Abstract
Optimal perioperative antiplatelet therapy in patients with coronary stents undergoing surgery still remains poorly defined and a matter of debate among cardiologists, surgeons and anaesthesiologists. Surgery represents one of the most common reasons for premature antiplatelet therapy discontinuation, which is associated with a significant increase in mortality and major adverse cardiac events, in particular stent thrombosis. Clinical practice guidelines provide little support with regard to managing antiplatelet therapy in the perioperative phase in the case of patients with non-deferrable surgical interventions and/or high haemorrhagic risk. Moreover, a standard definition of ischaemic and haemorrhagic risk has never been determined. Finally, recommendations shared by cardiologists, surgeons and anaesthesiologists are lacking. The present consensus document provides practical recommendations on the perioperative management of antiplatelet therapy in patients with coronary stents undergoing surgery. Cardiologists, surgeons and anaesthesiologists have contributed equally to its creation. On the basis of clinical and angiographic data, the individual thrombotic risk has been defined. All surgical interventions have been classified according to their inherent haemorrhagic risk. A consensus on the optimal antiplatelet regimen in the perioperative phase has been reached on the basis of the ischaemic and haemorrhagic risk. Aspirin should be continued perioperatively in the majority of surgical operations, whereas dual antiplatelet therapy should not be withdrawn for surgery in the case of low bleeding risk. In selected patients at high risk for both bleeding and ischaemic events, when oral antiplatelet therapy withdrawal is required, perioperative treatment with short-acting intravenous glycoprotein IIb/IIIa inhibitors (tirofiban or eptifibatide) should be taken into consideration.
Introduction
The number of patients with coronary stents undergoing surgery is increasing significantly. Premature discontinuation of antiplatelet therapy, especially if it occurs within the first months after stent implantation, is associated with a higher risk of stent thrombosis, a feared complication that might have dramatic clinical consequences1-6. On the other hand, antiplatelet therapy can significantly raise intraoperative haemorrhagic risk in surgical or endoscopic procedures7.
Perioperative management of antiplatelet therapy is often arbitrary and may be controversial for cardiologists, surgeons and anaesthesiologists. In recent years, international cardiological, anaesthesiological and haematological societies have proposed guidelines and joint position papers on the management of antiplatelet therapy in patients undergoing non-cardiac surgery8-18. However, some limitations of these recommendations are evident. Elective surgical procedures should be postponed until completion of the mandatory dual antiplatelet regimen, aspirin therapy should be stopped only if haemostasis is difficult to control during surgery, and a multidisciplinary approach is required (e.g., cardiologist, anaesthesiologist, haematologist, and surgeon) to determine the patient’s risk and to choose the best strategy13. However, little support is provided with regard to managing antiplatelet therapy in the perioperative phase in case of semi-elective or urgent surgical or endoscopic procedures, the definition of perioperative bleeding risk is not provided, and the suggested multidisciplinary approach on an individual basis does not allow for a standard approach. Moreover, guidelines shared with cardiologists, surgeons and anaesthesiologists are lacking, although the surgeon’s point of view is crucial. The management of the risk ratio between bleeding and thrombosis requires an exact knowledge of risk stratification defined for each condition, coupled with offering the minimal surgical impact. The purpose of this manuscript is to provide practical recommendations for a tailored and standardised antiplatelet treatment management, even in difficult or unusual scenarios, that are specific to each type of surgery (cardiac and non-cardiac), which has been elaborated from a previously reported consensus document from the Italian Society of Interventional Cardiology (GISE) and the Italian Association of Hospital Cardiologists (ANMCO)19.
THE GISE-ANMCO CONSENSUS DOCUMENT
To overcome the aforementioned limitations of existing guidelines, the Italian Society of Interventional Cardiology (GISE) and the Italian Association of Hospital Cardiologists (ANMCO) promoted the creation of a consensus document with regard to the optimal antiplatelet regimen in patients with coronary stents undergoing surgical and endoscopic procedures. The Writing Committee was composed of clinical and interventional cardiologists, surgeons and anaesthesiologists, who met seven times in Milan and contributed equally to its creation19. Most of the members of the Writing Committee were delegates of the most important national societies of cardiologists, surgeons and anaesthesiologists. Cardiologists defined the thrombotic risk on the basis of procedural features, such as stent type, time from percutaneous coronary interventions (PCI) to surgery, and clinical features, such as acute coronary syndrome at the time of PCI, previous stent thrombosis, concomitant diabetes, renal impairment, and low cardiac ejection fraction. Surgeons classified all interventions according to the haemorrhagic risk as low, medium, and high. Finally, on the basis of both ischaemic and thrombotic risk, an agreement with regard to the most appropriate antiplatelet therapy in the perioperative phase was reached for each procedure.
