
Abstract
Aims: Prior percutaneous coronary intervention (PCI) is increasingly encountered in acute coronary syndrome (ACS) patients, with uncertain significance. We sought to evaluate the impact of prior PCI in ACS patients.
Methods and results: Patients with ACS enrolled in the prospective PROSPECT registry underwent three-vessel intravascular ultrasound and virtual histology evaluation after successful PCI of the culprit lesion(s). We identified patients with prior PCI (>6 months before index ACS) and compared their outcomes to those without prior PCI. Time-to-event for major adverse cardiac events (MACE) was estimated up to three years, and the independent association between prior PCI and MACE was evaluated in a multivariable model. Among 696 patients enrolled, 77 (11.1%) had prior PCI. They were older and more likely to have prior myocardial infarction, chronic kidney disease, and congestive heart failure. At three years, patients with prior PCI had significantly higher rates of cardiac death, rehospitalisation for worsening angina, and MACE (adjusted HR=1.73 [95% CI: 1.09, 2.75], p=0.02), independent of other comorbidities and intravascular ultrasound findings.
Conclusions: Prior PCI was noted in over 10% of patients with ACS and was associated with higher mortality and morbidity, independent of other comorbidities. Prior PCI should be considered a high-risk feature when evaluating ACS patients.
Introduction
Patients with known coronary artery disease (CAD) and prior percutaneous or surgical revascularisation have a higher incidence of subsequent death or myocardial infarction (MI) than those without CAD1. Percutaneous coronary intervention (PCI) is currently performed in over 600,000 patients annually in the USA, and thus the likelihood of encountering patients with prior PCI is increasing2. However, no recent study has examined whether patients with prior PCI presenting with an acute coronary syndrome (ACS) have an increased risk of major adverse cardiac events (MACE), and, if so, what the aetiology of this risk is. In the Providing Regional Observations to Study Predictors of Events in the Coronary Tree (PROSPECT) study3, after successful PCI of the culprit lesion(s), patients with ACS underwent three-vessel greyscale intravascular ultrasound (IVUS) and virtual histology (VH) IVUS evaluation. Future MACE at untreated non-culprit lesions were best predicted by larger (>70%) plaque burden (PB), smaller (<4 mm2) minimal lumen area (MLA), and presence of a thin-cap fibroatheroma (TCFA). We used this data set to investigate the significance of prior PCI relative to MACE occurring within three years of the index ACS hospitalisation.
Methods
The PROSPECT study has been described in detail3,4. In brief, 697 patients presenting with ACS (recent ST-segment elevation MI [STEMI], non-ST-segment elevation MI [NSTEMI], or unstable angina with electrocardiogram changes) were enrolled in the USA and Europe from 2004 to 2009 after successful PCI of all lesions believed to be responsible for the clinical presentation and after completion of any other planned interventions (culprit lesions) in ≤2 major epicardial coronary arteries. Quantitative coronary angiography (QCA) and VH-IVUS imaging of all thee coronary arteries were then performed as previously described4-6. Patients with prior PCI could be enrolled if it had occurred >6 months before the index ACS event; patients with prior coronary artery bypass grafting (CABG) or with STEMI <24 hours were excluded.
Patients were followed for a median of 3.4 years. C-reactive protein (CRP) levels were drawn at baseline, one month, and six months. The primary endpoint was the occurrence of MACE at three years, consisting of cardiac death, cardiac arrest, MI, or rehospitalisation for unstable or progressive angina, as independently adjudicated by a committee blinded to imaging results. Stent thrombosis was adjudicated according to Academic Research Consortium definitions7. Each adverse event was adjudicated to have arisen from either a previously treated culprit lesion responsible for the index ACS event or an untreated non-culprit lesion, or was considered indeterminate in origin in the absence of follow-up angiography.
QUANTITATIVE CORONARY ANGIOGRAPHY
QCA measurements were performed for non-culprit vessels (and their branches) ≥1.5 mm in diameter with the use of proprietary methods modified from QCA-CMS version 7.0 (Medis medical imaging systems bv, Leiden, The Netherlands) as previously described3. The reference vessel diameter, minimal lumen diameter, and diameter stenosis were calculated for each vessel. All lesions with ≥30% visual diameter stenosis were analysed according to the study protocol.
