Abstract
Many patients who undergo PCI subsequently undergo CABG. Until recently, however, there has been little data in the literature describing the effect of previous PCI on subsequent CABG outcome in terms of mortality or major adverse cardiovascular events (MACE). There are, for obvious reasons, no randomised trials addressing this question. This article summaries the limited observational evidence which does exist and which consistently reports an increase in hospital mortality and MACE in patients with prior PCI.
Introduction
While best evidence still supports CABG as the optimal revascularisation strategy in most patients with multivessel1 and left main stem2 coronary artery disease there is relatively little information in the literature regarding the influence of prior PCI on the outcome of patients who eventually undergo CABG. In the last four years, three large observational studies have reported that prior PCI adversely affects the outcome of CABG in terms of hospital mortality and major adverse cardiac events (MACE)3-6. As at least one-third of patients with prior PCI will eventually undergo CABG7, these findings, if real, have major implications both clinically, in deciding the optimal initial revascularisation strategy in patients with multi-vessel coronary artery disease, and economically in terms of cost-effectiveness for health services. In the absence of evidence from randomised clinical trials and meta-analyses this article reviews the existing available evidence.
The evidence (three studies)
STUDY 1 (Table 1) In 2005 Hassan and colleagues3 compared clinical outcome in 6,032 patients undergoing CABG between 1996 and 2000 of whom 5,113 had no prior PCI and 919 who did have prior PCI. From this overall population they undertook a propensity matched analysis of 919 patients who had prior PCI and 919 who had no prior PCI (Table 1). Both groups were well matched with respect to age, gender, diabetes, the presence of unstable angina, the use of a pre-operative balloon pump, ventricular function and the presence of left main and/or triple vessel coronary disease.
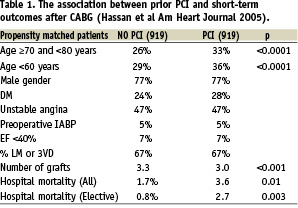
The mean number of bypass grafts was higher (p<0.001) in the patients having no prior PCI (3.3) compared to those with prior PCI (3.0). As shown in Table 1 the overall hospital mortality was significantly less (p<0.01) in the no PCI group (1.7%) than in the prior PCI group (3.6%) and for elective patients the figures were respectively 0.8% and 2.7% (p=0.003).
Multiple logistic regression analysis showed that significant predictors of an increased odds ratio (OR) for hospital mortality were an age over 80 (OR=4.7), cardiogenic shock (OR=4.1), emergency or salvage operation (OR=2.3), prior PCI (OR=1.9), impaired left ventricular function with EF <0.4 (OR=1.7) and female gender (OR=1.5). It is notable that with the exception of prior PCI all the other pre-operative characteristics are very well recognised risk factors for increased mortality.
STUDY 2 (Table 2a and 2b) In 2006 Thielmann and colleagues4 reported hospital outcome in 2,626 patients with no prior PCI, 360 patients with one prior PCI and 289 patients with more than one prior PCI (Table 2a). The groups were similar with respect to age, gender, diabetes, unstable angina, ventricular function and the percentage with left main and three vessel coronary artery disease. The number of prior stents used in these three groups were, respectively, 0, 1.3 and 3.6 (p <0.001) but there was no difference in the number of bypass grafts performed in each groups (mean of three grafts).
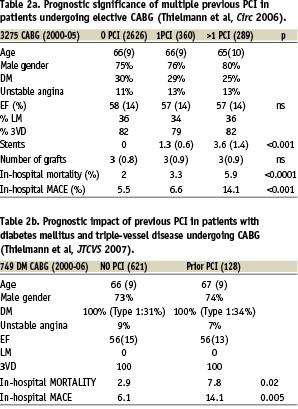
In-hospital mortality increased from 2% to 3.3% and 5.9% respectively in the three groups (p<0.0001) as did in-hospital MACE at 5.5%, 6.6% and 14.1% respectively (p<0.001). Multiple logistic regression analysis showed an increased odds ratio (OR) for death with chronic obstructive pulmonary disease (OR=2.7) and more that one prior PCI (OR=2.2). In propensity matched patients with more than one prior PCI the OR for death was 3.0 (95% CI 1.5–6; p<0.0002). Likewise logistic regression analysis for overall in-hospital MACE showed that more than one prior PCI had an OR of 2.2 and for propensity matched patients the OR was 2.3 (95% CI 1.5–7; p<0.0004).
In a sub-group analysis of 749 diabetic patients from the same cohort of patients5, 621 had no prior PCI and 128 had prior PCI (Table 2b). Again, the groups were well matched with respect to age, gender, unstable angina, ventricular function and the presence of left main and triple vessel coronary artery disease. The in-hospital mortality for no prior PCI was 2.9% versus 7.8% for prior PCI (p<0.02) and in-hospital MACE was respectively 6.1% and 14.1% (p<0.005). Multiple logistic regression analyses reported that prior PCI increased the overall risk of death with an OR of 2.5 and in propensity matched patients by an OR of 3.0 (95% CI 1.1 – 7.9 p<0.03); for in-hospital MACE the overall OR was 2.5 in patients with more than one prior PCI and in propensity matched patients the OR was 2.3 (95% CI 1.0– 5.2 <0.02).
