Acute coronary syndrome (ACS) predominantly arises from vulnerable lipid-rich plaques (LRPs). Despite contemporary systemic medical therapy, 30% of ACS patients have a recurrent event within 5 years, prompting interest in local prophylactic treatment options aimed at plaque stabilisation1. Near-infrared spectroscopy (NIRS) combined with intravascular ultrasound (IVUS) enables the identification of LRPs at risk for future coronary events2. Paclitaxel-coated balloons (PCBs) allow for targeted intracoronary pharmacological treatment without leaving behind a permanent implant. Preclinical studies have shown the potential of PCBs to serve as a local plaque-stabilising therapy3. We therefore investigated the safety and feasibility of using a PCB as a pre-emptive treatment for non-flow-limiting, non-culprit LRPs in patients presenting with non-ST-segment elevation ACS (NSTE-ACS).
The Intravascular Identification and Drug-Eluting Balloon Treatment of Vulnerable Lipid-Rich Plaques (DEBuT-LRP) study was an investigator-initiated, first-in-human proof-of-concept study conducted at the Amsterdam University Medical Centers (ClinicalTrials.gov: NCT04765956). The study design and rationale have been previously published4. Briefly, patients with NSTE-ACS underwent three-vessel IVUS-NIRS (Makoto TVC-MC10 imaging system, with a 50 MHz catheter [both Infraredx]) after successful percutaneous coronary intervention (PCI) of all flow-limiting culprit lesions, with confirmation through intracoronary functional testing in case of uncertainty. Complete inclusion and exclusion criteria are provided in Supplementary Table 1. An LRP was defined as a region with a maximum lipid core burden index (LCBI) in a 4 mm segment (maxLCBI4mm) ≥3252. Patients with at least 1 LRP directly underwent PCB treatment under nominal pressure inflation (SeQuent Please NEO [B. Braun]) for the entire LRP length including 5 mm margins. In patients with multiple LRPs, only one was treated with the instruction to preferably target the one with the highest maxLCBI4mm. LRPs in the left main or previously stented segments with 5 mm margins were excluded from PCB treatment. Repeat IVUS-NIRS was performed after PCB inflation. Clinical follow-up was performed at 1, 9 and 12 months. Repeat coronary angiography with three-vessel IVUS-NIRS was done at 9-month follow-up. All images were analysed at an independent core laboratory (MedStar Cardiovascular Research Network, Angiographic and Invasive Imaging Core Lab, Washington, D.C., USA). The primary endpoint was the change in maxLCBI4mm in the PCB-treated LRP from baseline to 9-month follow-up. Core laboratory methodology, secondary endpoints and endpoint definitions have been previously described4. Statistical analyses were performed using SPSS Statistics, version 28.0 (IBM). We used the Wilcoxon signed-rank test for the primary and secondary imaging endpoints. The absolute difference in units between baseline and follow-up imaging measurements was described as the median with interquartile range (IQR), and the relative risk in median with IQR as a percentage. The McNemar test was used for paired categorical data.
Between January 2021 and September 2022, 65 patients were screened for inclusion, of whom 45 patients underwent IVUS-NIRS imaging after successful PCI of flow-limiting lesions (Supplementary Figure 1). Out of 26 patients with >1 LRP, 20 patients were enrolled in the study to undergo PCB treatment of 1 LRP. Six were excluded because of an unsuitable LRP location, as defined by the protocol. Baseline characteristics are displayed in Supplementary Table 2 and Supplementary Table 3, and procedural and angiographic characteristics are shown in Supplementary Table 4 and Supplementary Table 5. PCB treatment was performed under a median inflation pressure of 6 atmospheres (IQR 6 to 6) for 74 seconds (IQR 60 to 90). Three procedural complications occurred. One patient experienced distal embolisation after PCB treatment, which was resolved after intracoronary nitroglycerine and adenosine administration, resulting in swift restoration of Thrombolysis in Myocardial Infarction (TIMI)-3 flow. Alternatively, this could have been spasm (Moving image 1, Moving image 2, Moving image 3, Moving image 4 demonstrate the case of this patient undergoing PCB treatment of an LRP in the left anterior descending artery, after successful treatment of a significant stenosis in the right coronary artery). No IVUS-NIRS-related complications occurred. Two patients experienced procedural complications due to PCI of significant lesions, not related to IVUS-NIRS or PCB treatment, with one wire dissection requiring bailout stenting and one distal embolisation of the culprit lesion only.