IVUS AND VH-IVUS ANALYSIS
Greyscale and radiofrequency IVUS of the left main and proximal 6-8 cm of each major epicardial coronary artery were performed using motorised catheter pullback at 0.5 mm/s as previously described3,4. Off-line greyscale and VH-IVUS analysis were performed by 1) QCA-CMS (Medis medical imaging systems) for contouring, 2) pcVH version 2.1 (Volcano Corp., Rancho Cordova, CA, USA) for contouring and VH data output, and 3) proprietary qVH version 2.5 (developed and validated at Cardiovascular Research Foundation, New York, NY, USA) for segmental qualitative assessment and data output. Quantitative IVUS measurements included external elastic membrane, lumen, plaque plus media cross-sectional area, and PB (defined as plaque plus media divided by external elastic membrane). An IVUS lesion was defined as a segment with ≥3 consecutive frames with ≥40% PB. VH-IVUS plaque components were reported as cross-sectional area and percentages of total plaque area. Lesions were classified into five main phenotypes: 1) VH-IVUS-derived TCFA (VH-TCFA), 2) thick-cap fibroatheroma, 3) pathological intimal thickening, 4) fibrotic plaque, and 5) fibrocalcific plaque.
STATISTICAL ANALYSIS
Outcomes were analysed in patients with versus patients without prior PCI. Prior PCI patients were further categorised as having had prior intervention of the culprit lesion responsible for the index ACS or of a non-culprit lesion unrelated to the presenting ACS. Continuous variables are presented as median with interquartile range and were analysed with ANOVA. Categorical variables are presented as proportions and were analysed with chi-square tests. Kaplan-Meier time-to-event estimates were compared with the log-rank test. Cox regression analysis was performed to evaluate the independent relationship between prior PCI and three-year MACE (either total MACE, or MACE adjudicated to originally treated culprit lesions or to untreated non-culprit lesions). In addition to prior PCI, the models also included worst non-culprit lesion by QCA (diameter stenosis >70%, 50-70%, or <50%), the presence of any lesion with IVUS MLA <4 mm2, the presence of any lesion with IVUS PB >70%, the presence of any lesion classified as a VH-TCFA, diabetes mellitus (DM), age, chronic kidney disease (creatinine clearance <60 mL/min), and prior MI. All analyses were performed with SAS version 9.2 (SAS Institute, Cary, NC, USA). A two-sided p-value <0.05 was considered significant.
Results
PATIENT AND BASELINE CHARACTERISTICS
Prior PCI status was unknown in one patient, and thus the current study population consists of 696 patients, 77 (11.1%) of whom had prior PCI. Baseline characteristics are shown in Table 1. Patients with prior PCI were older, had a higher incidence of aspirin use, and more commonly had risk factors for CAD (except DM). Among the 77 subjects with prior PCI, 43 (56%) had prior intervention of the ACS culprit lesion, and 34 (44%) had prior intervention of a non-culprit lesion. These two groups had similar baseline characteristics, except for prior MI and single-vessel disease which were more common in the non-culprit lesion prior PCI group (62.5% vs. 32.6%, p=0.01, and 79.4% vs. 53.5%, p=0.02, respectively).
