Abstract
Aims: To test the efficacy of sequential application of drug-coated balloon (DCB) and bare metal stent (BMS) for treatment of de novo coronary lesions, comparing the sequence of application (DCB first vs. BMS first).
Methods and results: In a multicentre pilot trial, 26 patients with de novo coronary lesions were randomised to receive a paclitaxel-coated balloon application followed by BMS implantation (DCB first) or vice versa (BMS first). Quantitative coronary angiography (QCA) and optical coherence tomography (OCT) were performed post-procedure and at six months, with OCT % neointimal volume obstruction as primary endpoint. Longitudinal geographical miss was only observed in DCB first (23.1 vs. 0.0%, p=0.220). Implantation of BMS first resulted in fewer malapposed struts (p=0.013) but similar coverage at six months. No significant difference was found regarding the primary endpoint (25.5 vs. 24.9%, p=0.922), mean thickness of coverage (261 vs. 225 μm, p=0.763), late loss (0.53 vs. 0.45 mm, p=0.833), binary restenosis (27.3 vs. 16.7% in-segment, p=0.640) or clinical endpoints.
Conclusions: Sequential application of DCB and not pre-mounted BMS for treatment of de novo coronary lesions results in efficient inhibition of neointimal hyperplasia. The sequence of application (DCB first vs. BMS first) does not seem to influence the outcome, except for better apposition in BMS first.
Abbreviations
BMS bare metal stent
DCB drug-coated balloon
DES drug-eluting stent
ISA incomplete stent apposition
MACE major acute cardiovascular event
MLA minimal lumen area
MLD minimal lumen diameter
NASB non-apposed side branch struts
NIH neointimal hyperplasia
OCT optical coherence tomography
PCI percutaneous coronary intervention
PES paclitaxel-eluting stent
QCA quantitative coronary angiography
RVD reference vessel diameter
SES sirolimus-eluting stent
TLR target lesion revascularisation
TVR target vessel revascularisation
Introduction
Drug-eluting stents (DES) have efficiently reduced the restenosis rates to 7.9-8.9 % at nine months1, due to the sustained elution of an antiproliferative agent that inhibits neointimal hyperplasia. However some reports have suggested an eventually higher incidence of late stent thrombosis2-5. In all these cases, the common pathological finding was an incomplete neointimal healing6 with incomplete endothelialisation of the metallic struts7. In DES the antiproliferative drug is eluted from the struts, creating a peri-strut gradient that plays against a proper healing. Likewise, the polymer containing and releasing the drug might induce inflammation and thrombosis8-10.
Drug-coated balloons (DCB) represent an alternative to DES for inhibiting neointimal hyperplasia. DCB transfer the drug evenly along the vessel wall, instead of creating a peri-strut gradient, what seems a more favourable scenario for complete endothelialisation of the struts. However this technology must circumvent two limitations: first, the marked hydrophobicity of the antiproliferative drugs hinders their diffusion in a hydrophilic milieu like the vessel wall; second, the transfer time is very short, compared to the sustained elution of DES. A hydrophilic carrier with affinity for the drug facilitates its transfer onto the vessel wall. This mechanism would explain why the combination of paclitaxel with the contrast media iopromide during injection for coronary angiography results in a therapeutic effect inhibiting neointimal hyperplasia11-13, even though the contact time with the vessel wall is limited to a few seconds: the hydrophilic iopromide would act as carrier for the hydrophobic paclitaxel, facilitating its transfer into the tissue up to the adventitia14. Once in the tissue, paclitaxel would bind to fixed lipophilic compounds, becoming resistant to wash-out and exerting a prolonged effect14.
In swine coronary overstretch models, DCB combining paclitaxel with a hydrophilic iopromide-based carrier have proven dose-dependent reduction of the neointimal area, with complete endothelialisation of all the struts and reduction of inflammatory markers15. In the clinical setting the same device was superior to plain balloon angioplasty16,17 and to paclitaxel-eluting stent (PES)18 for the treatment of in-stent restenosis. For de novo coronary lesions, the combination of DCB with BMS results in larger inhibition of neointimal hyperplasia than a sirolimus-eluting stent (SES) in porcine coronary overstretch models19. These studies used a DCB with a hydrophilic iopromide-based carrier, and BMS pre-mounted on the DCB. There is scarce information about the efficacy of this combination in the clinical setting. Moreover, the effect of sequential application of DCB and BMS for treatment of de novo coronary lesions, and the impact of the sequence (DCB first vs. BMS first) are unknown. Hypothetically, sequential application might increase the risk of “geographical miss” (mismatching between the DCB-treated and the stented segments) compared to pre-mounted devices, especially if DCB is applied first. On the other hand, application of DCB first might enhance the diffusion of the drug onto the vessel wall, with better contact than in the presence of an interposed stent.
