
Abstract
Aims: The aim of this study is to validate a novel monorail infusion catheter for thermodilution-based quantitative coronary flow measurements.
Methods and results: Based on the principles of thermodilution, volumetric coronary flow can be determined from the flow rate of a continuous saline infusion, the temperature of saline when it enters the coronary artery, and the temperature of the blood mixed with the saline in the distal part of the coronary artery. In an in vitro set-up of the systemic and coronary circulation at body temperature, coronary flow values were varied from 50-300 ml/min in steps of 50 ml/min. At each coronary flow value, thermodilution-based measurements were performed at infusion rates of 15, 20, and 30 ml/min. Temperatures and pressures were simultaneously measured with a pressure/temperature sensor-tipped guidewire. Agreement of the calculated flow and the measured flow as well as repeatability were assessed. A total of five catheters were tested, with a total of 180 measurements. A strong correlation (ρ=0.976, p<0.0001) and a difference of –6.5±15.5 ml/min were found between measured and calculated flow. The difference between two repeated measures was 0.2%±8.0%.
Conclusions: This novel infusion catheter used in combination with a pressure/temperature sensor-tipped guidewire allows accurate and repeatable absolute coronary flow measurements. This opens a window to a better understanding of the coronary microcirculation.
Introduction
Quantitative measurement of volumetric coronary blood flow (ml/min) during catheterisation in humans is currently not possible. Surrogate methods, such as intracoronary Doppler, have high variability and are operator-dependent. In addition, normal values of coronary flow reserve vary considerably. Since microvascular resistance can be defined as the ratio between coronary pressure and flow, the lack of a reliable technique to measure volumetric coronary blood flow has hampered the quantitative assessment of the coronary microcirculation as well. Consequently, reliable data regarding microvascular dysfunction are limited.
The index of microcirculatory resistance (IMR)1,2 has been proposed to obtain a semi-quantitative measure to describe the status of the microcirculation. In calculating IMR, the mean transit time of a bolus of saline infused into the coronary artery is used as a surrogate for flow. IMR has recently been shown to bear important prognostic information in patients with ST-segment elevation myocardial infarction (STEMI) and non-STEMI3-5.
A more robust and operator-independent method to quantify volumetric coronary blood flow based on the principle of thermodilution with continuous infusion of saline has been described and validated in animals and in humans6,7. The method requires a specially designed infusion catheter that ensures complete and homogeneous mixing of continuously infused saline with the blood in the coronary artery. Unfortunately, a suitable infusion catheter is presently not available. To overcome this limitation we designed a novel monorail infusion catheter that ensures complete and homogeneous mixing of saline with blood, and can be used as standard PCI equipment. In the present study, validation and repeatability of thermodilution-based coronary flow measurements were performed in vitro with different infusion rates over a wide range of coronary flow values.
Methods
THERMODILUTION-DERIVED CORONARY BLOOD FLOW
As described earlier6, coronary blood flow (Qb, ml/min) can be calculated by continuous intracoronary infusion of saline using the equation:
![]() (1)
(1)
where Qi is the preset infusion rate of saline by an infusion pump (in ml/min); Tb is the temperature of blood in the distal coronary artery before infusion of saline; Ti is the temperature of the infused saline when it exits the infusion catheter; and T is the temperature of the homogenous mixture of blood and saline in the distal part of the coronary artery during infusion. The constant cp relates to the difference between the specific heats and densities of blood and saline. When saline is infused in blood, this constant equals 1.088. In practice, the temperature of blood (Tb, ~37°C, ~100°F) is taken as a reference and the other temperatures are measured with respect to that value. Therefore, Ti and T stand for relative temperatures compared to Tb. Equation 1 then becomes:
![]() (2)
(2)
Since, in clinical practice, Qi is chosen between 15 and 30 ml/min, the last part of equation 2 subtracts between 1.2 and 2.4 ml/min from the calculated coronary blood flow and can therefore be neglected. Consequently, the equation is further simplified to:
![]() (3)
(3)
In our in vitro set-up, the specific heat and density of circulating warm water and that of the infused water at room temperature are almost identical. Hence, cp approaches 1.00. Moreover, since we do not use blood in our in vitro set-up, it is more appropriate to mention the thermodilution-derived flow Qthermo instead of Qb. The equation for measuring coronary blood flow by continuous intracoronary infusion of saline becomes, finally:
![]() (4)
(4)
INFUSION CATHETER DESIGN
To allow thermodilution-derived flow measurements in clinical practice, an infusion catheter must fulfil a number of prerequisites: a) the device should be usable as standard PCI equipment; b) the infused saline should be homogenously and completely mixed with the blood to obtain a correct mixing temperature (T); c) when the sensor is pulled into the infusion catheter, it must be possible to measure the temperature of the saline accurately when it is entering the coronary artery (Ti); and d) the presence of the catheter should influence the coronary blood flow as little as possible.
