
Abstract
Aims: The optimal duration of dual antiplatelet therapy after an acute coronary syndrome (ACS) is still unknown and debated. We sought to assess the incidence of adverse clinical events beyond 12 months after an ACS in patients treated by percutaneous coronary intervention (PCI) and clopidogrel.
Methods and results: Among 1,592 consecutive ACS patients treated by PCI enrolled in the RECLOSE 2-ACS study and without event within one year, 1,310 (82%) patients presented at least one risk factor such as age ≥65 years, diabetes, prior myocardial infarction (MI), chronic kidney disease and multivessel coronary disease. The primary endpoint rate (the composite of cardiac death, MI, stroke and any urgent coronary revascularisation) was 3.7% per year after the first 12 months. The adverse event rate beyond 12 months was higher in patients with at least one risk factor as compared with patients without (8.1% vs. 1.8%, p<0.001). Each additional risk factor was associated with a relative risk for long-term adverse events of 1.66 (95% CI: 1.41-1.96; p=0.0001). Independent predictors of late events were age ≥65 years (OR 2.11, 95% CI: 1.38-3.37, p=0.002), insulin-treated diabetes mellitus (OR 2.29, 95% CI: 1.41-3.71, p=0.001), chronic kidney disease (OR 1.93, 95% CI: 1.21-3.09, p=0.006), prior MI (OR 2.71, 95% CI: 1.85-3.97, p=0.0001), and multivessel coronary disease (OR 1.53, 95% CI: 1.18-1.97, p=0.01).
Conclusions: Patients at risk of adverse events beyond 12 months after an ACS may be identified by simple clinical and angiographic characteristics such as age, diabetes, chronic kidney disease, prior MI and multivessel CAD. The risk of adverse events progressively increases with the number of these high-risk features.
Abbreviations
ACS: acute coronary syndrome
DAPT: dual antiplatelet therapy
DES: drug-eluting stent
HRPR: high residual platelet reactivity
LTA: light transmittance aggregometry
MACE: major adverse cardiovascular events
PCI: percutaneous coronary intervention
Introduction
Activated platelets have a key role in the development of atherothrombotic events leading to acute coronary syndromes (ACS)1, and dual antiplatelet therapy (DAPT) represents a cornerstone in secondary prevention after an acute myocardial infarction. In particular, in addition to aspirin, current practice guidelines recommend a platelet P2Y12 receptor inhibitor for 12 months after an ACS2,3. The routine 12-month DAPT length recommendation is derived largely from the designs of previous trials4-6, but it has a weak biological basis, and the comparisons of different DAPT lengths are scarce and not rigorous in ACS patients. Moreover, in current clinical practice DAPT is frequently prolonged beyond one year in patients considered at high risk of thrombosis7. So far, optimal DAPT length after an ACS is a matter of debate8-11. The PEGASUS trial showed that DAPT with aspirin and ticagrelor is able to prevent recurring ischaemic events well beyond 12 months after a myocardial infarction, at the price of increased major bleedings8. Data on the comparison of 12-month with more prolonged DAPT or, more appropriately, on the clinical impact of DAPT length individually tailored on the basis of ischaemic and bleeding risk profile are completely lacking. Finally, the adverse event rates beyond 12 months in ACS patients treated by percutaneous coronary intervention (PCI) are limited, but probably heterogeneous and dependent on patients’ risk profiles. Thus, we sought to assess adverse events occurring beyond 12 months in ACS patients receiving invasive management and clopidogrel treatment enrolled in the Responsiveness to Clopidogrel and Stent Thrombosis 2-ACS (RECLOSE 2-ACS) study12.