The manuscript provides practical recommendations that are specific to each type of surgery. The methodology is aimed at allowing for a tailored and standardised management even in difficult or unusual scenarios.
This document is an elaboration from the previous Italian consensus document19. As distinct from the Italian published version, the present manuscript also received the endorsement of the Italian Society of Anaesthesiology. Anaesthesiologists contributed significantly to the paper, thus providing a multidisciplinary approach with the additional advantage of recommendations coming from different perspectives. Of note, due to lack of evidence from clinical trials, the present consensus document derives mostly from experts’ opinion, which represents the main limitation. It has now been officially endorsed by 16 cardiology, anaesthesiology and surgery societies. A free English application for I-phone and I-pad can be downloaded at the site https://itunes.apple.com/us/app/stent-sur
gery/id551350096?mt=8.
“STENT AND SURGERY”: THE DIMENSION OF THE PROBLEM
The number of PCI is increasing worldwide20,21. Every year more than one million PCI are performed in the USA and Europe20,21. In more than 85% of cases a coronary stent is implanted22, and prolonged antiplatelet therapy is mandatory after stent implantation. The most common causes of discontinuation are surgery and bleeding events which are often associated with a poor prognosis23.
The management of antiplatelet drugs in the perioperative period is relevant, both from an epidemiologic and a clinical point of view. It has been estimated that 4-8% of patients undergo surgery within the first year after coronary stent implantation and 23% within five years22. The withdrawal and sometimes also the maintenance of antiplatelet therapy may have dramatic consequences7,24. Surgery can lead to inflammatory, hypercoagulable and hypoxic states which are associated with plaque instability and perioperative arterial thrombosis22. On the other hand, bleeding risk might be 3.4 times higher during dual antiplatelet therapy compared to aspirin alone25.
ASSESSMENT OF THE PERIOPERATIVE ISCHAEMIC RISK (THE CARDIOLOGIST’S POINT OF VIEW)
Aspirin can significantly reduce the risk of cardio-cerebrovascular events in secondary prevention26. Abrupt discontinuation of aspirin therapy can be associated with a “rebound” effect27 and surgical interventions increase coagulation per se28. Previous studies demonstrated that perioperative discontinuation of aspirin therapy is associated with a significant increase in major adverse cardiac events (MACE)27,29. Also, in coronary artery bypass grafting (CABG), maintenance of aspirin in the perioperative phase is associated with a significant reduction of mortality30,31.
Data on the effect of the association of aspirin and clopidogrel are lacking and derive mostly from post hoc analyses of randomised trials and from registries32,33.
The incidence of perioperative MACE is high, especially if surgery is performed early after coronary stenting34.
The increase of MACE might, in part, be due to the perioperative discontinuation of antiplatelet therapy35-37. In Schouten’s series, the MACE rate was 2.6% in the overall population, which increased to 13.3% in patients undergoing early surgery37. However, the protective effect of perioperative antiplatelet therapy did not emerge in other studies38,39. These (apparently) discordant data might be explained by a bias in patient selection: antiplatelet therapy maintenance might identify a population at high risk for MACE, which seems likely to be the result of complex unidentified interactions between clinical and surgical risk factors. Previous studies demonstrated that the risk of perioperative MACE is higher within the first months after stent implantation40, even though data are not consistent41. In a recent study by Wijeysundera and colleagues42, the overall rate of 30-day events was 2.1%. It demonstrated that elective non-cardiac surgery could be performed reasonably safely in carefully selected patients when at least six months have elapsed since DES implantation and from 46 to 180 days after BMS implantation.