Therefore, we designed a novel catheter based on the structure of a conventional monorail balloon catheter with an outer diameter of 0.84 mm (Figure 1A). In this design, the conventional balloon part was replaced by the non-blow-moulded pre-shape of the balloon. It consists of a 25 cm long monorail inner lumen for the 0.014” pressure/temperature wire and an outer lumen running over the whole length of the catheter for saline infusion. This is the inflation lumen of the conventional balloon catheter. There are four outer holes with a dimension of 100 µm (infusion holes 1-4) between the outer lumen and the surface, made by ultrashort-pulse laser ablation. The number of four side holes was chosen to enable easy infusion of saline and maintain the strength of the catheter. Two pairs of infusion holes are located at 7 and 8 mm from the tip and positioned at 0°, 180° and 90°, 270°, respectively (Figure 1B, Figure 1C). These holes aim to provide homogenous mixing of saline with blood, which is not achieved when using a standard infusion catheter with only an open end hole (Figure 2, Moving image 1). There are two 50 µm inner holes (sensor holes) between the outer and the inner lumen. Sensor holes are located 9 mm from the tip and positioned at 90° and 270°, respectively. These holes provide a direct contact between the infused saline and the temperature sensor of the guidewire, when the latter is pulled back into the catheter, and so they enable the precise assessment of the temperature of saline at the very moment it enters into the coronary artery (Ti). Larger sensor holes would compromise the strength of the catheter and could cause loss of indicator through the monorail lumen. The hub is standard sized, and compatible with any automated pump injector used for the continuous injection of saline at room temperature. The infusion catheter (RayFlow®) recently obtained CE approval and is manufactured by Hexacath Inc., Paris, France.
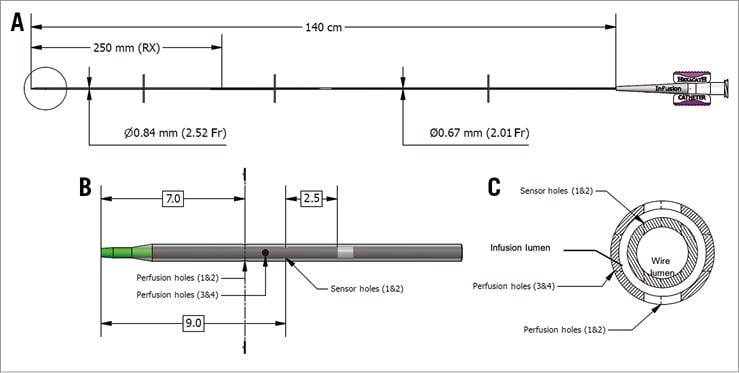
Figure 1. RayFlow infusion catheter. A) Schematic drawing and dimensions of the design of the infusion catheter. B) Close-up of the tip (indicated in the circle in A), highlighting the four infusion holes and the two sensor holes. C) The cross-section of the catheter tip at the most distal infusion holes (1 & 2), as indicated by the dashed line in B. The lumen for the guidewire and for the infusion as indicated.

Figure 2. Injection patterns of different catheters. A) Injection of black ink into the coronary vessel through a standard catheter with an open end hole. Incomplete mixing is observed. B) Injection through the novel monorail infusion catheter into the coronary vessel. Infusion of ink is observed through the side holes with proper mixing (Moving image 1).
