stellant; Medrad Inc. [now Bayer], Warrendale, PA, USA) filled with saline at room temperature (between 21 and 22°C). The infusion catheter was purged, loaded on the pressure/temperature wire and advanced into the first millimetres of the artery to be investigated. In 27 patients (27 coronary arteries), a Doppler flow velocity wire (FloWire®; Philips/Volcano, San Diego, CA, USA) was connected to a Doppler system (FlowMap®; Philips/Volcano)) and advanced in the distal part of the artery, at the same location as the pressure/temperature wire. The phasic and means tracings of the average peak velocity (APV) signals of the coronary flow were displayed on the screen and electronically stored on the physiologic tracings recorder (Mac-Lab™ Hemodynamic Recording System; GE Healthcare, Chicago, IL, USA), along with the pressure tracings and the electrocardiogram (ECG). High-quality Doppler signals were obtained in all patients only after having buckled the distal tip of the wire to stabilise the Doppler crystal and to read the flow in a retrograde mode. In five patients, this manoeuvre necessitated the use of a microcatheter, which was subsequently retracted into the guide catheter. Doppler signal tracings with artefact were excluded from the analysis.
SALINE INFUSION RATES
In all 88 vessels, infusion rates of 10 mL/min of saline at room temperature were used during 60 to 90 s. Thereafter, hyperaemia was achieved through an infusion rate of 20 mL/min in all but 4 vessels, in which an infusion rate of 15 ml/min was used (3 non-dominant left circumflex artery [LCx] and 1 right coronary artery [RCA]). In 10 vessels in which a Doppler wire was also used, infusion rates of 6 mL/min and 8 mL/min were used in addition to the rates of 10 and 20 mL/min.
PHYSIOLOGIC PARAMETERS
ECG, phasic and mean central aortic pressure (Pa, in mmHg), and phasic and mean distal coronary pressure (Pd, in mmHg) were continuously recorded on the Mac-Lab and CoroFlow systems (n=88). Average peak velocity of coronary blood flow (APV, in cm/s, n=27) was simultaneously and continuously recorded on the Mac-Lab system.
Distal temperature was continuously recorded, along with phasic and mean central aortic pressure and phasic and mean distal coronary pressure, and synchronised with the tracings recorded on the Mac-Lab system (n=88).
Absolute coronary flow is derived from continuous thermodilution (Qthermo ) calculated by the previously validated equation:
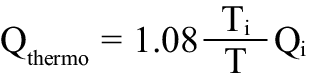
in which Qi is the infusion rate of saline by an infusion pump (in mL/min), Ti is the temperature of the infused saline when it exits the infusion catheter, and T is the temperature of the homogenous mixture of blood and saline in the distal part of the coronary artery during infusion. Ti and T are both relative to the normal blood temperature before the start of the infusion. The constant 1.08 relates to the difference between the specific heats and densities of blood and saline. Details of the measurements have been given previously.
PRESENCE OR ABSENCE OF HYPERAEMIA
To assess the presence or absence of hyperaemia in response to the various infusion rates, changes in pressure gradient (Pa-Pd, in mmHg), changes in the ratio between Pd and Pa (Pd/Pa), as well as changes in Doppler-derived APV were assessed at baseline and during infusion of saline at the various rates.
An example of simultaneous tracings of phasic and mean central aortic pressure, distal coronary pressure, APV and thermodilution during the infusion of 10 mL/min and of 20 mL/min is given in Figure 1.