INTRA-OPERATIVE MANAGEMENT (THE ANAESTHESIOLOGIST’S POINT OF VIEW)
In the modern anaesthesia scenario, anaesthesiologists are facing a double challenge: the choice of the best and safest anaesthesiological technique for the patient, and how to manage haemostasis in the perioperative period.
Contrary to common belief, at present there is no evidence about a real superiority of a single anaesthesia technique in patients with coronary artery disease43-46, neither regarding inhalation vs. intravenous general anaesthesia nor general vs. loco-regional or blended techniques. Nevertheless, there is a certain agreement towards preferring blended or loco-regional anaesthesia whenever possible due to its intrinsic better control of perioperative pain and ability to lower sympathetic stimulation47,48. However, loco-regional anaesthesia might have an intrinsic and unavoidable risk when performed in patients on antiplatelet therapy49. The field of loco-regional anaesthesia is greatly affected by antiplatelet therapy, especially in terms of neuraxial techniques, due to the increased risk of catastrophic neurological events in the presence of abnormal bleeding status. Nowadays, it is well known that a safe neuraxial technique can be safely performed in patients on aspirin therapy49. By contrast, dual antiplatelet therapy with aspirin and clopidogrel during the week preceding a surgical intervention is an accepted contraindication to any form of regional anaesthesia18,43,47,49. Spinal haematoma has been described during clopidogrel treatment45, but the precise risk of spinal or epidural haematoma with dual antiplatelet therapy is unknown46.
Therefore, the latest recommendations of the American Society of Local Anaesthesia to stop clopidogrel seven days prior to surgery are based on clinical judgement and on isolated reports of epidural haematomas after spinal analgesia, combined spinal-epidural analgesia or both, rather than on results provided by clinical trials18,45,49,50. Afterwards, a loco-regional anaesthesia can be performed using the neuraxial technique in patients on aspirin therapy, whereas dual antiplatelet therapy represents a contraindication. If P2Y12 inhibitors cannot be discontinued, a general anaesthesia is advisable.
THE PERIOPERATIVE HAEMORRHAGIC RISK: THE SURGEON’S POINT OF VIEW
It is well known that antiplatelet therapy confers an increased risk of bleeding26,32. Conversely, the association between antiplatelet agents and perioperative bleeding risk has not been adequately addressed. The vast majority of the available data derives from registries or observational studies, which do not have sufficient statistical power.
A meta-analysis on the effects of low-dose aspirin on perioperative bleeding complications demonstrated that aspirin increased the frequency of bleeding complications by approximately 50%7. However, the definition used in the included studies was extremely heterogenous and often did not use a standard definition. Moreover, when surgeons were blinded regarding aspirin application, they could not differentiate patients on aspirin from patients off aspirin from bleeding behaviour alone51. The authors concluded that, with the possible exception of intracranial neurosurgery and transurethral prostatectomy, where bleeding-related fatalities after aspirin ingestion were reported7,24,52, low-dose aspirin increases bleeding only quantitatively. Additionally, only a few studies analysed in the meta-analysis were randomised, and therefore low-dose aspirin might be considered simply a risk indicator for increased comorbidity with an increased bleeding risk per se53. Only one double-blind randomised trial has investigated the perioperative bleeding risk in patients undergoing non-cardiac surgery while on 75 mg aspirin therapy29. No significant increase of bleeding events was identified in those patients taking aspirin as compared with those who were not on antiplatelet therapy. In Albaladejo’s series, major and minor haemorrhagic complications were observed in 9.5% of patients35. Most bleedings were at the surgical site (85.2%) and were associated with repeat surgery in 18.5% of patients. The death rate in patients with bleeding complications was 12.0% (95% CI: 6.6 to 19.7). Another study37 demonstrated a very low rate of excessive blood loss during surgery (1%), whereas blood transfusion was required in 24% of patients who continued vs. 20% of those who discontinued antiplatelet therapy.