Validation tests
IN VITRO SET-UP
The physiologic representative experimental model simulates the left ventricle, the systemic and the coronary circulation, and has been described extensively elsewhere6,9.
In short, the model combines a piston pump, a left ventricular chamber and two valves. Originating from the left ventricular chamber, a polyurethane tube with the dimensions and mechanical properties of the native aorta leads to a system of compliances and resistances, creating physiological aortic pressure and flow patterns. The coronary circulation is modelled with polyurethane tubes of physiologic dimensions, branching off the aorta directly distal to the aortic valve. The coronary circulation is bifurcated into an epicardial compartment and a subendocardial compartment. The latter is exposed to left ventricular pressure, inducing periodic collapse during systole, similar to normal coronary physiology. The model is submerged in water which is kept at a constant temperature of 37.00±0.05°C by an external thermal bath and circulator (F34-HL; Julabo GmbH, Seelbach, Germany).
INSTRUMENTATION AND MEASUREMENT PROTOCOL
A pressure/temperature wire (PressureWire™ Certus™; St. Jude Medical, St. Paul, MN, USA) was advanced into the coronary artery. Thereafter, the novel monorail infusion catheter was advanced over the pressure/temperature wire with its tip placed in the proximal part of the main coronary branch. Care was taken that the sensor of the pressure/temperature wire was located approximately 6 cm distal to the tip of the infusion catheter as was done in previous experiments in animals and humans7. A perivascular ultrasound flow probe (4PSB; Transonic Systems Inc., Ithaca, NY, USA) was placed around the main branch of the coronary artery to measure coronary flow as reference. Using an adjustable clamp distal to the flow probe, the reference flow was set between 50 and 300 ml/min (Q) and varied in steps of 50 ml/min. These flow rates are based on previous experience in animals and in humans7. Continuous infusion of water at room temperature (Qi) was provided by an automated pump injector (Medrad® Stellant®; Medrad Inc., Warrendale, PA, USA) with a flow rate of 15, 25 or 30 ml/min. The pressures achieved for the different infusion rates were recorded. A schematic overview of the measurement set-up is shown in Figure 3. Five novel catheters were tested with a total of 180 measurements.
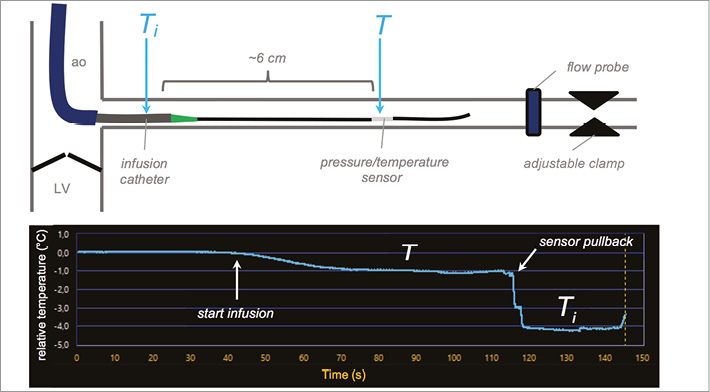
Figure 3. Schematic representation of set-up and example of measurement. A guiding catheter is positioned in the ostium of the coronary branch distal to the aortic valve that separates the left ventricle (LV) from the aorta (ao). The pressure/temperature wire is placed in the coronary branch with its sensor located approximately 6 cm distal to the tip of the infusion catheter. The perivascular flow probe and clamp are located distal to the pressure/temperature wire. The start of the infusion is indicated on the temperature tracing as well as the moment of the pullback of the sensor into the infusion catheter. The relative temperature T is measured distally, whereas Ti is measured after pullback of the sensor inside the infusion catheter as indicated.