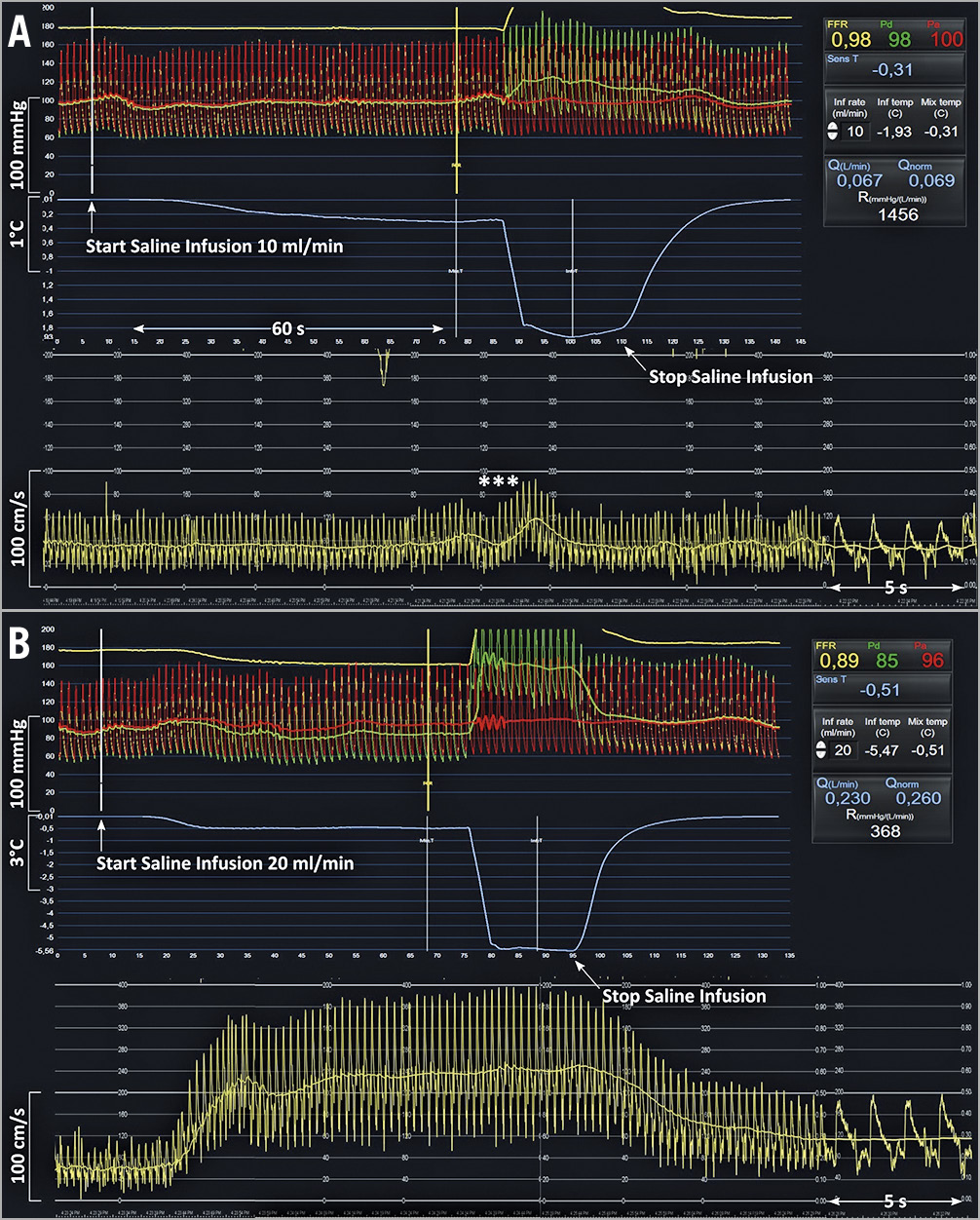
Figure 1. Example of simultaneous tracings of phasic and mean central aortic pressure, distal coronary pressure, average peak velocity and thermodilution during the infusion of 10 mL/min and of 20 mL/min of saline in the LAD of a 77-year-old male patient with mild wall irregularities. A) After the start and during the next 70 s of the infusion of saline at 10 mL/min through the RayFlow catheter located in the proximal LAD, no changes in distal coronary pressure, in Pd/Pa, or in APV were observed (reflecting a resting state). The slight oscillations in flow velocities (***) are caused by the pullback of the pressure/temperature wire. B) In contrast, after the start and during the next 70 s of the infusion of saline at 20 mL/min through the RayFlow catheter located in the proximal LAD, a decrease in Pd, a decrease in Pd/Pa, and an increase in APV were observed. After cessation of the intracoronary saline infusion, these indices returned to their baseline level. On the right-hand side, the CoroFlow software displays instantaneously all relevant parameters, including infusion rate, flow and resistance. APV: average peak velocity; LAD: left anterior descending coronary artery; Pa: aortic pressure; Pd: distal coronary pressure
CORONARY FLOW RESERVE
Absolute CFR, as derived from continuous thermodilution (CFRthermo), was defined as the ratio of absolute coronary flow during saline infusion at 20 mL/min to absolute coronary flow at 10 mL/min (or 6 mL/min or 8 mL/min, when available). Coronary flow velocity reserve (CFVR) was defined as the ratio of APV during infusion of saline at 20 mL/min to the APV at 10 mL/min (or 6 mL/min or 8 mL/min, when available).
DOSE-FINDING STUDY
In order to assess the optimal infusion rate of saline to measure resting flow by continuous thermodilution, both thermodilution and Doppler tracings were recorded at infusion rates of 6, 8, 10 and 20 mL/min in 10 arteries. The optimal infusion rate was selected based on two criteria: (1) infusion rate low enough to avoid microvascular dilation (reflected by the changes in Pd/Pa and in APV), (2) infusion rate high enough to allow reliable thermodilution tracings with an adequate signal-to-noise ratio. In these 10 arteries, CFRthermo was calculated by using the flow measured at 6, at 8 and at 10 mL/min as denominator and compared with the corresponding CFVR.