Methods
STUDY DESIGN
The RECLOSE 2-ACS study design has been described previously12. Briefly, it was an observational, single-centre cohort study of consecutive patients with ACS undergoing invasive treatment. All patients were treated with clopidogrel 600 mg loading dose, followed by a 75 mg daily dose on top of aspirin. Platelet reactivity after clopidogrel loading was prospectively assessed for every patient with light transmittance aggregometry (LTA), and patients with high residual platelet reactivity (HRPR; defined as an ADP 10 test ≥70%) received tailored antiplatelet therapy, generally consisting of a 150 mg clopidogrel maintenance dose. Long-term DAPT (>12 months) was strongly recommended. The study was approved by the local ethics committee. All patients gave informed consent. The present study is based on a retrospective post hoc analysis of the RECLOSE 2-ACS study.
PATIENT POPULATION
Of 1,789 patients enrolled in the RECLOSE 2-ACS study, 197 were excluded because they were lost to follow-up within one year (n=17), experienced new events within one year (n=137), or had an indication to chronic anticoagulant therapy (n=43). The remaining 1,592 patients were included in the present analysis (Figure 1). Almost all patients (97%) were on aspirin. Based on the PEGASUS trial study design8, patients were classified as at high risk in the presence of at least one of the following five characteristics: age ≥65 years, diabetes mellitus, chronic kidney disease (creatinine clearance <60 mL/min), prior myocardial infarction, and multivessel CAD.
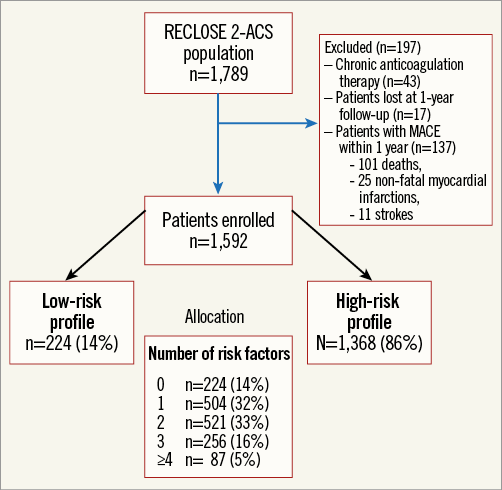
Figure 1. Study flow chart.
ENDPOINTS
The primary endpoint of this study was a composite of major adverse cardiovascular events (MACE: cardiac death, non-fatal myocardial infarction, urgent coronary revascularisation, and stroke) beyond one year after the index ACS event. Individual components of the primary endpoint, stent thrombosis, and TIMI major bleeding were secondary endpoints with previously defined definitions12.
FOLLOW-UP
All patients had scheduled follow-up at one, six, and 12 months from the ACS, and annually thereafter. All other possible information gathered from hospital readmission charts or by referring physicians, relatives, or municipality vital registries, were entered into the prospective database.
STATISTICAL ANALYSIS
Discrete data are expressed as frequencies, and continuous data as mean±SD or median and interquartile range, as appropriate. The χ² test was used to compare categorical variables, and the unpaired two-tailed Student’s t-test or Mann-Whitney rank-sum test was used to test differences between continuous variables. Survival curves were generated with the use of the Kaplan-Meier method, and the difference between groups was assessed by log-rank test. Multivariable regression analysis to evaluate the independent contribution of clinical, angiographic, procedural, and platelet reactivity variables to the primary endpoint was performed by the forward stepwise Cox proportional hazards model. The variables entered into the model were as follows: age ≥65 years, male sex, smoking habits, diabetes mellitus requiring or not requiring insulin treatment, hypertension, prior myocardial infarction, chronic kidney disease, multivessel coronary artery disease (CAD), left ventricular ejection fraction ≤40%, use of drug-eluting stents (DES), total stent length, HRPR, and clopidogrel therapy length (months). The proportional hazard assumption was assessed and satisfied graphically by plotting log (-log) survival curves against log survival time for each predictor category and verifying whether the curves were parallel and, in addition, using time-dependent covariates. We performed sensitivity analysis in order to test how robust the model was relative to the included population by assessing the effect of adding patients excluded due to events within one year and looking for recurrent events after the first 12 months according to the number of risk factors. The DAPT score13 was calculated for each patient enrolled in our study. Moreover, MACE and bleeding rates at long-term follow-up were calculated using the Kaplan-Meier method in high (≥2) versus low (<2) DAPT score patients, and compared with the log-rank test. Discrimination was assessed by calculating the area under the receiver operating characteristic curve and expressed as the C statistic. A p-value <0.05 was considered significant. All tests were two-sided. Analyses were performed with SPSS statistical package, Version 19 (IBM Corp., Armonk, NY, USA).