During every step of the measurement protocol the temperature was first set to zero (reference temperature comparable to 37°C or 100°F in humans), then infusion was started with the injector and the temperature in the distal coronary artery decreased. After reaching a steady state, the temperature of the mixture of the hot circulating water and cold infused water T was recorded for ~30 sec. Thereafter, the sensor was pulled back into the infusion catheter to obtain Ti, as depicted in Figure 3. The recording was stopped and the sensor was advanced again to perform a second measurement to assess repeatability. Distal coronary pressures were continuously monitored before and during the continuous infusion.
STATISTICAL ANALYSIS
Correlation was calculated using the Spearman’s rank correlation coefficient. Agreement between calculated (Qthermo) and measured (Q) flow was assessed by Bland-Altman plots of the differences between the techniques. Repeatability was also assessed by a Bland-Altman plot. Coronary pressure values before and during infusion were compared with a paired t-test. Data are expressed as mean and standard deviation or as median and range as appropriate.
Results
The instrumentation itself was uncomplicated and straightforward for all infusion catheters tested. The range of temperatures measured in the distal coronary artery was 0.20 to 4.63°C below the reference temperature. The range of temperatures measured at the tip of the infusion catheter was 3.82 to 8.59°C below the reference temperature.
The pressure needed to achieve the desired infusion rates increased from 10.9 bar (range 8.5 to 15.0 bar), 19.4 bar (range 15.0 to 21.4 bar), and 24.0 bar (range 16.1 to 24.7 bar) for infusion rates of 15, 25, and 30 ml/min, respectively. These pressure values were within the regular pressure safety margins of the catheter (<500 psi ≈34 bar) and no problems were observed during infusion.
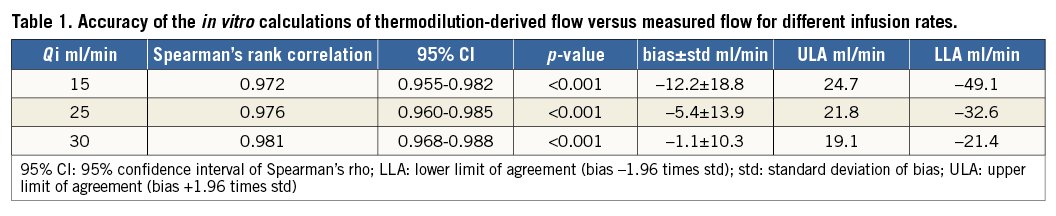
Calculated flow (Qthermo) showed a strong overall correlation with measured flow (Q) (ρ=0.976, 95% CI: 0.968 to 0.982; p<0.0001) (Figure 4A). The Bland-Altman plot showed an overall bias of –6.5±15.5 ml/min (Figure 4B). The figures for the separate infusion rates are shown in Table 1. A better agreement was found for higher infusion rates for all coronary flow values. Mild underestimation of the measured flow was observed mainly for higher coronary flow values in combination with low infusion rates.
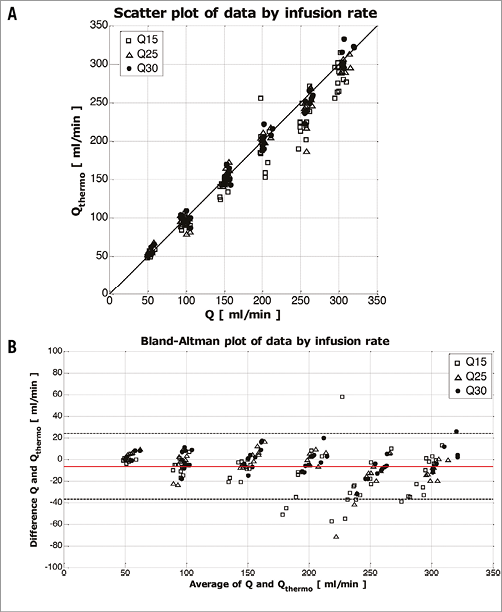
Figure 4. Data by infusion rate. A) Scatter plot between calculated (Qthermo) and measured flow (Q) and line of identity. B) Bland-Altman plot of the difference between Qthermo and Q shown for the three different infusion rates (Qi). The average bias –6.5 ml/min is shown as the red line and the limits of agreement are indicated by the dashed lines (upper and lower limits of agreement [bias±1.96· standard deviation] are 24.0 ml/min and –37.0 ml/min, respectively).