Follow-up imaging was performed at 272 days (IQR 256 to 281) in 18 patients (2 patients refused). No complications occurred. A total of 17 patients had analysable IVUS-NIRS images prior to PCB treatment and after 9 months. The primary endpoint of the median maxLCBI4mm of the PCB-treated LRPs significantly decreased from 397 (IQR 299 to 527) at baseline to 211 (IQR 106 to 349) at follow-up (p<0.001), which was an absolute change of −131 (IQR −315 to −80) and a relative change of −42% (IQR −71 to −14) (Central illustration, Table 1, Supplementary Figure 2). No significant differences were observed for the IVUS-derived area measurements. The minimum lumen area (MLA) increased numerically, but not significantly, from 6.7 mm2 to 7.8 mm2 within the maxLCBI4mm segment (p=0.23). A total of 14 patients had analysable IVUS-NIRS images at all 3 timepoints (before PCB treatment, after PCB treatment and after 9 months) and these outcomes are displayed in Supplementary Table 6. On a vessel basis, the maxLCBI4mm within the entire PCB-treated vessel significantly decreased from 431 (IQR 362 to 519) at baseline to 331 (IQR 206 to 461) at follow-up (p=0.002), which was an absolute change of −111 (IQR −223 to –54) and a relative change of –24.6% (Supplementary Table 7, Supplementary Figure 3). The maxLCBI4mm within the untreated vessels showed no significant difference between baseline (136 [IQR 98 to 243]) and 9-month follow-up (105 [IQR 61 to 217]; p=0.11). A case example is displayed in Supplementary Figure 4. Site-reported imaging outcomes are displayed in Supplementary Table 8.
Over a median follow-up period of 398 days (IQR 394 to 439), a total of 5 patients (25%) had a cardiovascular event (Supplementary Table 9). There were no deaths. No index LRP-related events occurred. One patient had a type 1 myocardial infarction attributable to a definite stent thrombosis of the culprit NSTE-ACS lesion, occurring 6 days after index PCI due to antiplatelet therapy non-adherence. Three patients had unplanned visits to the emergency department due to angina. Two of them were discharged the same day after ruling out myocardial infarction. The third patient stayed overnight and underwent repeat coronary angiography, 34 days after the index PCI, where no significant stenosis was observed. However, at 9-month follow-up this same patient underwent repeat revascularisation for significant in-stent restenosis of the culprit NSTE-ACS lesion. Two patients experienced a bleeding event requiring medical intervention (one epistaxis, i.e., Bleeding Academic Research Consortium [BARC] 2 bleeding, and one gastrointestinal bleed with a haemoglobin drop of 2.2 mmol/L requiring endoscopic intervention, i.e., BARC 3b bleeding).
In summary, we demonstrated that prophylactic PCB-treatment of LRPs was safe and feasible and resulted in a significant reduction of the maxLCBI4mm at 9-month follow-up, with no reduction of the maxLCBI4mm in plaques that were not PCB-treated. No PCB-treated LRP-related events occurred during 1 year of follow-up.
Even in the current era of potent systemic antithrombotic, lipid-lowering and anti-inflammatory therapies, recurrent ischaemic cardiovascular events occur in >30% of patients within 5 years after ACS1. Approximately half of patients presenting with ACS have additional vulnerable plaques, increasing the risk for recurrent coronary events5. Therefore, local treatment of a vulnerable plaque on top of systemic medical therapy deserves further study in an effort to reduce future adverse events. Prophylactic PCI of vulnerable non-culprit plaques with bioresorbable vascular scaffolds (BVS) was shown to improve intracoronary imaging outcomes in the PROSPECT ABSORB Trial6. No significant reduction of clinical events was found, although the study was not powered for clinical outcomes. The PREVENT trial (ClinicalTrials.gov: NCT02316886) is evaluating preventive stenting with either BVS or drug-eluting stents in patients with vulnerable plaques. Nonetheless, ethical concerns arise regarding stent-related complications for non-flow-limiting lesions. Therefore, drug-coated balloons may be a safe and effective alternative, allowing local pharmacological treatment of vulnerable plaques without leaving behind a permanent implant.