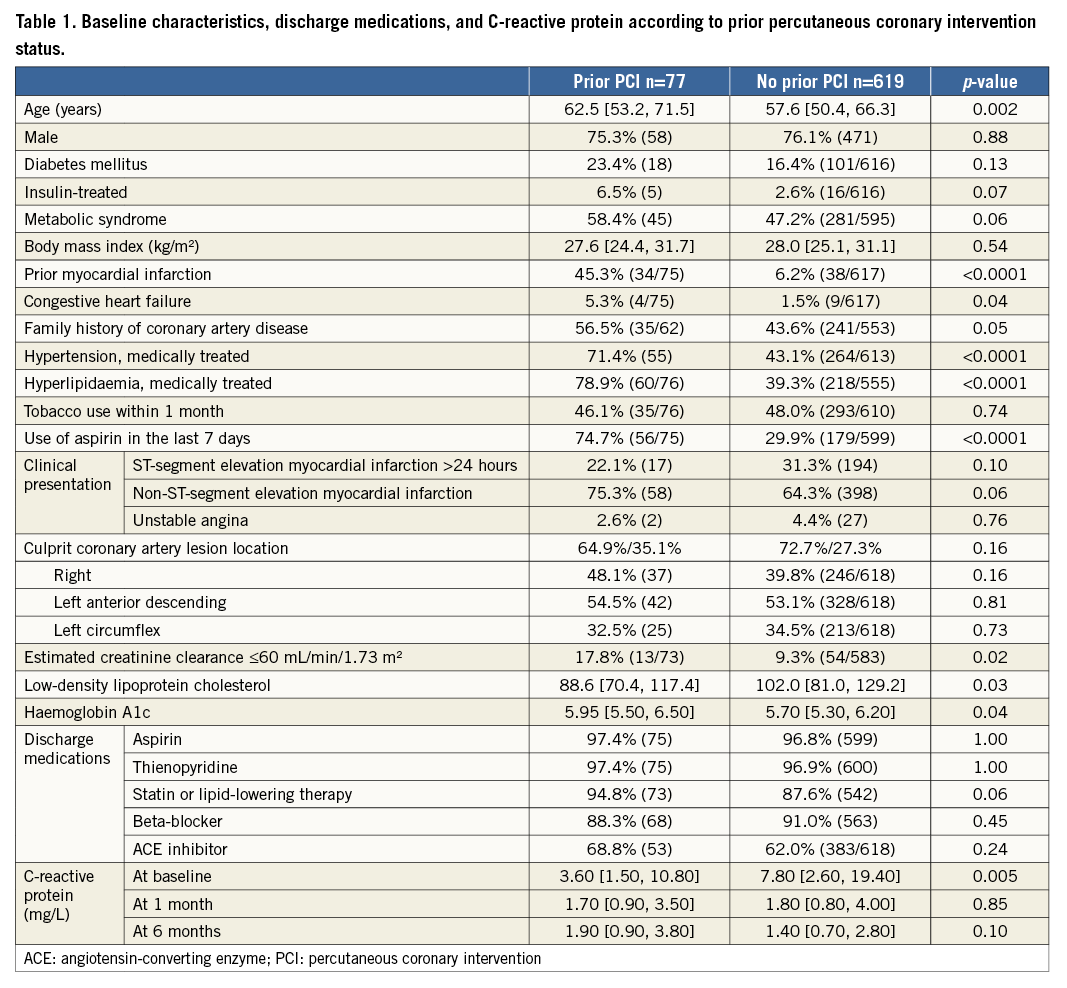
Rates of guideline-directed medical therapy usage at discharge in prior PCI and no prior PCI groups were comparable. CRP levels at baseline were significantly higher in the patients without prior PCI, but the levels at one month and at six months did not vary significantly between the two groups.
QCA AND IVUS ANALYSIS
The number and characteristics of non-culprit lesions with visually estimated diameter stenosis >30% were not significantly different between the two groups, with the exception of greater eccentricity in the prior PCI group. The angiographic characteristics of non-culprit lesions are shown in Table 2.