For assessment of repeatability, a scatter plot of the two consecutive measurements was made and the relative difference between two consecutive flow calculations was plotted against the average of the two calculated flow values (Figure 5A, Figure 5B). The average bias between the two measurements was 0.2%±8.0%.
Coronary pressure increased minimally, though significantly, during infusion by 1.5±2.0 mmHg (p<0.001).
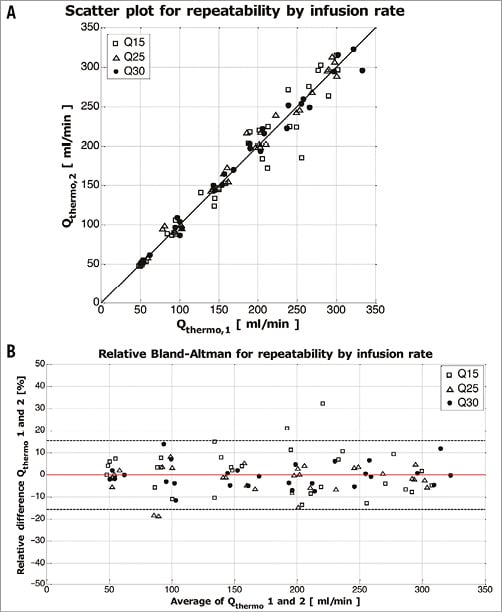
Figure 5. Repeatability by infusion rate. Scatter plot and line of identity (A) and Bland-Altman plot (B) for repeatability. The average bias as indicated by the red line was –0.2% and the accompanying upper and lower limits of agreement (bias±1.96·standard deviation) were 15.4 and –15.8% as indicated by the dashed lines.
Discussion
The present data indicate that this novel monorail infusion catheter allows an accurate assessment of absolute coronary blood flow by the principle of thermodilution and continuous infusion of saline. In addition, the test/re-test repeatability is excellent. The technique does not deviate significantly from standard PCI techniques. Because coronary pressure is measured simultaneously with the flow by the pressure/temperature wire, this technique also allows the quantitative assessment of microvascular resistance (expressed in dynes·s·cm–5). This might open new research avenues on the coronary microcirculation, improve the understanding of this part of the coronary circulation, and quantify the effect of therapeutic interventions.
A number of practical and conceptual points, related to the novel infusion catheter and to the principle of thermodilution, need to be discussed. First, this novel catheter is a monorail system. Therefore, it can easily be advanced over a 0.014” guidewire with pressure/temperature sensor, while already connected to the infusion pump and purged outside the body. Therefore, the instrumentation is easy and safe, and the measurements take only a few minutes. This is a major advancement with respect to the former over-the-wire system that was used in the validation work of the method6,7. Second, the sensor of the pressure/temperature wire should be positioned between 3 and 6 cm distal to the tip of the infusion catheter. Such a position guarantees complete mixing of the blood and the infused saline (Moving image 1) and avoids significant loss of indicator (heating of the cold saline) into the wall (Figure 2). A uniform temperature was found between 3 and 6 cm distal to the tip of the infusion catheter. This was reassuring in relation to the assumption that mixing was complete and no heat was lost to the surrounding tissue. Third, from a conceptual point of view, it should be emphasised that the coronary flow is measured at the location where the saline enters the coronary artery and not where the temperature T is measured. In other words, flow measured by this technique represents the blood flow distal to the tip of the infusion catheter. Fourth, the outer diameter of the catheter is 0.84 mm, corresponding to an area stenosis of less than 10% in a coronary segment 3 mm in diameter, and less than 15% in a coronary segment 2.5 mm in diameter. Therefore, it is unlikely that its presence will significantly decrease absolute coronary flow. In addition, should it happen, it can be determined and easily corrected by multiplying flow by the ratio of fractional flow reserve measured with and without the infusion catheter in situ as described earlier7. Finally, only maximal hyperaemic flow is obtained by this technique, not resting flow. Consequently, minimal microvascular resistance is obtained. However, this is not a limitation because it is in fact the minimal resistance that is clinically important and determines the state of the microcirculation. Indeed, the main function of the microcirculation is the regulation of myocardial blood flow by adjusting the total coronary resistance. Under baseline conditions and in the absence of significant epicardial resistance, microvascular resistances are high. Microvascular resistance will decrease to allow oxygen supply to match oxygen needs, either to compensate for the presence of an epicardial stenosis or to adjust to increased needs, or both. Therefore, the potential of the microcirculation to adjust the resistance (i.e., minimal microvascular resistance) appears to be the most important metric to gauge the functional status of the microcirculation. In humans, preliminary data suggest that the infusion of saline at room temperature at a rate of 15-30 ml/min through the specially designed catheter induces maximal hyperaemia. One could argue that coronary flow could further increase due to the infusion of saline. In the case of maximal hyperaemia, and thus minimal microvascular resistance, this could only be the case if distal coronary pressure increases. In our experiments, we did not find a clinically significant increase in coronary pressure.