STATISTICAL ANALYSIS
Normality of the distribution of the values was tested by using density plots and histograms. Continuous data were summarised as mean±standard deviation (SD) or median with interquartile range, as appropriate. Categorical data were presented as numbers with percentages. Measured temperatures and haemodynamic indices at baseline and during saline infusion were compared with the paired t-test or the Wilcoxon matched pairs signed-rank test, as appropriate. The agreement between haemodynamic indices derived from Doppler and thermodilution was assessed using Pearson correlation, Passing-Bablok regression and Bland-Altman analysis. Analyses were performed with R version 3.5.2 (R Foundation for Statistical Computing, Vienna, Austria). A p-value <0.05 was considered statistically significant.
Results
PATIENT CHARACTERISTICS
Sixty-seven patients (96 coronary arteries) were considered for inclusion. In 35 of them, a Doppler wire and a guidewire equipped with a pressure/temperature sensor were used. In 8 of them (23%, 8 arteries) it was not possible to obtain stable and good quality Doppler tracings during the entire study protocol, so that the final study population consisted of 59 patients (Supplementary Figure 1). In 14 of them, a severe lesion was present in a contralateral artery; in 45 only mild diffuse atherosclerosis was present at angiography. Mean age was 64.1±11.8 years; 89% were male. Mean ejection fraction (EF) was 58±8% with 52 (88.1%) patients presenting with normal EF (>55%), 6 (10.2%) with slightly reduced EF (between 35% and 55%) and 1 (1.7%) with severely reduced EF (<35%). A total of 88 arteries were studied. There were 39 left anterior descending arteries (LAD), 19 LCx, and 30 RCA. The baseline characteristics of the patients are summarised in Supplementary Table 1. No complications occurred related to the invasive measurements.
RESTING VERSUS HYPERAEMIC INDICES
In 88 vessels, thermodilution-derived absolute flow and resistances were measured at a saline infusion rate of 10 mL/min and during hyperaemia (obtained by an infusion rate of 20 mL/min). In 4 smaller-sized arteries (3 LCx and 1 RCA), transient bradycardia was observed during the infusion rate of 20 mL/min. In these arteries, hyperaemic measurements were recorded by using an infusion rate of 15 ml/min. Detailed figures of Pd/Pa, coronary blood flow velocity, absolute flow and absolute microvascular resistance as stratified by artery are given in Table 1.
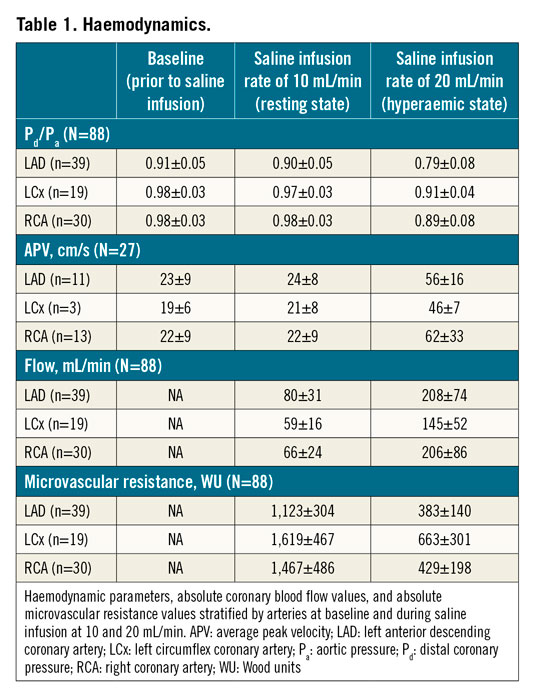
Overall, Pd/Pa at baseline and during saline infusion of 10 mL/min were similar (0.95±0.05 versus 0.94±0.05, mean difference 0.005 [−0.01 to 0.02], p=0.495). Pd/Pa decreased significantly during saline infusion of 20 mL/min (0.85±0.09, p<0.001 versus both baseline and saline infusion of 10 mL/min, n=88) (Figure 2A). Similarly, there was no difference in APV at baseline and during saline infusion of 10 mL/min (22±8 versus 23±8 cm/s, mean difference −0.98 [−6.04 to 4.07], p=0.597, n=27). APV increased during saline infusion of 20 mL/min (58±25 cm/s, p<0.001 versus both baseline and saline infusion of 10 mL/min, n=27) (Figure 2B).