Results
PATIENT POPULATION, TREATMENT AND OUTCOMES
Of 1,592 study patients, 1,368 (86%) with at least one risk factor were included in the high-risk group and 224 (14%) in the low-risk group (no risk factor). Their baseline clinical and angiographic characteristics are summarised in Table 1. The two study groups showed significant differences in almost all baseline characteristics. In particular, high-risk profile patients presented a higher prevalence of cardiovascular risk factors, NSTEMI as ACS presentation, Killip class III or more, and HRPR, and also had greater CAD severity.
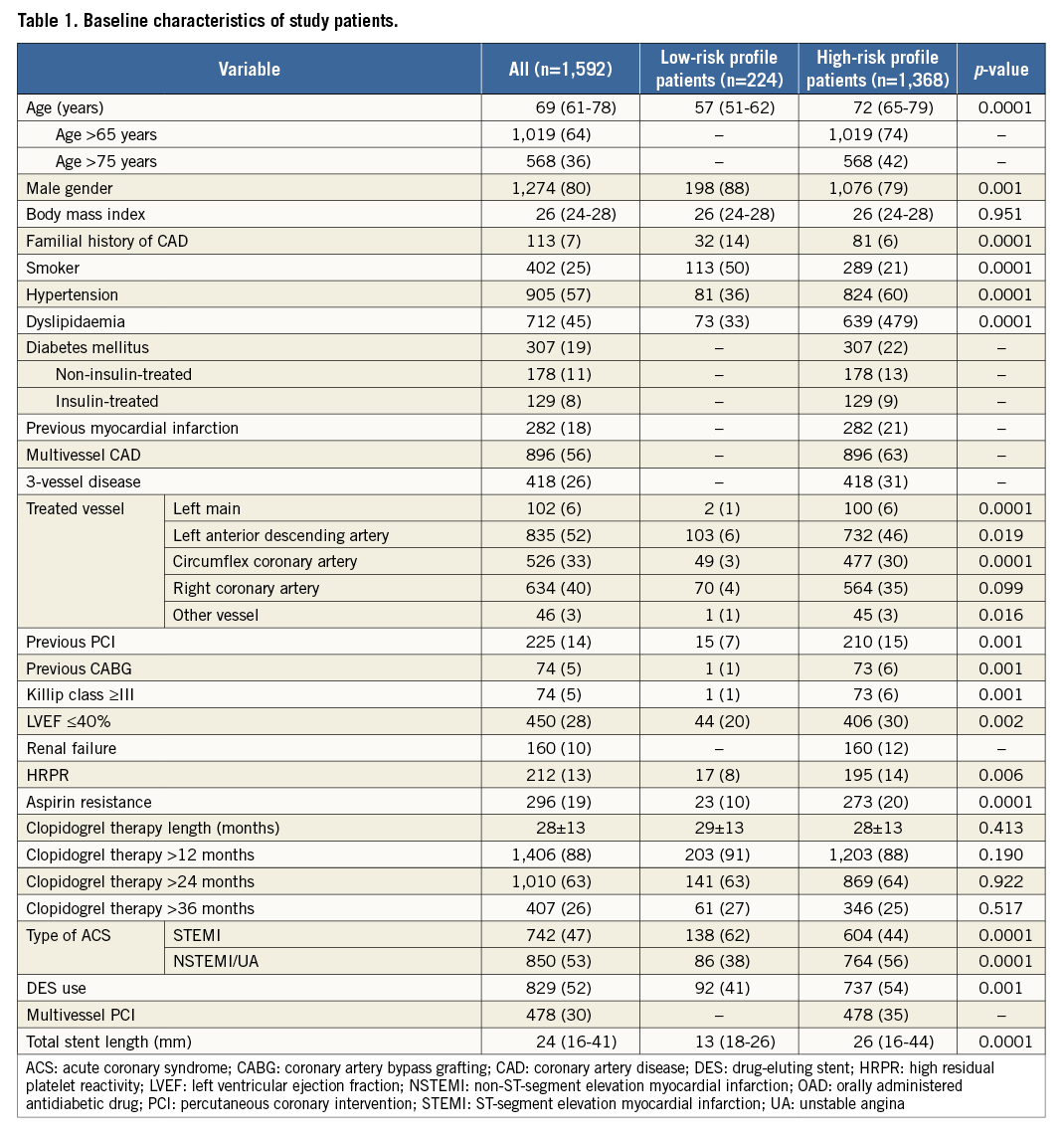
Adjusted Kaplan-Meier curves for the primary endpoint in high- and low-risk profile patients are reported in Figure 2. Table 2 summarises the clinical outcomes beyond one year in the low- and high-risk groups. MACE rates beyond 12 months were higher in patients with as compared with without at least one risk factor (8.1% vs. 1.8%, p=0.001). The difference in MACE rate was mainly driven by fatal events (cardiac mortality: 0.9% in low-risk patients and 4.3% in those at high risk; p=0.013), while there was no significant difference either in the other components of the primary endpoint or in the frequency of stent thrombosis (p=0.104).
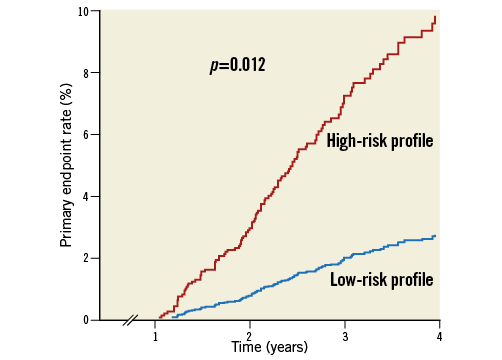