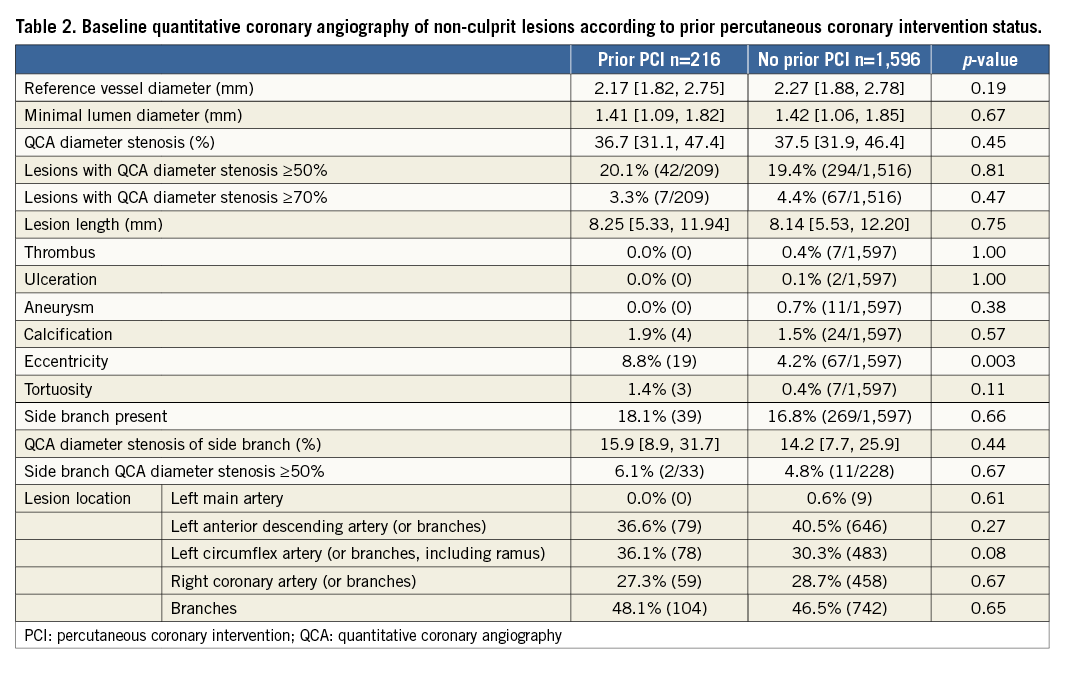
After successful PCI of culprit lesions, IVUS of the coronary tree demonstrated a similar number of untreated lesions in the coronary tree in patients with and without prior PCI (Table 3). However, patients with prior PCI more often had untreated regions of atherosclerosis with characteristics associated with higher risk for future non-culprit lesion-related MACE3 including MLA ≤4 mm2 and PB ≥70%. By VH-IVUS, however, there was no significant difference in the incidence of VH-TCFAs in these two groups. Among prior PCI patients, there were no differences between patients with culprit lesion versus non-culprit lesion prior PCI with respect to plaque characteristics, except that the non-culprit lesion prior PCI group was more likely to have at least one lesion with PB ≥70% (63.3% vs. 34.1%, p=0.01).
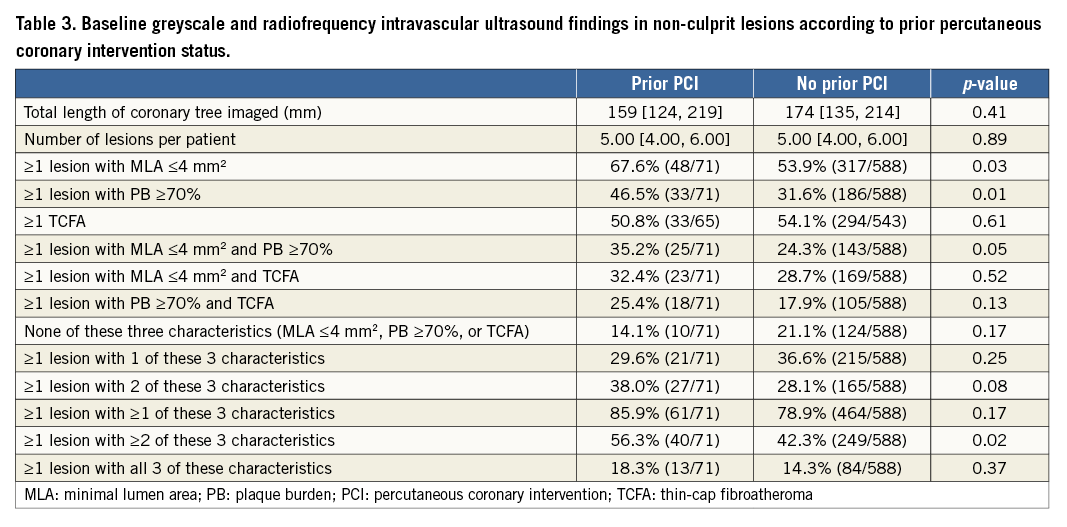
CLINICAL OUTCOMES
Median follow-up was 3.4 years (minimum two years). At three years, patients with prior PCI had significantly higher rates of MACE than patients without prior PCI (32.3% vs. 19.1%, p=0.005) (Table 4, Figure 1). Culprit lesion-related MACE, non-culprit lesion-related MACE, and indeterminate MACE occurred with greater frequency in the prior PCI group. The incidence of death (9.9% vs. 2.8%, p=0.002) and cardiac death (7.1% vs. 1.2%, p=0.001) was also higher in the prior PCI cohort, although most deaths were categorised as having an indeterminate relationship with the ACS culprit lesion(s), since these patients rarely had a follow-up angiogram. Stent thrombosis and hospitalisation for worsening angina were also increased in the prior PCI groups, events predominantly attributed to recurrent disease arising from the ACS index-treated culprit lesion(s). There were no significant differences in outcomes between patients with prior PCI of culprit versus non-culprit lesions (data not shown). Importantly, patients with prior PCI of non-culprit lesions also had significantly more events than those without any prior PCI (MACE: 38.4% vs. 19.1%, p=0.005; cardiac death: 6.6% vs. 1.2%, p=0.02), suggesting that the excess events are not only related to re-PCI of culprit lesions which experienced (in-stent) restenosis.