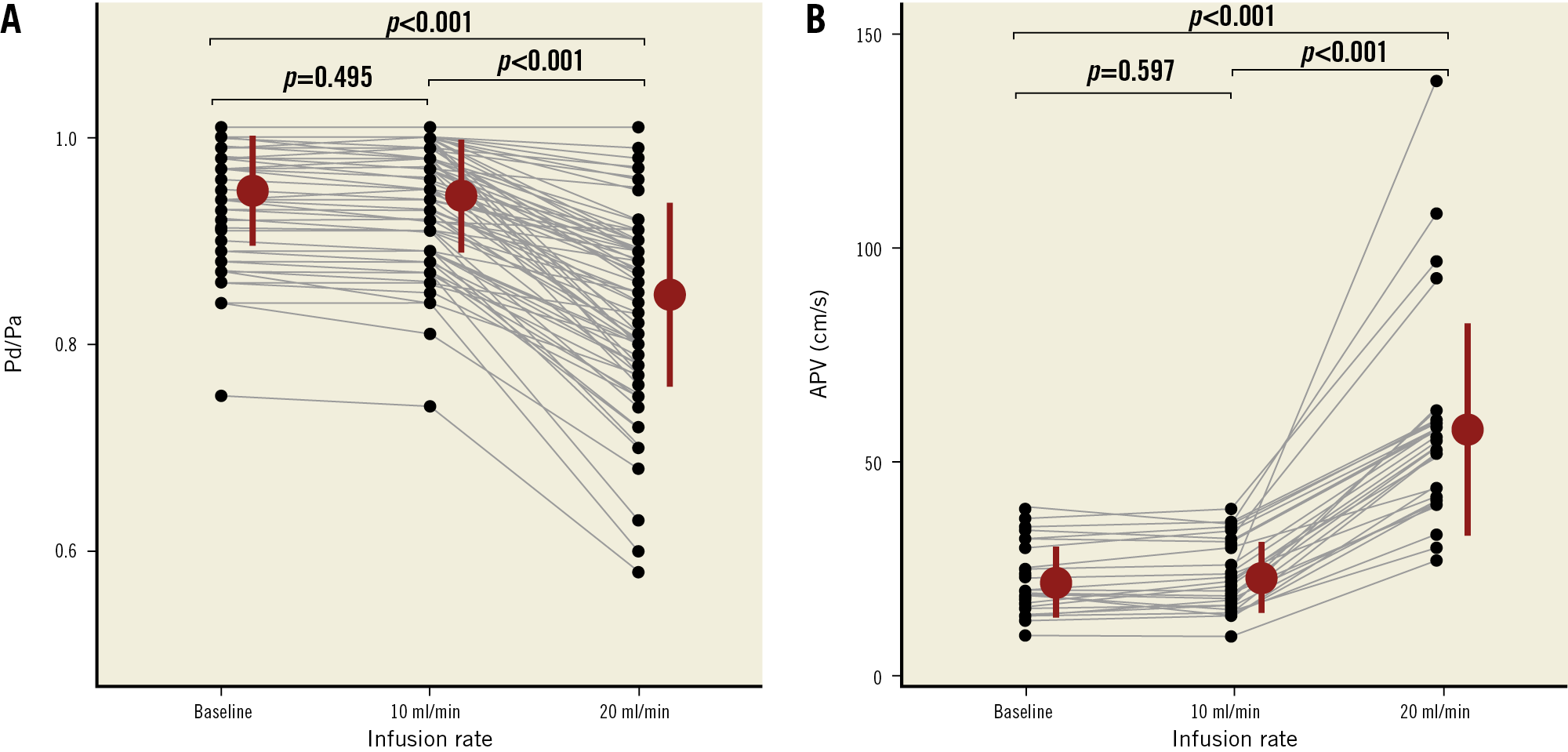
Figure 2. Individual values, mean and SD of Pd/Pa (A) and APV (B) observed at baseline and during saline infusion at 10 and 20 mL/min. APV: average peak velocity; Pa: aortic pressure; Pd: distal coronary pressure
Overall, there was a significant increase in absolute coronary flow during saline infusion of 20 mL/min as compared to 10 mL/min (194±78 versus 71±27 mL/min, respectively, p<0.0001).
CFRTHERMO VERSUS CFVR
Average values of CFRthermo and of CFVR were not significantly different (2.78±0.91 versus 2.76±1.06, mean bias of −0.02 [limits of agreement: −0.95 to 0.91], p=0.935). In addition, there was a strong correlation between the individual values of CFRthermo and of CFVR (Pearson r=0.90, 95% CI: 0.78-0.95, p<0.001). No systematic or proportional differences were observed between CFRthermo and CFVR (Passing-Bablok coefficient A 0.03, 95% CI: −0.61 to 0.55, and coefficient B 1.01, 95% CI: 0.79 to 1.30) (Figure 3).