Methods
The De Novo Pilot study (NCT00934752) was a multicentre, prospective, single-blind, open-label randomised trial assessing the performance of the Moxy DCB (Lutonix Inc., Maple Grove, MN, USA) in combination with an independent not pre-mounted BMS for treatment of de novo coronary lesions, comparing the effect of the sequence of application (DCB first vs. BMS first) on the extent of neointimal hyperplasia (NIH) at six months.
Study population and allocation to treatment
The study enrolled patients with stable/unstable angina or with documented silent ischaemia, and one de novo coronary stenosis ≥50% and <100%, ≤18 mm length, with a reference vessel diameter (RVD) ≥2.5 and ≤3.25 mm and amenable for percutaneous coronary intervention (PCI). Exclusion criteria included: 1) myocardial infarction or thrombolysis in previous 72 hours; 2) history of stroke within the past six months; 3) intervention required in >2 coronary lesions, or in one additional lesion lying in the same vessel as the study lesion; 4) coronary intervention within 60 days before the index procedure or planned after it; 5) any previous intervention on the target coronary vessel; 6) left ventricular ejection fraction <25%; 7) target lesion located in the left main coronary artery, or involving bifurcation of vessels ≥2.5 mm; 8) planned use of adjunctive coronary devices (e.g., cutting-balloon, atherectomy).
Patients were screened for eligibility before entering the procedure. All potentially eligible patients provided informed signed consent for enrolment. Final inclusion was done after verifying the eventual successful treatment of the non-study lesion and after the guidewire had crossed the target lesion without complications. Patients were randomly allocated on a 1:1 basis to receive treatment with Moxy DCB before BMS (DCB first) or after BMS (BMS first) using computer generated-sequences, in blocks stratified by centre.
The study was conducted in accordance with the Good Clinical Practice, Declaration of Helsinki guidelines and local regulations, and the protocol was approved by the Ethical Committees of the centres involved in the trial: Erasmus MC, Rotterdam; Academic MC, Amsterdam and Catharina Ziekenhuis, Eindhoven, NL.
Study endpoints and sample size calculation
The primary endpoint of the trial was the in-stent percent neointimal volume obstruction at six months assessed by optical coherence tomography (OCT). No evidence about the expected magnitude of the effect was available when the trial was designed, and therefore no formal sample size calculation based on the primary endpoint could be done. Based on unpublished data from other ongoing OCT trials, a minimum number of 10 patients per treatment arm was considered necessary to provide reliable and non-trivial results, and to detect a significant deviation in any of the arms from the results obtained with DES.
Secondary endpoints of the study included OCT endpoints (apposition at baseline and at six months; coverage at six months), quantitative coronary angiography (QCA) endpoints (late lumen loss, percent diameter stenosis, binary restenosis defined as diameter stenosis ≥50%) and clinical endpoints (composite of cardiac death, myocardial infarction [MI] and clinically-driven target lesion revascularisation [TLR]; stent thrombosis; major/minor bleeding).
Study devices
The DCB used in this study was the Moxy catheter (Lutonix, Maple Grove, MN, USA), model 9001. It is a standard rapid exchange semi-compliant balloon, coated by paclitaxel at a surface concentration of 2 μg/mm2, and by a proprietary hydrophilic non-polymeric carrier. The device was available at 2.5 and 3.0 mm diameter, and at 18 and 30 mm length for this study. All patients were stented with the Multi-link Vision/MiniVision stent (Abbott Vascular, Santa Clara, CA, USA). It is a cobalt-chromium BMS with a strut thickness of 81μm, available at 2.5, 2.75 and 3.0 mm diameter, and at 15, 18 and 23 mm length for this study.