Paclitaxel is an antiproliferative and anti-inflammatory drug that reduces plaque burden and inflammation when locally delivered onto atherosclerotic lesions, based on preclinical data3. We studied, for the first time, the safety and efficacy of PCB treatment in LRPs and found that PCB treatment led to a significant decrease of the maxLCBI4mm after 9 months of follow-up, which appeared safe with no treatment-related complications. No significant change in IVUS measurements was seen, which could indicate that PCB treatment changes plaque composition but not plaque size. To address possible microembolisations overestimating the treatment effect, we compared the maxLCBI4mm before and after PCB treatment and found no significant difference, while the observed treatment effect of PCB mainly occurred up to 9-month follow-up (Supplementary Table 6). While these findings might indicate a possible role for pre-emptive PCB treatment in plaque stabilisation, further randomised studies are essential to investigate the impact of this prophylactic treatment on clinical outcome and to compare the results with a control group receiving guideline-directed medical therapy alone to assess whether there is an additional effect of PCB treatment on top of lipid-lowering medication.
Several limitations must be acknowledged. First, the sample size is small and there was no control arm. However, patients can be considered their own control with LRPs that were not PCB treated. Second, at 1-year follow-up, 15% were not taking any lipid-lowering medication, and no routine cholesterol measurements were performed during follow-up. Lastly, only 10% of patients were female, which may be a result of the small sample size.