Figure 2. Kaplan-Meier curves depicting adverse event (primary endpoint: the composite of cardiac death, myocardial infarction, stroke, and any urgent coronary revascularisation) rates beyond one year after an acute coronary syndrome in patients with low- (no risk factor) and high-risk (at least one risk factor) profile adjusted for differences in baseline characteristics.
PREDICTORS OF ADVERSE EVENTS
At Cox multivariable analysis, age ≥65 years (OR 2.11, 95% CI: 1.38-3.37, p=0.002), insulin-treated diabetes mellitus (OR 2.29, 95% CI: 1.41-3.71, p=0.001), chronic kidney disease (OR 1.93, 95% CI: 1.21-3.09, p=0.006), prior MI (OR 2.71, 95% CI: 1.85-3.97, p=0.0001), and multivessel coronary disease (OR 1.53, 95% CI: 1.18-1.97, p=0.01) were all independent predictors of MACE (study primary composite endpoint). Among the RECLOSE 2-ACS study population, the DAPT score showed poor discrimination (C statistic: MACE model, 0.58 [95% CI: 0.52-0.63]; major bleeding model, 0.60 [95% CI: 0.49-0.71]). The MACE rates beyond 12 months from the index event were greater, but not significantly different, among the high DAPT score patients compared with the low DAPT score patients (12.9% high-score patients vs. 7.8% low-score patients [OR 1.34, 95% CI: 0.93-1.94], p=0.115). Rates of major bleedings were significantly different by score (1.8% in the high-score patients vs. 4.2% in the low-score patients [OR 0.34, 95% CI: 0.17-0.92], p=0.026).