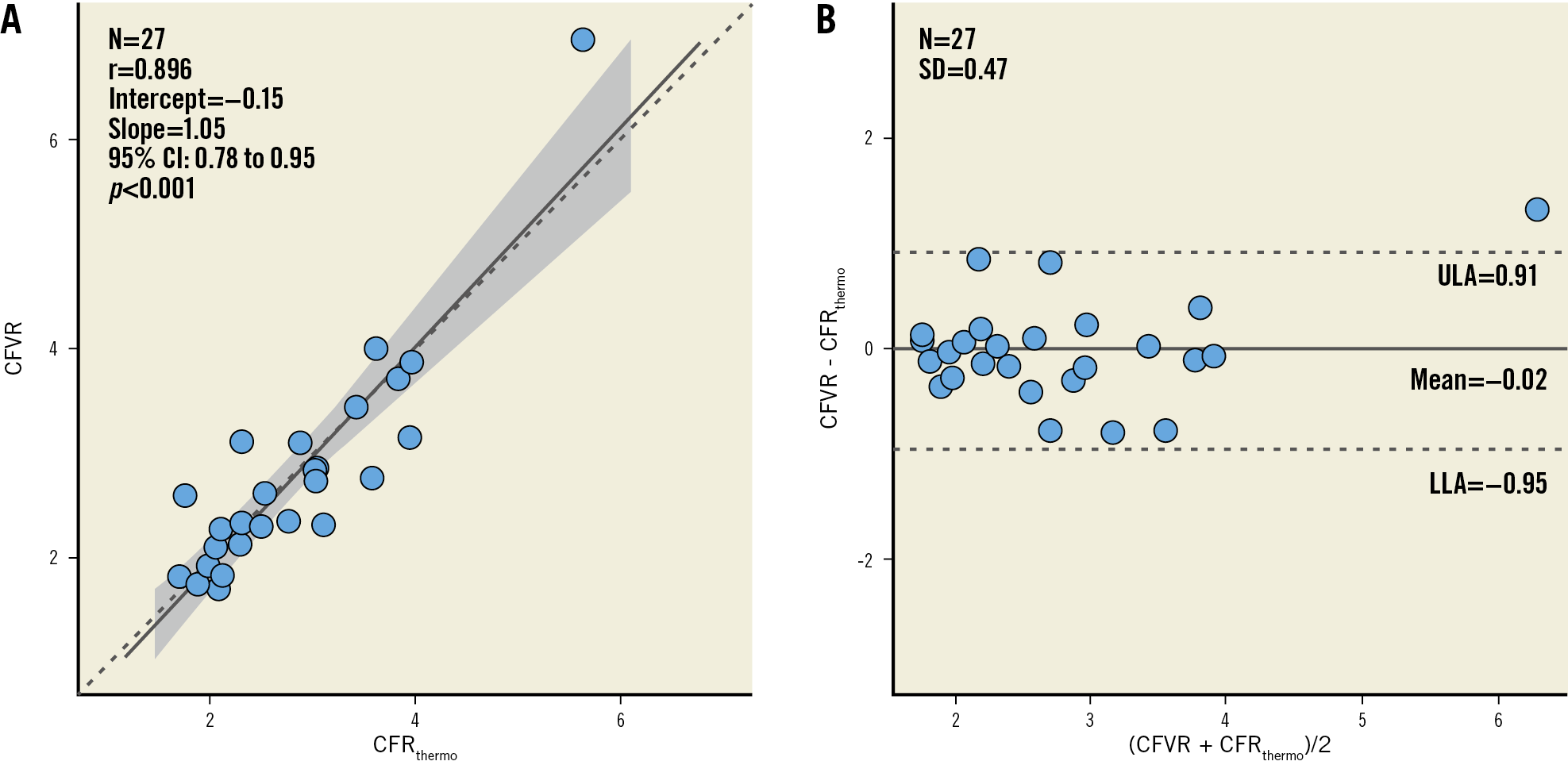
Figure 3. Correlation (A) and agreement (B) between thermodilution-derived coronary flow reserve (CFRthermo) and coronary flow velocity reserve (CFVR).
DOSE FINDING
In 10 vessels (3 LAD, 6 RCA, 1 LCx), simultaneous thermodilution and Doppler measurements were obtained at baseline, and during saline infusion at rates of 6, 8, 10 and 20 mL/min. Both Pd/Pa and APV did not change during saline infusion at 6, 8 and 10 mL/min as compared to baseline. Pd/Pa values (mean±SD) were 0.94±0.05 (baseline), 0.94±0.06 (6 ml/min), 0.94±0.06 (8 ml/min), 0.93±0.07 (10 mL/min), and 0.82±0.13 (20 mL/min). APV values (mean±SD) were 25±10.7 cm/s (baseline), 25.1±10.2 cm/s (6 mL/min), 27±10.8 cm/s (8 mL/min), 25.6±11.6 cm/s (10 mL/min), and 56.9±26.5 cm/s (20 mL/min). With 20 mL/min, Pd/Pa decreased significantly and APV increased significantly compared to baseline (p=0.031 and p=0.002, respectively) (Figure 4). However, with 6 mL/min, the decline in distal temperature during saline infusion was inconsistent and the thermodilution tracings were unstable in 6 out of 10 cases.