Description of the intervention
Before the intervention all subjects received aspirin 100-325 mg and clopidogrel 75 mg daily for three days or in a loading dose of 300 mg. Use of glycoprotein IIb/IIIa inhibitors was left at the operator’s discretion. Intravenous heparin or another thrombin inhibitor was administered to maintain an activated clotting time ≥250 seconds (or ≥200 seconds if a glycoprotein IIb/IIIa inhibitor was being administered) during the procedure. The interventions were performed with a ≥6 Fr guiding catheter. Systematic predilatation of the target lesion was mandatory regardless the allocation to treatment. The implanted BMS had to cover the whole target lesion length. The DCB should extend at least 2 mm beyond the distal and proximal margins of the stent and of the segment exposed to predilatation; a single DCB inflation ≥30 seconds was mandatory. If necessary, post-dilatation could be performed with the DCB catheter or with other shorter compliant or non-compliant balloon. After optimisation of the result, intracoronary nitroglycerine was administered and final angiography and OCT pullback were recorded. Optimisation of the result based on OCT images was strongly discouraged.
Follow-up
Subjects with a single study-lesion were kept on dual antiplatelet therapy with aspirin and clopidogrel for three months. In case a non-study lesion had been also treated during the same procedure, duration of the antiplatelet therapy could be extended to meet the requirements of the devices employed.
Clinical follow-up visits were scheduled at 30 days, 6, 12 and 24 months. Angiographic and OCT follow-up were performed at six months.
QCA analysis
QCA analysis was performed with the CAAS II system20 (Pie Medical BV; Maastricht, The Netherlands) in a corelab setting (Cardialysis BV; Rotterdam, The Netherlands). An in-DCB region of interest was defined as that coronary segment between the two radiopaque markers of the DCB during inflation. In-segment region comprised the in-DCB segment plus 5 mm proximal and 5 mm distal. MLD was automatically detected by the software. RVD at the point of MLD was calculated by the software by interpolation. Percent diameter stenosis was calculated as: (1-[MLD/RVD])*100.
OCT study and analysis
OCT pullbacks were obtained post-procedure and at six months follow-up with a Fourier-domain C7 system, using a Dragonfly catheter (Lightlab Imaging, Westford, MS, USA) at a rotation speed of 100 frames/sec using non-occlusive technique21. After infusion of intracoronary nitroglycerine, the optical catheter was withdrawn by a motorised pullback at a constant speed of 20 mm/second, while Iodixanol 320 contrast (VisipaqueTM, GE Health Care, Cork, Ireland) was infused through the guiding catheter at a continuous rate of 2-6 ml/sec.
OCT pullbacks were analysed offline in a core-laboratory (Cardialysis BV, Rotterdam, The Netherlands) by independent investigators blinded to the allocation and to the clinical and procedural characteristics of the patients, using proprietary software (Lightlab Imaging; Westford, Massachusetts, USA). Cross-sections at 1 mm intervals within the stented segment and 5 mm proximal and distal to the stent edges were analysed. Lumen and stent areas were calculated in each analysed cross-section. A metallic strut typically appears as a bright signal-intense structure with dorsal shadowing. Apposition was assessed strut by strut at baseline and follow-up by measuring the distance between the strut marker and the lumen contour22. The marker of each strut was placed at the endoluminal leading edge, in the mid-point of its long-axis, and the distance was measured following a straight line connecting this marker with the centre of gravity of the vessel. Struts located at the ostium of side branches, with no vessel wall behind, were labelled as non-apposed side-branch (NASB) struts and excluded from the analysis of apposition. Struts were classified as malapposed (ISA, incomplete stent apposition) during the statistical analysis if their distance to lumen contour was ≥100 μm, threshold resulting from rounding up the sum of the strut thickness (81 μm) plus the axial resolution of OCT (14 μm). Tissue coverage thickness was measured only at follow-up from the marker of each visible strut to the adluminal edge of the tissue coverage, following a straight line connecting the strut marker with the centre of gravity of the vessel. A strut was considered non-covered when the thickness of coverage was 0 μm. If the thickness of coverage was ≥60 μm for any of the struts in the cross-section, neointimal hyperplasia (NIH) area was calculated (Figure 1). From lumen, stent and NIH areas and stent length, the corresponding volumes were calculated. In-stent percent neointimal volume obstruction (primary endpoint) was calculated as: (NIH volume / Stent volume) *100.