In conclusion, in this first-in-human proof-of-concept study, pre-emptive treatment of non-flow-limiting, non-culprit vulnerable lipid-rich plaques with a paclitaxel-coated balloon resulted in a significant reduction of the lipid burden (maxLCBI4mm) without overt safety concerns. Future randomised controlled trials are warranted to investigate the potential prognostic impact of this novel treatment strategy to improve clinical outcomes.
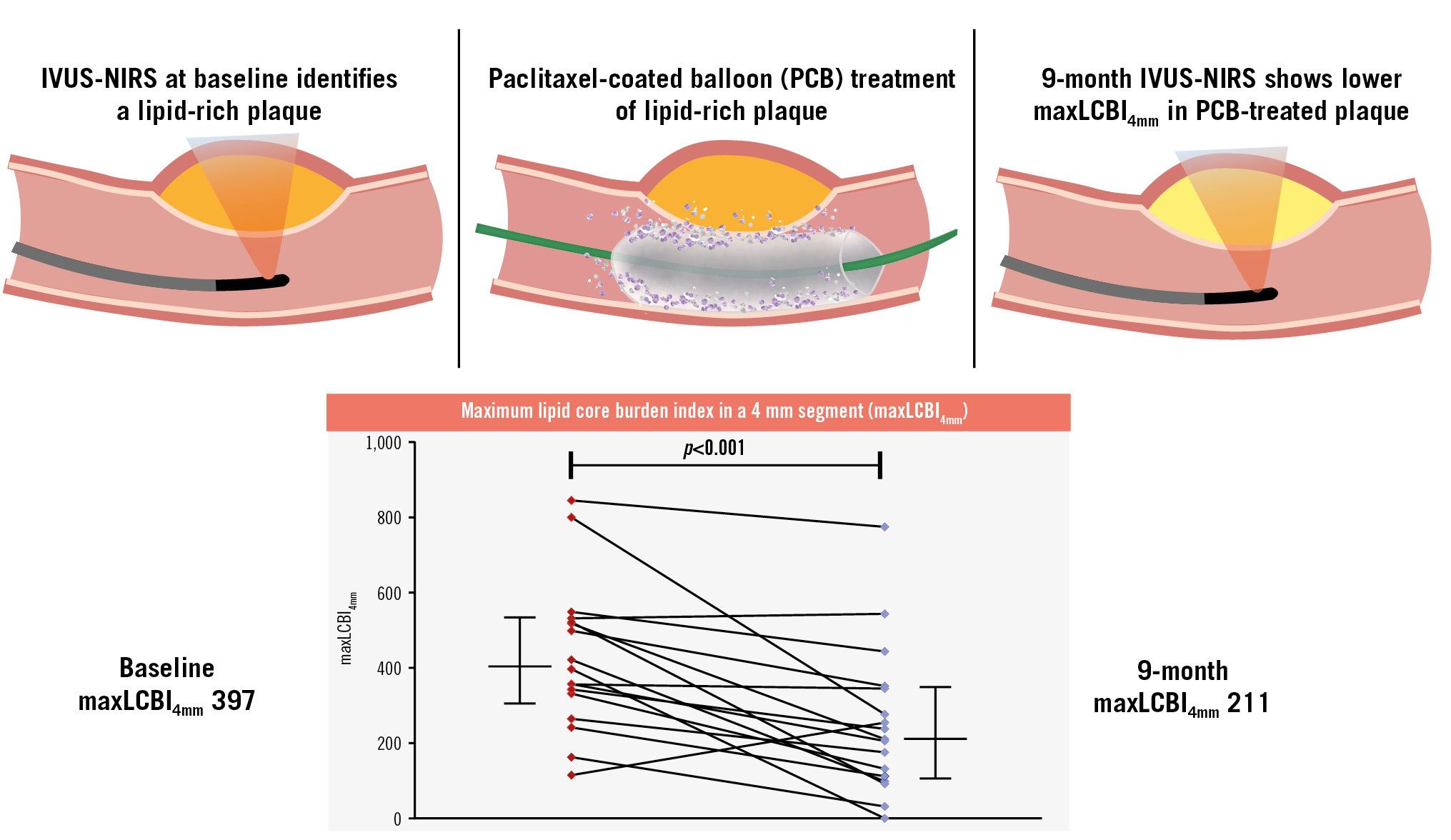
Central illustration. DEBuT-LRP study. Schematic overview of the DEBuT-LRP study with the primary outcome of change in maxLCBI4mm from baseline to 9-month follow-up in paclitaxel-treated lipid-rich plaques. In the DEBuT-LRP study, patients presenting with non-ST-segment elevation acute coronary syndromes underwent intravascular ultrasound combined with near-infrared spectroscopy (IVUS-NIRS). Lesions with a maxLCBI4mm ≥325 underwent additional paclitaxel-coated balloon treatment. Patients underwent repeat IVUS-NIRS after 9-month follow-up, and the maxLCBI4mm was significantly reduced compared with baseline. Red dots indicate the baseline measurement of maxLCBI4mm of the individual patients, and blue dots indicate the 9-month measurement. On both sides of the graph the median with interquartile range is displayed as horizontal lines. maxLCBI4mm: maximum lipid-core burden index in a 4 mm segment
Table 1. Core laboratory-adjudicated 9-month imaging outcomes in PCB-treated LRPs.