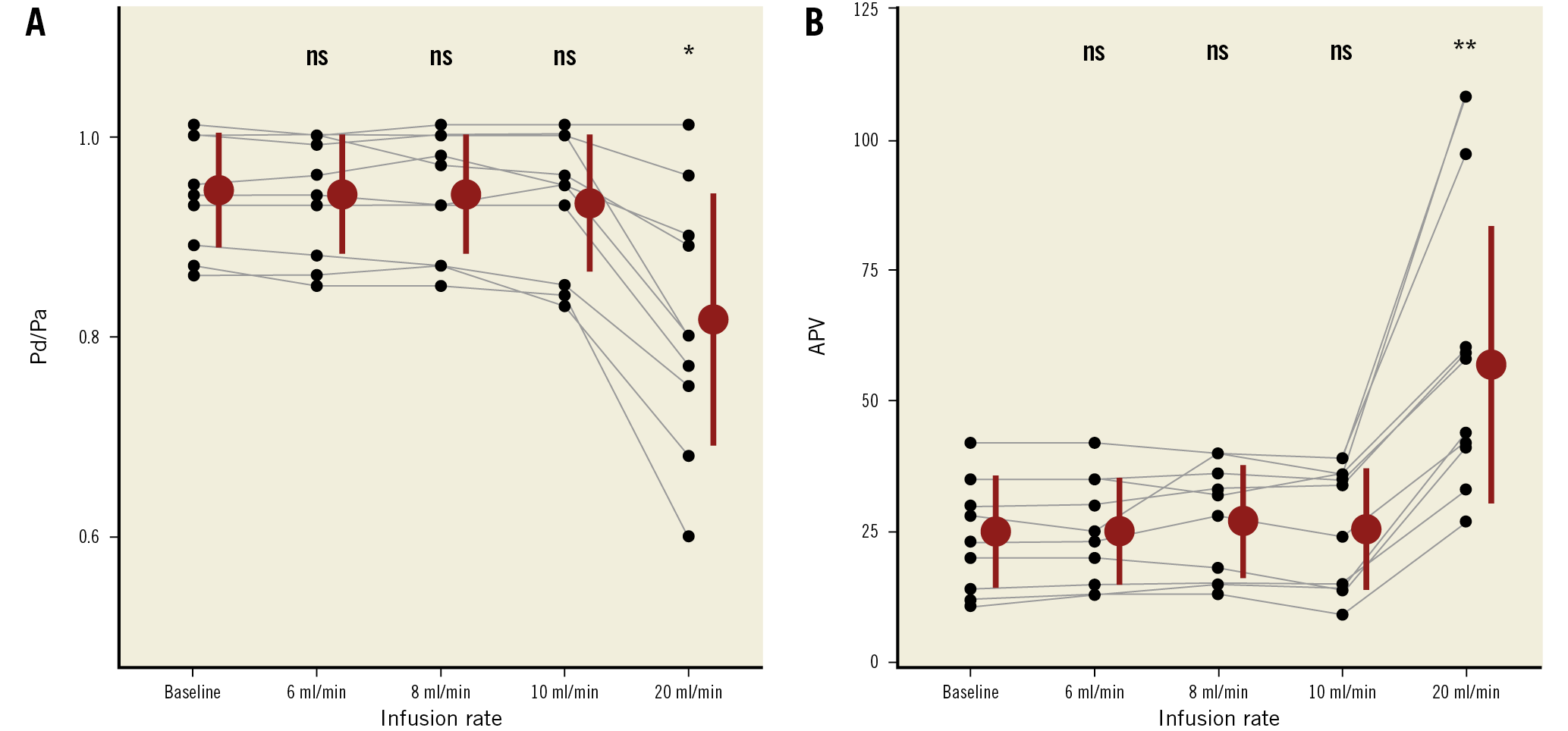
Figure 4. Individual values of of Pd/Pa and APV in the “dose-finding study” (6, 8, 10, 20 mL/min). ns: p ≥0.05. * p<0.05. ** p<0.001. APV: average peak velocity; Pa: aortic pressure; Pd: distal coronary pressure
Discussion
SUMMARY OF FINDINGS
The present data can be summarised as follows. (1) In addition to hyperaemic flow and resistance, continuous coronary thermodilution also enables measurement of resting absolute coronary blood flow and microvascular resistance. With infusion rates of 8 to 10 mL/min, but not with 6 mL/min, a stable thermodilution signal was obtained without increase in flow as compared to baseline. (2) The excellent agreement between CFR based on thermodilution (CFRthermo) and Doppler wire-derived CFVR indicates that resting measurements are accurate. (3) Continuous coronary thermodilution with infusion rates of 10 and 20 ml/min can be used to calculate CFR and microvascular resistance reserve.