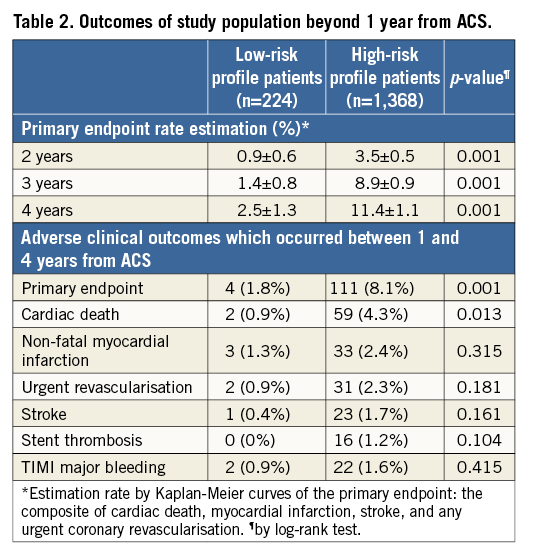
CLINICAL OUTCOME AND NUMBER OF RISK FACTORS
Of 1,368 high-risk profile patients, 504 (32%), 521 (33%), 256 (16%) and 87 (5%) had one, two, three or four or more risk factors, respectively. Event rate curves stratified by number of risk factors are reported in Figure 3. The overall estimated long-term MACE prevalence for patients with 0, 1, 2, 3, ≥4 risk factors was 3.2±1.3, 6.3±1.3, 10.5±1.7, 15.4±3.2, 34.6±7.6 (p=0.001), respectively. The primary endpoint rate progressively increased with the number of risk factors and each additional high-risk factor was associated with a relative risk for long-term MACE of 1.66 (95% CI: 1.41-1.96; p=0.0001). In the sensitivity analysis including patients with an event within the first year after the index ACS, the results did not significantly differ and each additional risk factor was associated with a relative risk for long-term MACE of 1.50 (95% CI: 1.34-1.68; p<0.0001). In addition, the association between the number of risk factors and event rates beyond 12 months persisted after the exclusion of patients showing HRPR on clopidogrel (n=248, RR: 1.54 [1.27-1.86]; p=0.0001) or those who discontinued clopidogrel ≤12 months (n=186, RR: 1.62 [1.35-1.95]; p=0.0001).
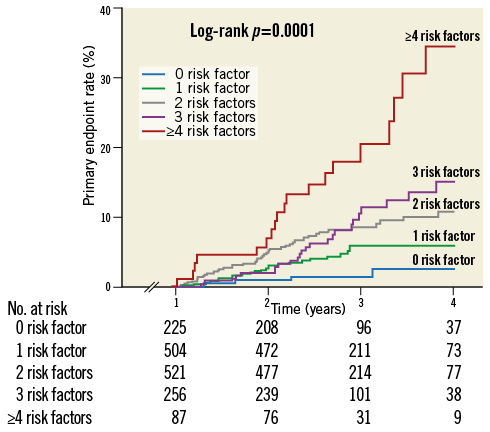
Figure 3. Kaplan-Meier curves depicting adverse event (primary endpoint: the composite of cardiac death, myocardial infarction, stroke, and any urgent coronary revascularisation) rates beyond one year after an acute coronary syndrome according to the number of risk factors (0, 1, 2, 3, ≥4 risk factors).
One year after an ACS, TIMI major bleeding rates were low and not significantly different between high- and low-risk patients (0.9% vs. 1.6%, p=0.415). Moreover, major bleeding estimation rates did not significantly increase with the number of risk factors (Figure 4).