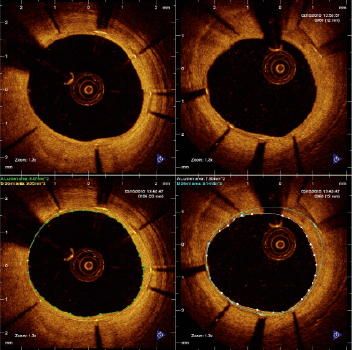
Figure 1. Examples of cross-sections in the optical coherence tomography studies six months after treatment with the combination of Moxy DCB and BMS (upper panel): neointimal hyperplasia (NIH) area is calculated as [stent area - lumen area] (lower panel).
To summarise the spatial distribution of the uncovered struts along the stents, “spread-out vessel graphics” were created by correlating the longitudinal distance from the distal edge of the stent to the strut (abscises) with the angle where the struts were located in the circular cross-section with respect to the centre of gravity of the vessel (ordinates), taking as reference 0º the position at three o’clock. The resultant graphic represented the stented vessel, as if it had been cut longitudinally along the reference angle 0º and spread out on a flat surface.
Assessment of longitudinal and axial mismatch (geographical miss)
Longitudinal geographical miss, defined as presence of ballooned or stented segments not covered in their whole length by the DCB application, was assessed by angiography in both treatment groups, using the stent and the edge markers of the corresponding balloons as references.
Axial geographical miss, defined as inability of the inflated DCB to contact the vessel wall at some regions of the stented segment, was subject to exploratory assessment in the group B (stent first), by means of graphics comparing the final stent area with the nominal area of the inflated DCB per cross-section. Thus, in those portions where stent area was bigger than the nominal inflated DCB area, axial geographical miss would be more likely to occur. This graphics were contrasted vs. the NIH area distribution along the stent, to explore a potential association between axial geographical miss and the extent of NIH.
Statistical analysis
Results are reported as mean±standard deviation for continuous variables, and as count (percent) for nominal variables. Continuous variables were compared with U-Mann-Whitney’s test. Nominal variables were compared with Pearson’s chi-square, or Fisher’s exact test if the expected frequency was <5 in any cell.
In the OCT per strut analysis, the proportions of uncovered and ISA struts were analysed using multi-level logistic regression models with random effects at three different levels: 1) treatment arm, 2) patient, 3) stent. Mean thickness of coverage was analysed using a multi-level linear regression model with random effects at the same three levels, after logarithmic transform. Overlap segments were considered as separate units of clustering.
Clinical endpoints followed a hierarchical events model. Backward step logistic regression and proportional hazards Cox regression were used for 30 days and six months results, respectively.
All statistical analyses were performed according to the intention-to-treat principle, using the SAS v8.2 package (SAS Institute Inc.; Cary, NC, USA).
Results
Figure 2 shows the flow chart of the study. Between the 24th of June and the 15th of December 2009, 26 patients were enrolled and randomised. Two patients, both in the DCB-first group, withdrew consent after randomisation, one of them before the 30 days visit, the other one between 30 days and six months. One of the angiographies and OCT studies in the BMS-first group were lost. One OCT study in each group was considered of insufficient quality to be analysed. One patient in BMS-first underwent implantation of other type of stent than the one established per protocol (Skylor, Invatec S.p.a.; Roncadelle, Brescia, Italy). Considering the similar characteristics of both types of stent, the steering committee decided not to exclude the patient from the analysis. Tables 1 and 2 show the baseline clinical and procedural characteristics of the patients, with no significant imbalance. Longitudinal geographical miss was only found in DCB first, although the difference did not reach statistical significance.
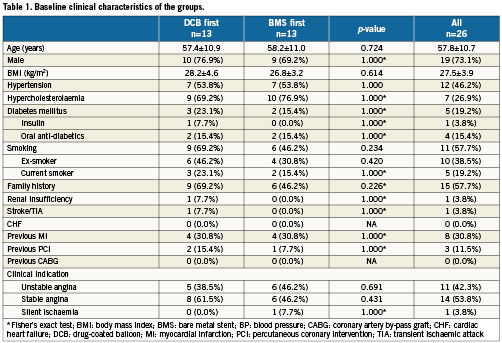
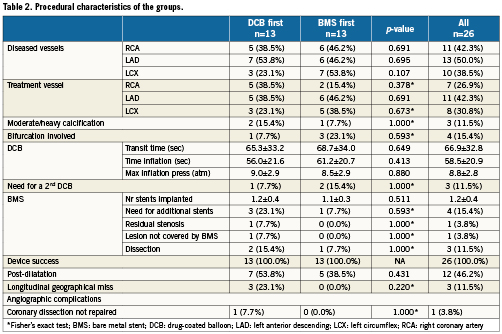
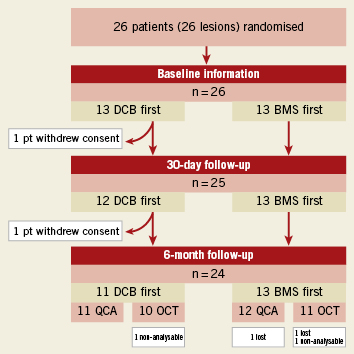
Figure 2. Flow chart of the study patients, with allocation to treatment and loss at follow-up.