In earlier work performed in vitro4, in animals2 and in humans2,5, the concept of continuous intracoronary thermodilution of saline was proposed as a method to quantify hyperaemic volumetric coronary blood flow in mL/min and minimal microvascular resistance in Wood units (WU). The accuracy of these hyperaemic measurements was recently validated against [15O]H2O-PET6. The present study extends this method of absolute flow measurement to calculation of resting blood flow. This means that, for the first time, true volumetric flow reserve can be calculated invasively in humans.
ASSESSMENT OF CORONARY FLOW AND RESISTANCE
Even though FFR is conceptually a ratio of two flows, its calculation is based on pressure measurements, a parameter much easier to measure than flow. In contrast, to assess the microcirculation, resistance must be quantified. The latter is the ratio of driving pressure and flow.
Nowadays, only PET can quantify resting and hyperaemic myocardial perfusion (flow per unit of tissue mass) and therefore it is considered the standard of reference. However, cardiac flow PET is not widely available. Moreover, during PET, distal coronary pressure cannot be measured, thus precluding the quantification of microcirculatory resistance.
Invasive, wire-based techniques assessing surrogates of flow have been developed.
Doppler flow velocity measurements11,12 introduced invasive measurements of CFVR in humans13. Their main advantage is to provide a continuous and highly sensitive assessment of APV of coronary flow. In the present study, we took advantage of this sensitivity to exclude small changes in flow that might have been induced by the low infusion rates of saline. Flow velocity measurements when combined with distal coronary pressures have been proposed as indices of microvascular resistance. Yet, intracoronary Doppler measurements remain difficult to perform14. In an all-comers study, the quality of the tracings was considered suboptimal in one third of cases15. In the present study, flow velocity measurements could not be obtained at the various phases of the protocol in 23% of patients. In contrast, continuous thermodilution flow measurements could be obtained in 100% of cases.
A second invasive approach based on bolus thermodilution has been proposed16,17. After a hand injection of 3-4 mL of saline through the guiding catheter, a thermodilution curve is recorded by a guidewire equipped with a pressure/temperature sensor, and the mean transit time (Tmn), a surrogate of flow, is calculated. Tmn is inversely proportional to flow. Based on Tmn and Pd, the index of microcirculatory resistance (IMR) has been proposed by Fearon et al18. Thermodilution-derived Tmn is easier to obtain, both at rest and during hyperaemia, than the corresponding Doppler flow velocity recordings and continuous thermodilution measurements13. However, when compared side by side with [15O]H2O-PET-derived CFR, the variability of CFR derived from bolus thermodilution appeared to be significantly higher than CFR derived from Doppler coronary flow velocity14. In addition, both CFVR and CFR derived from bolus thermodilution require the administration of adenosine for induction of hyperaemia. Continuous thermodilution-derived assessment appears to be robust, highly reproducible, operator-independent, and safe for the quantification of absolute coronary blood flow in order to investigate subtle changes in microvascular resistance. It is important to notice that the flow is measured at the spot where saline is administered in the proximal part of the vessel and not in the distal part of the artery where the temperature is measured.
The basic principle of dye-dilution techniques is that the indicator – in this case the “cold” of saline – is homogeneously mixed in the volume to be measured, and can therefore be considered a constant fraction of this volume. If the amount of the indicator is known – in this case the infusion rate and the temperature of saline when it enters the coronary artery – a simple rule of three makes it possible to derive the absolute flow in mL/min. In the present study, we observed fluctuations of the temperature recorded in the distal part of the artery when saline was infused in the proximal part of the artery at a rate of 6 mL/min. This phenomenon is probably due to an incomplete mixing of saline with blood and was not observed with 8 and 10 mL/min and to a too low signal-to-noise ratio.
ABSENCE OF HYPERAEMIC RESPONSE
Another important principle of continuous dye-dilution techniques is that the indicator itself should not modify the flow to be measured. The absence of changes in Pd/Pa and in APV between baseline conditions and infusion of saline was considered as proof of the absence of hyperaemic response to the infusion of saline. Pragmatically, to measure resting flow by continuous thermodilution, it is advised to use an infusion rate of 10 mL/min in large arteries (typically the LAD) and 8 mL/min in smaller arteries (typically the RCA or non-dominant LCx).