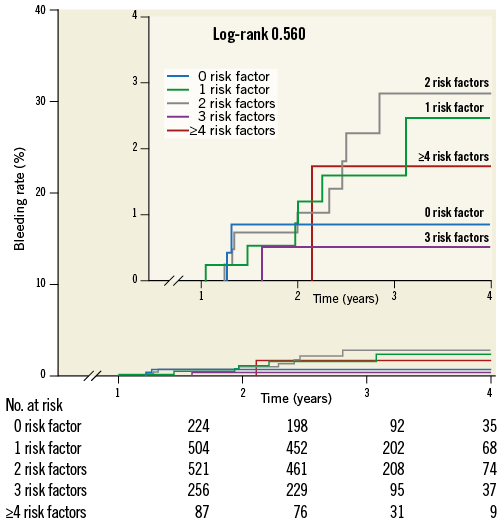
Figure 4. Kaplan-Meier curves depicting major bleeding rates beyond one year after an acute coronary syndrome according to the number of risk factors (0, 1, 2, 3, ≥4 risk factors).
Discussion
The main findings of the present study may be summarised as follows:
1. Event rates beyond one year from the index ACS event are relatively low but not negligible.
2. Patients at risk of adverse events beyond 12 months after an ACS may be identified by simple clinical and angiographic characteristics, such as age, diabetes, prior myocardial infarction, chronic kidney disease and multivessel CAD.
3. The risk of adverse events progressively increases with the number of these high-risk features.
4. The association between adverse events one year after ACS and the number of risk factors persists after the exclusion of patients showing HRPR on clopidogrel or of those who discontinue clopidogrel ≤12 months.
Until now, ACS patient outcomes have been assessed mainly within the first 12 months from the index event, while information on long-term event rates is limited. Patients with events within one year from the index event were excluded from the analysis because, in these cases, DAPT prescription is driven by the recent ACS event. In our large prospective registry of consecutive ACS patients treated with PCI, and event-free during the first year after the index event, the adverse event rate was relatively low (around 3.7% per year) but clearly not negligible. Predictors of adverse events beyond 12 months were five well-known risk factors for patients with ACS, comprising advanced age, insulin-treated diabetes mellitus, prior myocardial infarction, chronic kidney disease and multivessel CAD. Almost identical risk factors have been selected as additional high-risk clinical characteristics for the inclusion criteria in the PEGASUS trial8, due to the expected association with increased long-term adverse event rates. In the PEGASUS trial, the use of ticagrelor >1 year after an acute myocardial infarction resulted in a reduced ischaemic event rate, at the price of increased major bleeding events, with a disappointing overall risk/benefit profile. The need for a more accurate stratification of long-term patient risk emerged from the PEGASUS trial. In fact, at subgroup analysis, no single risk factor indicated a category of patients which might benefit from prolonged/resumed ticagrelor therapy8. From our study, it emerged that most ACS patients (86%) present at least one of the aforementioned risk factors and can be considered at increased risk of an event late after the index event. Odds ratios for adverse clinical events after one year from the index event were similar and around 2 for each risk factor. Accordingly, the risk of an adverse event progressively increased with the number of risk factors, which appeared to have a synergistic impact, with each additional risk factor being associated with a 66% relative risk long-term increase in MACE at long-term follow-up. As a consequence, patients with two, three and four or more risk factors presented remarkably high event rates beyond 12 months after the ACS. At the same time, bleeding event rates did not increase with the number of these risk factors, at least not in similar proportions. The strong association of risk factors with the long-term risk of MACE seems to carry important prognostic information. Thus, the simple number of risk factors in a given patient or the inclusion of these high-risk features in more elaborate and complete risk scores might help to select ACS patients who may profit the most from prolonged DAPT to optimise secondary prevention strategies, assuming the higher bleeding risk associated with prolonged DAPT. Unfortunately, in the DAPT trial, among patients not sustaining major bleeding or ischaemic events one year after PCI, a prediction score assessing late ischaemic and bleeding risks to inform dual antiplatelet therapy duration showed modest accuracy in derivation and validation cohorts13, as confirmed by our analysis.