| PCB (n=18) | Median absolute difference | Median relative difference, % | p-valuea | ||
|---|---|---|---|---|---|
| Baseline | 9 months | ||||
| NIRS findings | |||||
| Within PCB segmentb | |||||
| Maximum LCBI4mm | 397(299 to 527) | 211(106 to 349) | –131( −315 to −80) | −42(−71 to −14) | <0.001 |
| Maximum LCBI4mm ≥325 | 13(77) | 5(29) | - | - | 0.008c |
| LCBI of target lesion segment | 178(144 to 264) | 87(33 to 147) | −90(−150 to −50) | −57(−76 to −30) | <0.001 |
| IVUS findings | |||||
| Within maximum LCBI4mmb | |||||
| Plaque burden, % | 47(40 to 53) | 44(39 to 52) | −1.3(−5.6 to 5.9) | −2.9(–11.1 to 11.7) | 0.71 |
| Mean vessel area, mm2 | 15.3(10.9 to 18.9) | 16.9(12.7 to 19.8) | 0.4(−0.5 to 2.0) | 3.0(−2.6 to 17.4) | 0.23 |
| Minimal lumen area, mm2 | 6.7(5.1 to 8.6) | 7.8(4.9 to 9.9) | 0.4(−0.4 to 2.1) | 9.4(−7.2 to 21.5) | 0.23 |
| Mean plaque area, mm2 | 6.7(5.1 to 9.6) | 6.9(5.2 to 9.8) | 0.5(−0.3 to 0.9) | 6.8(−8.5 to 13.2) | 0.30 |
| Within PCB segmentb | |||||
| Plaque burden, % | 43(36 to 49) | 42(37 to 45) | −1.2(−4.7 to 1.7) | −2.8(−9.8 to 5.3) | 0.19 |
| Mean vessel area, mm2 | 15.4(10.9 to 19.2) | 15.5(12.0 to 19.6) | 0.3(−0.7 to 1.0) | 2.5(−4.4 to 8.6) | 0.38 |
| Minimal lumen area, mm2 | 5.3(4.1 to 8.4) | 5.8(4.6 to 8.1) | 0.1(−0.9 to 1.1) | 2.4(−15.2 to 28.2) | 0.82 |
| Mean plaque area, mm2 | 6.1(4.5 to 8.7) | 6.3(5.2 to 8.4) | 0.0(−0.6 to 0.7) | 0.3(−9.4 to 14.7) | 0.82 |
| Angiographic findings | |||||
| Within PCB segment | |||||
| Minimum lumen diameter, mm | 1.7(1.3 to 2.2) | 2.0(1.6 to 2.4) | 0.0(−0.3 to 0.5) | 2.7(−15.0 to 32.3) | 0.36 |
| Reference vessel diameter, mm | 2.7(2.2 to 3.3) | 2.7(2.3 to 3.0) | 0.0(−0.4 to 0.3) | −1.0(−12.7 to 13.4) | 0.82 |
| Diameter stenosis, % | 35(22 to 44) | 26(19 to 35) | −3.0(−16.0 to 6.0) | −10.0(−45.8 to 24.3) | 0.28 |
| Data are given as median (IQR) or number of patients (%). ap-value of difference from baseline to follow-up as derived from the Wilcoxon signed-rank test. bData available in 17 patients. cp-value of difference as derived from the McNemar test. IQR: interquartile range; IVUS: intravascular ultrasound; LCBI: lipid-core burden index; LRP: lipid-rich plaque; NIRS: near-infrared spectroscopy; PCB: paclitaxel-coated balloon | |||||
Funding
The collaborative project DEBuT-LRP is co-funded by the PPP Allowance made available by Health~Holland, Top Sector Life Sciences & Health, to stimulate public-private partnerships (grant number: TKI-LSH-DT2019-AMC-24409), together with in-kind and in-cash grants from B. Braun, Infraredx, and Amsterdam UMC. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper, and its final content.
Conflict of interest statement
R. Delewi has received educational grants from Edwards Lifesciences and Boston Scientific, outside the submitted work. H.M. Garcia-Garcia has received research grants from Philips, Abbott, Boston Scientific, Corflow, Neovasc, MedAlliance, Medis, and Biotronik; speaker fees from ACIST, Medis, and Boston Scientific; and honoraria for participation in the Abbott Advisory Board. J.P.S. Henriques has received research grants from Health~Holland, B. Braun, and Infraredx/Nipro to conduct the current study; and research grants from ZonMw, AstraZeneca, and Abbott, outside the submitted work. B.E.P.M Claessen has received speaker fees from Abiomed; and consultancy fees from Amgen, Sanofi, Boston Scientific, and Philips, outside the submitted work. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Case example: LAD prior to PCB treatment.
Moving image 2. Case example: PCB inflation in the proximal LAD.
Moving image 3. Case example: LAD after PCB inflation in the proximal LAD, with loss of the distal anatomy of the LAD possibly due to distal embolisation or coronary spasm.
Moving image 4. Case example: End result after intracoronary nitroglycerine and adenosine injections.