EFFECT OF MASS
Since pressure is uniformly distributed in a given volume, in normal coronary arteries distal coronary pressure is equal – or very close – to central aortic pressure, regardless of myocardial mass. This is why using pressure is a convenient way to evaluate epicardial stenoses. In contrast, absolute flow and resistance are linearly related to myocardial mass. The larger the mass, the greater the flow and the smaller the microvascular resistance. Since myocardial mass is not known and is markedly variable between individuals and between vascular territories, a large spread of normal values of flow and resistance is anticipated. Normalising hyperaemic values of flow and microvascular resistance with baseline measurements is one way to circumvent this limitation, and constitutes the basis of the concept of flow or resistance “reserve”. Flow reserve, therefore, expresses the number of times flow can increase above the baseline value. Similarly, resistance reserve expresses the number of times resistance can decrease below baseline resistance.
Study limitations
A number of limitations should be taken into account. First, the measurements were obtained in vessels with only mild stenoses at angiography. Since the relationship between pressure gradient and flow is relatively flat in case of mild stenosis, only marked increases in flow are expected to translate into a decrease in Pd/Pa ratios. Accordingly, this index is not sensitive to exclude mild degrees of hyperaemia. This was addressed by simultaneous Doppler-flow velocity assessment in a subset of patients. Doppler is extremely sensitive to even minimal changes in flow velocity and failed to document changes in flow velocity at a saline infusion rate of 10 ml/min as compared to baseline conditions.
Second, it is theoretically possible that the volume of infused saline should be subtracted from the calculation of measured flow. This might well influence the resting flow more than hyperaemic flow because during hyperaemia the microvasculature is supposed to have reached its maximal volume. More data are needed to explore these aspects and how they might influence the results of the thermodilution-derived flow measurements. The fact that flow velocity did not increase between baseline and during the infusion of 10 mL/min argues against a significant error related to the volume of saline.
Third, the present results were obtained in arteries with mainly non-significant stenoses. More work is needed to understand the validity of these measurements in stenotic arteries. However, from the practical point of view, investigating the microcirculatory function only makes clinical sense in patients without significant epicardial stenoses.
Fourth, ideally, the order of the interventions should have been alternated at random. For the sake of the duration of the study protocol, we elected to be pragmatic and to use escalating infusion rates, as is generally performed in “dose-finding” studies.
Fifth, the number of patients was limited, especially the number of patients in the “dose-finding” subgroup.
Sixth, measurements were not blinded in this study and the possibility of bias in agreement cannot be excluded. However, this bias is reduced by the fact that, even though the absolute flow was measured during saline infusion and the APV was recorded simultaneously, flow velocity values are visible in real time while thermodilution-derived absolute flow values are only visible at the end of the measurements.
Conclusions
Continuous intracoronary thermodilution is able to quantify absolute coronary blood flow at rest. Together with flow measurements performed at hyperaemia, continuous thermodilution offers, for the first time, the possibility of assessing truly quantitative flow and resistance reserve measurements in humans.
|
Impact on daily practice The present data indicate that continuous intracoronary thermodilution of saline at low infusion rates (8-10 ml/min) is able to quantify absolute coronary flow (in mL/min) and resistance (in Wood units) at rest. Accordingly, metrics of coronary microvascular function can now be derived from absolute measurements in an operator-independent manner. |
Funding
M. Kodeboina, S. Fournier, G. Di Gioia and J. Sonck are supported by a research grant from the CardioPaTh PhD programme. G. Di Gioia is financially supported by UniNA and Compagnia di San Paolo in the frame of the STAR programme.
Conflict of interest statement
C. Collet reports receiving research grants from Biosensors, HeartFlow Inc., Shockwave Medical, Pie Medical, Siemens, Medis Medical Imaging and Abbott Vascular, and consultancy fees from Opsens, Boston Scientific, Medyria, HeartFlow Inc., and Philips/Volcano. B. De Bruyne has received grants from Abbott Vascular, Boston Scientific and Biotronik, institutional consulting fees from Abbott and Boston Scientific, and is a shareholder in Philips, Siemens, GE, Bayer, HeartFlow, Edwards Lifesciences, and Ceyliad. S. Fournier declares speaker fees from Bayer, Amgen, and Biotronik, and consulting fees (advisory boards) from Bayer, and CathWorks. M. van ’t Veer declares speaker fees from Abbott. N. Pijls declares institutional research grants from Abbott and Hexacath, consultancy for Abbott, Opsens and General Electric, and minor equity in Philips, ASML, HeartFlow and GE. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