Table 3 presents the results of the QCA analysis. In spite of randomisation, patients allocated to BMS-first had significantly smaller vessels than patients in DCB-first (RVD: 2.41 vs. 2.81 mm, p=0.026, respectively). Late loss was non-significantly different between the groups (0.45 vs. 0.53 mm in-DCB, p=0.833).
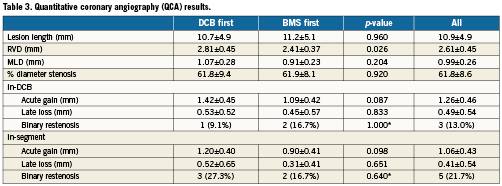
Table 4 presents the OCT in-stent areas and volumetric analysis. Lumen and stent areas parallel the QCA findings of smaller vessels in BMS-first. There was no significant difference in in-stent % NIH volume obstruction (primary endpoint of the trial) between DCB-first and BMS-first groups (25.5 vs. 24.9%, p=0.922, respectively). No correction for stent volume was required for the primary endpoint, because % NIH volume obstruction is by definition corrected for stent size. Table 5 presents the OCT areas and volumetric analysis of the stent edges. The exploratory assessment of axial geographical miss in BMS-first (Figure 3) did not show any clear association between axial DCB-BMS mismatch and the extent of local NIH. In the per-strut analysis, apposition immediately post-implantation tended to be worse in DCB first compared to BMS first (Table 6). Although the absolute proportion of ISA struts was substantially reduced in both groups at six months, the difference became then significant (0.1 vs. 2.3%, p<0.0001). Also the proportion of uncovered struts tended to be higher in DCB-first than in BMS-first (9.1% vs. 5.3%, p=0.237, respectively), without significant differences in thickness of coverage (p=0.575). After correction for vessel size (mean stent area), the difference in proportion of ISA struts still remained significant at six months (p=0.013). The spread-out vessel charts summarise the spatial distribution and clustering of uncovered struts (Figure 4). Uncovered struts cluster in some subjects, in some regions within a stent, or around the overlap segment.
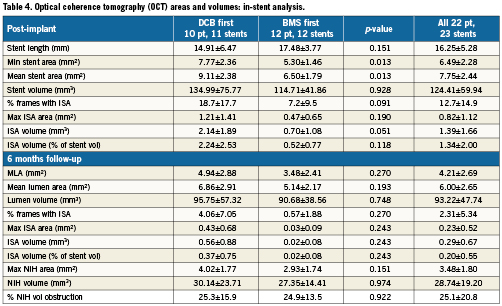
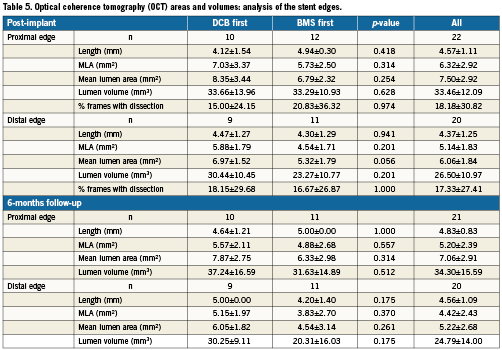
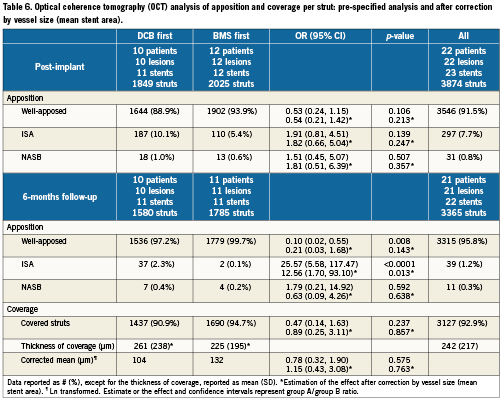
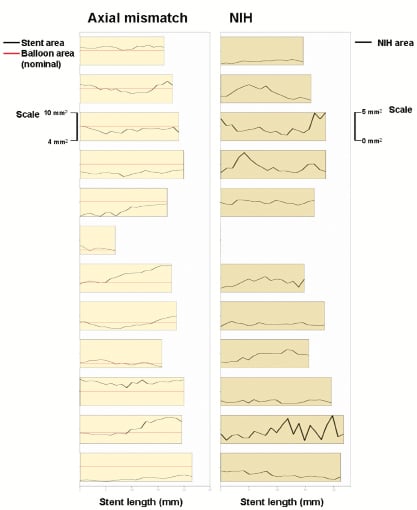
Figure 3. Exploratory assessment of axial geographical miss post-implantation (left panel) and its eventual association with local neointimal hyperplasia (NIH, right panel) in the group B of the study (BMS first). The bars in the left panel represent the length of each implanted stent. The black and red lines represent the stent area and the nominal area of the inflated balloon, respectively, in each cross-section. Thus, in those regions where the stent area is higher than the nominal inflated balloon area (black above red), axial mismatch would be more likely to occur. The black line in the right panel represents the local NIH area at six months in the corresponding stents. At first glance, no clear relation between NIH and axial geographical miss can be concluded.

Figure 4. Spread-out-vessel charts showing the spatial distribution of uncovered struts at six months in both treatment groups. The graphic summarises the clustering effect at the three levels: 1) allocation to treatment (right vs. left panel), 2) patient/lesion (bars are summaries per patient/lesion), 3) stent. The regional clustering within the stented region is also represented.