STUDY 3 (Table 3) Chocron and colleagues6 reported on 2,489 CABG patients operated between 1999–2004 from the IMAGINE Trial, a randomised trial of the ACE inhibitor Quinapril and placebo, which concluded that there was no difference in the time to the primary endpoint (a composite of cardiovascular death, resuscitated cardiac arrest, non-fatal myocardial infarction, coronary revascularisation, unstable angina requiring hospitalisation, documented angina not requiring hospitalisation, congestive heart failure requiring hospitalisation).
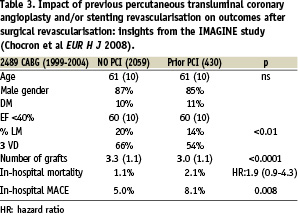
In a subsequent analysis of 2,059 CABG patients with no prior PCI and 430 with prior PCI, the patients were well matched with respect to age gender diabetes and ventricular function. In the prior PCI group there was a significantly (p<0.01) lower incidence of left main and three vessel coronary disease and a significantly lower number of bypass grafts (3.0 vs 3.3 p<0.0001). The in-hospital mortality was higher in the prior PCI group (2.1% vs 1.1%) with an OR of 1.9 (95% CI 0.9-4.3), but this did not reach statistical significance whereas the relative incidence of in-hospital MACE was 8.1% versus 5.0% (p=0.008).
Likely mechanism for adverse effects of prior PCI
In summary therefore these studies have consistently demonstrated an overall increase both in-hospital mortality (significantly greater in two studies) and in-hospital MACE (significantly greater in all studies) with prior PCI; these finding were maintained or exaggerated in those patients who were propensity matched for pre-operative risk factors.
There are several potential explanations for an inferior outcome in CABG patients with prior PCI. While one obvious explanation might be the presence of more severe coronary artery disease in the prior PCI group the studies actually suggest that there was more severe coronary disease in the group without prior PCI (and explaining referral for CABG as an initial strategy). Another possible explanation might be more severe left ventricular dysfunction in the prior PCI group but this is not supported by measurements of ejection fraction, which showed them to be similar in both groups. Two studies reported fewer bypass grafts in the prior PCI group implying support for the observation that incomplete revascularisation leads to impaired survival8; however, it is not clear whether fewer bypass grafts was due to the fact that some of the stented vessels remain patent (and therefore did not require bypass grafting) or whether the effects of stenting lead to a reduced ability to graft the vessel distally and the subsequent consequences of poorer distal run-off. Other possibilities are that the PCI leads to the inhibited development of collaterals or that the use of stents leads to generalised endothelial dysfunction; however while this is certainly true for drug eluting stents9 most of these studies used bare metal stents. In summary, while there seems to be a consistent observation of more adverse outcomes in patients with prior PCI the precise mechanisms have not been established from these studies.
Can PCI be used to safely delay the need for CABG?
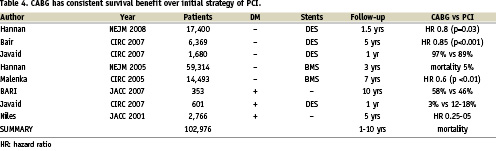
It is commonly asserted that PCI can be used to safely delay the need for CABG. However the existing literature has over eight registry studies involving over 100,000 patients with a mixture of patients with and without diabetes mellitus and treated with bare metal or drug-eluting stents and with follow-up ranging from one to ten years1 (Table 4). These studies consistently show that an initial strategy of PCI over CABG increases subsequent mortality by around 5 percentage points at three years. This strongly suggests that an initial strategy of PCI may not always be a safe option in terms of long-term outcome.
Will drug-eluting stents (DES) make a difference to these findings?
The majority of the patients in the studies quoted they received bare metal rather than drug-eluting stents (DES). While there is good evidence that DES reduce the incidence of restenosis, at least in more complex coronary lesions, there are several meta-analyses reporting that they do not improve clinical outcome over bare metal stents10. The assumption must therefore be that the use of DES would not make a substantial difference to the findings in the studies quoted previously. Almost certainly the reason that CABG offers a better survival benefit over bare metal stents and DES is that placing bypass grafts to the mid-coronary vessel not only deals with proximal coronary lesions regardless of their complexity, but that it also offers prophylaxis against the development of de novo disease. In contrast PCI, can only deal with suitable localised proximal culprit lesions, and offers no prophylactic benefit against the development of further disease.
Conclusion
Although there are no randomised trials or meta-analyses examining the effects of prior PCI on subsequent outcome in patients undergoing CABG, there appears to be consistent findings from several large observational studies, and particularly in propensity matched patients, that prior PCI increases both in hospital mortality and MACE by an odds ratio of two to three fold. While the precise mechanism for this remains to be elucidated, the consistency of the findings is striking and has two important implications. Not only does an initial strategy of PCI, rather than CABG, in patients with multivessel coronary artery disease increase mortality in all patients by around 5% at three years, but it subsequently increases the mortality in patients who eventually undergo CABG. This finding has important clinical implications for the individual patient and for the cost-effectiveness of both therapies in the wider health service. It underpins the need for patients with multivessel coronary disease to be advised by a multidisciplinary team to ensure a transparent decision process, real patient choice and genuine patient informed consent11.