The optimal duration of DAPT has been more extensively assessed in patients undergoing coronary drug-eluting stent (DES) implantation. A recent meta-analysis including 3,166 patients from 10 randomised trials underlined the fact that treatment with DAPT beyond one year after DES implantation in unselected patients may reduce recurring myocardial infarction and stent thrombosis, but it is associated with increased mortality because of an increased risk of non-cardiovascular mortality not counterbalanced by the reduction in cardiac mortality14. Thus, also in the setting of patients undergoing DES implantation, an individually tailored approach, carefully considering the individual benefit-risk profile in prescribing prolonged DAPT, seems to be the most appropriate and convenient secondary prevention strategy.
Limitations
Our study must be evaluated in the light of some limitations. First, study results are based on a post hoc analysis of a prospective registry and can only be considered hypothesis-generating. Second, we reported no difference in major bleeding rates beyond one year from ACS between risk groups; however, this study was probably underpowered for detecting differences in major bleeding rates, and this finding needs to be confirmed in larger cohorts. We also have to consider that bleeding risk may derive from comorbidities and predisposing conditions not included in the five key risk factors that we considered (i.e., age, diabetes, prior myocardial infarction, chronic kidney disease and multivessel CAD). In the present study, we documented that the relationship of the considered risk factors is strong with adverse ischaemic events beyond one year, but weak with late bleeding events. Third, our patients did not receive the two new P2Y12 inhibitors (i.e., prasugrel and ticagrelor) which were not available at the time of study enrolment. Thus, we are not able to speculate to what extent these results would be different using the new drugs rather than clopidogrel, and hence our results cannot be generalised to patients receiving prasugrel or ticagrelor. However, prolonged clopidogrel therapy was shown to be able to reduce event rates beyond 12 months from a myocardial infarction15. Our study findings were obtained in patients treated with prolonged clopidogrel therapy, and the association between adverse events one year after ACS and the number of risk factors persisted after the exclusion of patients showing HRPR on clopidogrel (poor responders to the drug) or those who discontinued clopidogrel ≤12 months (non-adherence to the drug). The favourable effect of prolonged DAPT with ticagrelor in the PEGASUS trial might derive from a class effect, since ticagrelor has been compared only with placebo8. On the other hand, the results obtained with ticagrelor in the PEGASUS trial should be extended with caution to other P2Y12 receptor antagonists, such as clopidogrel or prasugrel, since ticagrelor has several off-target effects able to impact on atherothrombotic events16,17. Fourth, all patients in our study had been treated with PCI; therefore, our results are not applicable to patients undergoing coronary artery bypass graft or to medically managed patients.
Conclusions
Patients at risk of adverse events beyond 12 months after an ACS may be identified by simple clinical and angiographic characteristics such as advanced age, diabetes, chronic kidney disease, prior myocardial infarction and multivessel coronary artery disease. The risk of late adverse events progressively increases with the number of these risk factors. Patients with multiple risk factors are those who might benefit the most from aggressive secondary prevention strategies, including prolonged DAPT, a concept that future trials need to validate.
| Impact on daily practice This study may help physicians to identify patients at highest risk of adverse events beyond 12 months from an ACS treated with PCI. |
Funding
This study was supported by an unrestricted grant from the Italian Health Ministry to the Tuscany Region for the Finalized Medical Research Program 2007.
Conflict of interest statement
G. Parodi reports receiving consulting or lecture fees from Daiichi Sankyo/Eli Lilly, AstraZeneca, Bayer and The Medicines Company. G. Tarantini reports receiving consulting or lecture fees from Daiichi Sankyo/Eli Lilly and AstraZeneca. R. Marcucci has received honoraria for lectures from Daiichi Sankyo/Eli Lilly and Merck Sharp & Dohme. N. Marchionni reports receiving consulting or lecture fees from Boehringer Ingelheim, Daiichi Sankyo, Eli Lilly and Bayer. D. Antoniucci reports receiving consulting fees from Daiichi Sankyo/Eli Lilly and The Medicines Company. The other authors have no conflicts of interest to declare.