Limitations
The slight underestimation of Q by Qthermo at high coronary flow rates and low infusion rates is of little clinical relevance because flow rates higher than 300 ml/min are rarely observed, even in large coronary arteries. In such large arteries, the infusion rate will be set rather high and, by doing so, a better signal to noise ratio is obtained for the measurements. However, infusion rates of saline higher than 30 ml/min should be avoided for two reasons. First, during our pre-testing at infusion rates of 40 ml/min, infusion pressures exceeded 35 bars, which is beyond the safety threshold indicated by the manufacturer. Second, high saline infusion rates should be avoided, especially in small coronary arteries because the dilution of arterial blood by saline might induce clinically relevant hypoxaemia and hypothermia. Finally, it is important to determine the lowest temperature of the saline when it enters into the coronary artery (Ti). For correct determination of this Ti, the sensor should be located 2.5 mm distal to the marker at the location of the sensor holes as indicated in Figure 1. Due to the lack of X-ray during the in vitro experiments, we pulled the sensor back a few millimetres into the infusion catheter until we found the lowest temperature. In this experimental setting, finding the location with the lowest temperature required subtle movements of the wire. Despite this source of error we achieved excellent repeatability for the determination of Ti with a relative difference of 0.4%±4.8% between the repeated measurements. In humans, these difficulties are not encountered because fluoroscopic control of the sensor position is available.
Conclusion
This novel infusion catheter, used in combination with a pressure/temperature sensor-tipped guidewire, allows repeatable absolute flow (in ml/min) and microvascular resistance (in dynes·s·cm–5 or in mmHg.min.ml–1) measurements. This opens a window to a better assessment and understanding of microcirculatory function and treatment of microcirculatory dysfunction.
| Impact on daily practice Hitherto, quantitative assessment of the coronary microvasculature has been impossible in humans. The development of a novel catheter for continuous thermodilution to be used in conjunction with a pressure/temperature sensor-tipped guidewire allows for the first time the calculation of absolute coronary flow and absolute microvascular resistance. |
Conflict of interest statement
E. Barbato reports an institutional consultancy for St. Jude Medical and Boston Scientific, and has received institutional research grant support from St. Jude, Boston Scientific, Abbott, Medtronic and Biotronik. N. Pijls reports a consultancy for St. Jude Medical, Opsens, and Boston Scientific, and has received institutional research grant support from St. Jude and Medtronic. B. De Bruyne reports an institutional consultancy for St. Jude Medical, Opsens and Boston Scientific, and has received institutional research grant support from St. Jude, Boston Scientific, Abbott, Medtronic and Biotronik. The other authors have no conflicts of interest to declare.
Supplementary data
Moving image 1. Examples of mixing of ink. First part: improper mixing, injection through end hole. Second part: proper mixing, injection through side holes.
Supplementary data
To read the full content of this article, please download the PDF.
Examples of mixing of ink. First part: improper mixing, injection through end hole. Second part: proper mixing, injection through side holes.