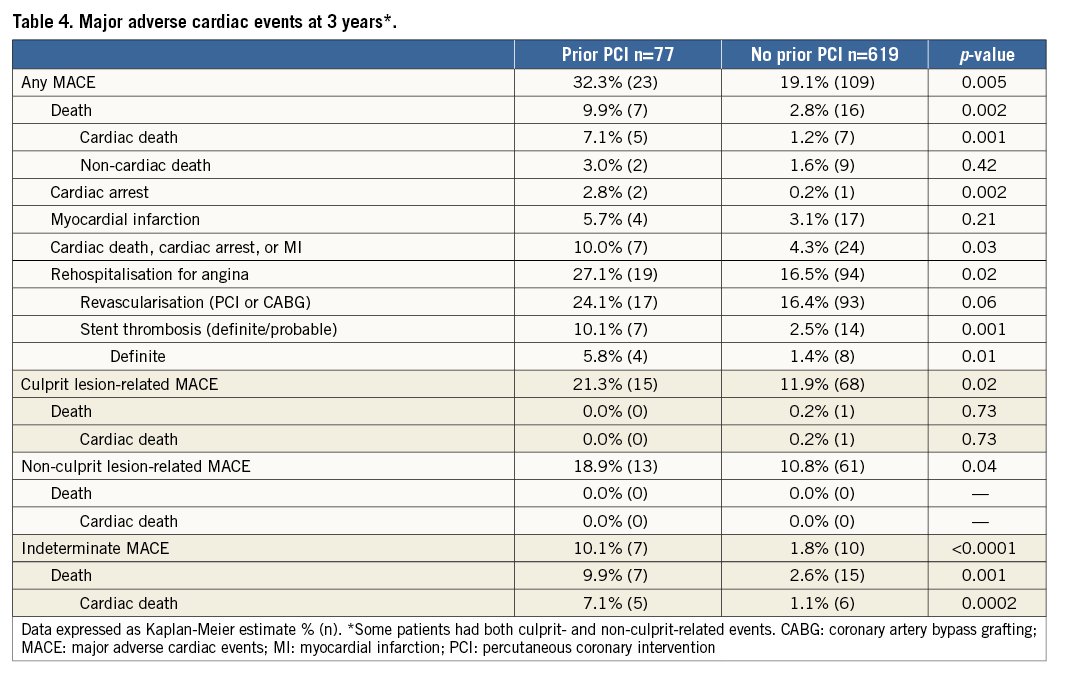
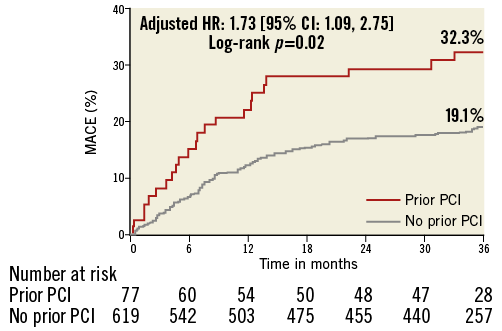
Figure 1. Incidence of MACE in patients with and without prior PCI. MACE: major adverse cardiac events; PCI: percutaneous coronary intervention
By multivariable analysis, prior PCI was significantly associated with three-year MACE (n=132, hazard ratio [HR] 1.73, confidence interval [CI]: 1.09-2.75, p=0.02). The only other independent correlate of MACE was the presence of any lesion with PB ≥70% (HR 1.81, 95% CI: 1.27-2.56, p=0.001). If insulin-treated DM was entered into the model rather than all DM, insulin-treated DM was also an independent correlate of MACE (HR 3.65, 95% CI: 1.77-7.52, p=0.005). Prior PCI was independently associated with culprit lesion-related three-year MACE as well (HR 1.85, 95% CI: 1.05-3.26, p=0.03), together with insulin-dependent DM (HR 3.95, 95% CI: 1.71-9.10, p=0.001), and PB ≥70% (HR 1.63, 95% CI: 1.05-2.54, p=0.03).