Table 7 summarises the clinical and safety secondary endpoints at 30 days and six months follow-up. Median follow-up time was 181 days (IQ range: 171-186.25): 176 days in group A (IQ range: 162.5-185), 181 days in group B (IQ range: 175-188).

Discussion
To the best of our knowledge this is the first randomised trial testing the efficacy of a DCB with an OCT primary endpoint. The results suggest that the sequential application of DCB and not pre-mounted BMS for the treatment of de novo coronary lesions is feasible and inhibits neointimal hyperplasia efficiently. The overall in-stent NIH volume obstruction (primary endpoint) and the mean thickness of coverage (25.1% and 242 μm, respectively) are comparable to the ones reported for paclitaxel-eluting stents (22.2-25.8%, 200-240 μm)23,24, lower than in some DES and far from those in BMS (53.9%, 530 μm)23. Also the proportion of uncovered struts (7%) is in the range of paclitaxel-eluting stents (5-7%), lower than in sirolimus eluting stents (8%), but higher than in BMS (1%)23,24. These OCT findings constitute an additional evidence of the biological effect exerted by DCB in the modulation of neointimal hyperplasia after stenting. Clinical and angiographic studies had already proven the concept consistently16-18, but this is the first time to quantify this effect with OCT, what will be interesting for the design of future studies.
The sequence “BMS first” translated into better apposition than “DCB first”, as reflected by significantly lower proportion of ISA struts and a non-significant trend to lower ISA areas and volumes. Although initially the sequence “BMS first” seemed to have also better coverage profile (higher proportion of covered struts at six months, with thinner tissue coverage), the log transform suggests that the neointimal coverage is actually comparatively thicker in this group, and the adjusted analysis suggests that these differences in coverage are mainly due to the smaller vessel size than to the allocation to treatment. Therefore both therapeutic strategies are comparable in terms of coverage at six months, but the sequence BMS first results in better apposition. Except from this advantage, there were no significant differences between treatment groups in the primary endpoint or in any of the remaining secondary endpoints. Thereafter the initial working hypothesis could not be confirmed. The results about the primary endpoint and struts coverage do not suggest that the application of DCB first actually results in better contact with the vessel wall, better transfer of the paclitaxel and therefore more effective action. Likewise, the idea that the implantation of BMS first would reduce the incidence of longitudinal geographical miss and hence be more efficient in real-world practice in spite of an eventually suboptimal contact between the DCB and the vessel wall, was not either confirmed: although no single case of geographical miss was certainly observed in the group “BMS first”, this did not seem to have any impact in any of the efficacy endpoints.
