We read the article by Hälg et al1 with great interest. We learned that patients on triple antithrombotic therapy have an elevated bleeding risk after stent implantation. Hälg et al state that the potential risk of embolic complications must be carefully balanced against the risk of major bleeding in this patient population. Unfortunately, Hälg et al only describe the elevated bleeding risk in their patient population on triple therapy.
We are also interested in whether the use of triple therapy has any influence on thromboembolic risk in the BASKET patient population. We would also like to know the rates of stent thrombosis, reinfarction, stroke and target vessel revascularisation. We believe that these rates could provide us very useful information. We agree that prospective clinical trials are needed in order to evaluate the best treatment strategy for patients on oral anticoagulant therapy (OAC) who undergo percutaneous coronary interventions (PCI). Unfortunately, all present recommendations are not based on randomised trials, but on expert opinion. In patients with the indication for chronic oral anticoagulation who need to undergo PCI, there are many possibilities, but the combinations of OAC + aspirin and aspirin + clopidogrel are unsafe because of elevated risk of stent thrombosis and stroke respectively2. Triple therapy, which is currently recommended, is known to elevate bleeding risk.
A last possibility is the combination of clopidogrel and OAC, which seems to be promising2. Therefore, a first randomised international multicentre open label trial was started on December 1, 2008 to assess the hypothesis that after PCI with stent implantation in patients on oral anticoagulant therapy, the combination of oral anticoagulation therapy & clopidogrel 75 mg/day is superior to triple therapy treatment because of the reduced risk of bleeding3. The primary outcome is the combination of TIMI and GUSTO minor and major bleeding up to 30 days and one year. The secondary outcomes are major adverse cardiac events (myocardial infarction, stent thrombosis, target vessel revascularisation, stroke). The sample size is 496. Because, to date, no randomised study has yet addressed this issue, the WOEST (What is the Optimal antiplatElet & anticoagulant therapy in patients with oral anticoagulation and coronary StenTing; clinical trials.gov:NCT00769938) trial will help to define new guidelines for patients with indication for long-term anticoagulation who need coronary stenting.
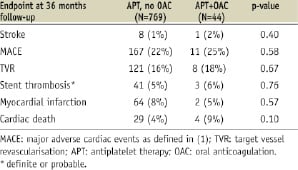
Drs Dewilde et al enquire about some additional endpoints in the group on oral anticoagulants in comparison to those without. They are summarised in the following table. Despite some trends in increased mortality, there were no significant differences found.
We agree that a dual combination of oral anticoagulation and clopidogrel might be an alternative to triple therapy, a hypothesis to be tested. Thus, we are looking forward to results of the WOEST study2.
Drs. Rubboli et al are correct in their remark concerning our paper when they point out that oral anticoagulation may merely be a marker of a higher bleeding risk, rather than the actual cause. Our data, in a strict sense, indeed show that the combination of oral anticoagulants with either single or dual antiplatelet therapy are associated with an increased late bleeding rate.
While the BASKET study was not intended to address the issue of bleeding, it offered a unique opportunity to study this important question in an unselected cohort of all-comers with a complete long-term follow-up. By using multivariate analysis, we tried to eliminate confounding factors as much as possible. Although some confounders may be unknown, the strong independent association of anticoagulants with late bleeding suggests an independent contribution. This is also in agreement with most studies on antithrombotic therapy, independent of underlying disease and agents used, showing that the stronger the antithrombotic therapy, the higher the bleeding risk3.
The risk in our study might have been even greater if all patients had remained on triple therapy throughout the entire follow-up period.
We agree that post hoc analyses as our own, and even prospective registries, do not provide a final conclusion on the bleeding risk of triple antithrombotic therapy. A randomised controlled trial would be needed, but it is unlikely that such a trial will be done. Thus, data such as ours help to assess the risks involved with interventions and associated drug therapies.
The conclusions drawn in our paper are valid and in agreement with current guidelines4. They are not altered by the reflections made by Rubboli et al. Accordingly, indications for oral anticoagulation should be critically reviewed in patients undergoing PCI and stent therapy. Patients with strong indications for oral anticoagulants (which may be questioned in many cases of atrial fibrillation with intermediate risk5) should preferably not be treated with drug eluting stents as the lower rate of restenosis may be outweighed by a higher rate of bleeding –and even mortality– due to anticoagulant and prolonged aggressive antiplatelet therapy. It seems wise to reduce potentially harmful therapies to a minimum.