Discussion
The principal findings from this study are the following. 1) Prior PCI occurred in ~10% of ACS patients enrolled in PROSPECT and was associated with a ~3.5-fold increase in mortality compared to patients without prior PCI. Patients with prior PCI also had substantially higher rates of cardiac arrest and rehospitalisation for unstable or progressive angina. 2) Prior PCI was significantly associated with MACE at three years, independently of other baseline and procedural characteristics. 3) The higher rate of MACE in patients with prior PCI was due to excess events related to both culprit and non-culprit lesions.
The results presented in this manuscript are novel and suggest that ACS patients with prior PCI should be viewed as a high-risk group with unique characteristics and attributes. Numerous divergent factors are at play in these patients. On the one hand, patients with prior PCI are more likely to receive risk factor modification therapy (including antiplatelet agents) compared to patients without known CAD. They may also be more likely to present earlier for therapy when recurrent angina (re)occurs, since they are familiar with the symptoms. These protective features may however be offset by the higher propensity for future events in patients with established vascular disease.
Few previous studies have examined the prognostic risk of prior PCI in patients presenting with ACS. Labinaz and colleagues evaluated the outcomes of patients with prior PCI enrolled in three large ACS trials evaluating the utility of glycoprotein IIb/IIIa inhibitors8. Among 24,166 patients, 3,012 (12.5%) had prior PCI at a median of two years before ACS (a rate quite similar to our study). The six-month rate of death was 4.7% versus 6.3% (p=0.0003) for the prior versus no prior PCI groups, respectively. Death or MI at six months occurred in similar proportions in the prior versus no prior PCI groups (14.5% vs. 14.6%, respectively, p=0.82). These data are very different from our study in which prior PCI patients had higher rates of death and MACE at three years. The discordance in findings may be due to the substantial improvements in medical and interventional therapy over the intervening 15 years. Moreover, patients in PROSPECT were nearly a decade younger than in the Labinaz study and had all been successfully revascularised during the index ACS hospitalisation. Santos et al9 analysed outcomes in 448 ACS patients treated between 1998 and 2000 in Portugal. Prior PCI, which was present in 30% of patients, was an independent predictor of death or MI only if PCI occurred within the four months preceding the ACS event (adjusted odds ratio [OR] 4.81, p=0.006). Restenosis was responsible for the majority of ACS events in these patients. Our study excluded patients with recent (<6 months) PCI, the time frame during which most restenosis occurs. Thus, our study provides new and important information on the prognostic risk relevant to a prior PCI group without early manifestations of restenosis.
In a more recent study, Elbarasi et al10 assessed the management patterns of NSTEMI in relation to prior coronary revascularisation (including PCI or CABG) from three Canadian registries (ACS I, ACS II, and Global Registry of Acute Coronary Events [GRACE]/expanded-GRACE). Of the 12,483 NSTEMI patients included in the registry, 1,773 (14.2%) had prior PCI only, 1,193 (9.5%) had prior CABG only, and 633 (5%) had both prior PCI and CABG. Patients with prior revascularisation were significantly older and suffered significantly more often from hyperlipidaemia and DM than those without prior interventions. Interestingly, prior PCI was independently associated with higher use of in-hospital cardiac catheterisation (adjusted OR 1.18, 95% CI: 1.04-1.34, p=0.008) and greater use of antiplatelet therapy within the first 24 hours of hospital admission (60.0% vs. 47.2%; p<0.001). Unfortunately, the authors did not report clinical outcomes beyond the acute hospitalisation phase10.