The results of this exploratory study suggest that the deployment of BMS first might ease the recognition of the target region and reduce the longitudinal geographical miss. However, this strategy might also result in an incomplete contact between the DCB and the vessel wall at some points, when the former is inflated inside the stent (axial geographical miss). The documentation of axial mismatch is more challenging. In this study we introduce a graphic method to assess axial geographical miss, as already explained, and explore its potential association with regional NIH. The results, however, do not suggest any direct relation in this respect. Likewise, although axial mismatch is a common finding among the patients in BMS-first, this does not entail worse outcome in any of the tested endpoints. It seems that geographical miss, either longitudinal or axial, influences the results at a lesser extent than currently believed. A potential explanation for this finding might be the diffusion kinetics of paclitaxel. Posa et al demonstrated in a coronary swine model that paclitaxel diffuses not only axially but also longitudinally into the vessel wall after DCB application25. Thus, a homogeneous inhibitory effect might be achieved, even though the contact with the vessel wall were suboptimal or the applications were slightly distant from the target point. Further investigation to clarify these findings is warranted.
The spread-out vessel charts offer an intuitive graphic representation of the spatial distribution and clustering of struts uncoverage. For instance, the effect of stent overlap can be easily understood with this representation. The graphic also depicts the complexity of healing after stenting, still poorly understood, with large inter-individual and regional variability within some patients. This marked clustering phenomenon highlights the importance of choosing an appropriate statistical method for the analysis of OCT data, in order to avoid misleading conclusions.
Limitations
This was a pilot study with small sample size, conceived to explore the effect of a novel DCB on the treatment of de novo coronary lesions. The results of several efficacy variables were in the expected ranges of paclitaxel-eluting stents, what is a relevant finding, but careful extrapolation of these results must be warned, because this was not a proper comparative study vs. a different device. Likewise, a bigger sample size might have contributed to understand better the role played by the sequence of application.
Randomisation resulted in a homogeneous distribution of all the control variables, except the vessel size. Although the primary endpoint was by definition corrected for vessel size, a statistical correction was required for the other efficacy endpoints. Sensitivity analysis including mean stent area as covariate circumvented this limitation in the per strut analysis. Mean stent area resulted to be a significant confounding factor for apposition (only affecting the proportion of NASB struts: the bigger the vessel, the more NASB struts) and for coverage (the bigger the vessel, the more proportion of uncovered struts and the thinner the coverage). The results of this sensitivity analysis, in which the inclusion of vessel size in the model significantly modified the magnitude of some effects, and in some cases even reversed the sense of the association, are also hereby reported.
Angiographic late loss was slightly higher than initially expected in this trial (overall in-stent 0.49 mm), despite the relatively small size of the vessels. Other paclitaxel-coated balloons with hydrophilic carriers had reported in-stent late loss of 0.09 and 0.19 mm for the treatment of in-stent restenosis16,18. Likewise, the rates of binary restenosis (overall in-segment 21.7%) at six months are clearly higher than previously reported by other DCB in other clinical scenarios (in-segment 5-7%)16,18. These findings might be related to the reduced paclitaxel dose of the Moxy balloon or to a less efficient transfer of the drug by the carrier. Further investigation will be required to better understand the reasons why this technology yields optimal results, comparable to paclitaxel-eluting stents, in some cases, but cannot avoid restenosis in others.
Conclusion
Sequential application of a paclitaxel-coated balloon in combination with a not pre-mounted BMS for the treatment of de novo coronary lesions is feasible and results in efficient inhibition of neointimal hyperplasia. The sequence of application (balloon first vs. BMS first) does not seem to influence the outcome, except for a significantly better apposition if the BMS is deployed first.
Funding
This trial has been sponsored by Lutonix Inc, Maple Grove, MA, USA. The corelab and CRO responsible for the analysis (Cardialysis BV; Rotterdam, The Netherlands) and the participating centres have received grants from the sponsor to run the trial.
Conflict of interest statement
P. Serruys, K. Kock and J. Koolen have received speakers’ fees from the sponsors. The other authors have no conflicts of interest to disclose.