Although there are few data to support the increased risk of MACE associated with prior PCI (Table 4), a number of mechanisms can be postulated. The greater incidence of high-risk features in non-culprit lesions of patients with prior PCI, such as PB ≥70% and MLA ≤4 mm2, can explain the higher rate of readmission with unstable angina and need for additional revascularisation. Furthermore, some of the MACE associated with the index ACS culprit lesions were also the result of more frequent stent thrombosis leading to MI and revascularisation, and occurred more frequently in the prior PCI cohort, regardless of whether the prior intervention was or was not in the culprit lesion. Although we do not have baseline IVUS of the culprit lesions, it is likely that those had more high-risk features in the prior PCI group, as was the case in the non-culprit lesions.
It is notable that patients with prior PCI had lower baseline CRP levels than patients without prior PCI, while, at one and six months from ACS, both groups had much lower and similar levels of CRP. It is unclear whether restenosis versus plaque rupture as a mechanism for the index ACS could explain this difference at least for patients with prior PCI in the index ACS lesion. In a prior analysis11, CRP levels >10 mg/L were associated with higher rates of non-culprit lesion MACE (19.0% vs. 7.2%, p=0.039) compared with normal CRP levels (<3 mg/L).
Limitations
The population enrolled in PROSPECT was rather unique in that patients had to survive the initial ACS presentation, undergo successful PCI of the lesion(s) deemed responsible for the event, and be candidates for a three-vessel IVUS study. Furthermore, recent PCI and CABG at any time were exclusion criteria, and the exact interval between the prior PCI and current ACS event was not collected. We also do not know the exact location of the prior PCI, the devices used during prior PCI or other procedural characteristics. Important prognostic data, such as left ventricular function, were missing in some patients. Because of the relatively small number of patients with prior PCI, limited power was present for a meaningful comparison of those patients with prior PCI of culprit lesions versus non-culprit lesions. Ultimately, no multivariable model can completely account for the differences that exist between the two groups, and our data only point to the fact that prior PCI identifies a sicker population of patients among those presenting with ACS.
Conclusions
Despite these limitations, we conclude that prior PCI is not an uncommon characteristic of patients with ACS, with a prevalence of >10%. Prior PCI, even when occurring >6 months earlier, and even after successful revascularisation of the ACS event, is independently associated with MACE within three years, including mortality. The increased rate of MACE in patients with prior PCI may be ascribed to more frequent recurrence at the treated culprit lesions (due to greater stent thrombosis and clinical restenosis), and more frequent symptomatic progression of untreated non-culprit lesions, in part due to more severe residual atherosclerosis (greater PB and smaller MLA). Prior PCI should be considered an important risk factor for future adverse outcomes in patients with ACS. Thus, aggressive surveillance and preventive measures should be emphasised in these patients prior to development of ACS.
Guest Editor
This paper was guest edited by Nick West, MA, MD, FRCP, Papworth Hospital, Cambridge, United Kingdom.
| Impact on daily practice Prior PCI occurred in ~10% of ACS patients enrolled in PROSPECT and was associated with a ~3.5-fold increase in mortality compared to patients without prior PCI, as well as substantially higher rates of cardiac arrest and rehospitalisation for unstable or progressive angina, independent of other baseline and procedural characteristics. The higher rate of MACE in patients with prior PCI was due to excess events related to both culprit and non-culprit lesions. In daily practice, prior PCI should be considered a high-risk feature when evaluating ACS patients. |
Acknowledgements
The authors would like to thank Dominic P. Francese, MPH, for his editorial assistance in the preparation of this manuscript.
Funding
While this substudy did not receive funding, the original PROSPECT study was funded by Abbott Vascular and Volcano.
Conflict of interest statement
A. Maehara has received grant support from Boston Scientific, is a consultant for Boston Scientific and ACIST, and has received speaker fees from St. Jude Medical. G.S. Mintz has received grant support from Volcano Corporation, and is a consultant forVolcano Corporation, Boston Scientific and InfraReDx. B. de Bruyne has received institutional consulting fees from St. Jude Medical. The other authors have no conflicts of interest to declare. The Guest Editor has no conflicts of interest to declare.