Data on the role of clopidogrel on perioperative bleeding risk are lacking. An increased haemorrhagic risk emerged in patients undergoing CABG while on clopidogrel therapy, which was reduced by stopping the drug at least five days prior to intervention33,54-57. However, published data are not consistent58. On the basis of these data, the latest guidelines on non-ST-elevation myocardial infarction of the European Society of Cardiology recommend the perioperative maintenance of clopidogrel in high-risk patients undergoing coronary artery bypass grafting (CABG) if coronary anatomy is complex, with special attention to reducing bleeding59,60. The bleeding risk in patients undergoing non-cardiac surgery while on antiplatelet therapy has been poorly investigated. The few available studies indicate an increased haemorrhagic risk39,61. Prostate biopsy and ureteroscopy can be performed in patients on aspirin therapy without a significant increase of major bleeding complications62-64. On the other hand, in case of transurethral prostatectomy aspirin seems to be associated with an increased risk of late bleeding events and a need for reintervention65,66. In case of abdominal surgery, therapy with clopidogrel significantly increases the post-intervention bleeding risk, but it does not seem to be associated with an increase of mortality due to haemorrhage or need for reintervention67. In patients with femoral fracture, perioperative clopidogrel therapy does not seem to be associated with a significant increase in mortality and morbidity68.
NEW ORAL ANTIPLATELET AGENTS
Prasugrel is a novel thienopyridine with a more rapid onset of action and a higher antiplatelet effect, as compared to clopidogrel, but it has been associated with an increased bleeding risk69,70. In the TRITON-TIMI 38 trial, in the subgroup of patients undergoing CABG within seven days after withdrawal of thienopyridines, the number of CABG-related bleeding events was fourfold higher in patients treated with prasugrel as compared to those treated with clopidogrel. Nevertheless, the risk of mortality was reduced70,71. Ticagrelor is a novel non-thienopyridine antiplatelet agent that inhibits the P2Y12 receptor, through a reversible binding mechanism of action. Like prasugrel, it is characterised by a more rapid onset of action, higher antiplatelet activity and clinical efficacy, as compared to clopidogrel. Ticagrelor does not increase overall bleeding events, but is associated with a significant increase of non-CABG-related bleeding72,73. As in the TRITON-TIMI 38 trial74, in the PLATO trial patients undergoing CABG within seven days after discontinuation of antiplatelet therapy showed a significant decrease of overall and cardiovascular mortality in the ticagrelor group. Apparently, this protective effect was not due to a different haemorrhagic risk, which was similar in both groups74.
In patients undergoing surgery in whom discontinuation of antiplatelet therapy is required, prasugrel and ticagrelor should be stopped seven and five days before intervention, respectively.
GUIDELINES: WHAT THEY SAY (AND DO NOT SAY)
Several guidelines and expert recommendations on the perioperative management of antiplatelet therapy have been published8-18. Of note, they derive mostly from expert opinion rather than from randomised studies. A multidisciplinary approach with cardiologists, anaesthesiologists and surgeons is recommended on an individual basis. The assessment of the ischaemic and haemorrhagic risk should be provided for each patient, in order to tailor the optimal perioperative antiplatelet regimen. If perioperative antiplatelet therapy discontinuation is required, bridge therapy with unfractionated or low molecular weight heparin is generally not recommended, as it might be associated with increased bleeding risk, without conferring an anti-ischaemic protective effect75.
Of note, the existing guidelines on perioperative antiplatelet therapy have the following limitations, which negatively affect their applicability in daily clinical practice: I) are not shared with cardiologists, surgeons and anaesthesiologists; II) do not provide a standard classification of surgical interventions, according to the haemorrhagic risk; III) do not provide a standard classification of the patient’s thrombotic risk; IV) do not provide general, practical advice on the optimal perioperative regimen on the basis of the surgical intervention and the ischaemic risk but rather recommend a risk/benefit evaluation on an individual basis; V) provide little support with regard to managing antiplatelet therapy in the perioperative phase in case of non-deferrable and/or high haemorrhagic risk interventions; VI) do not provide practical advice on the timing and modalities of antiplatelet therapy discontinuation and resumption.
DEVELOPMENT OF THE THROMBOTIC VERSUS BLEEDING RISK ALGORITHM
DEFINITION OF THROMBOTIC RISK
The genesis of stent thrombosis is multifactorial and is influenced by patient characteristics, coronary lesions, procedural features, coagulation cascade, and antiplatelet therapy9. Therefore, the difficulty of appropriate risk stratification for stent thrombosis becomes evident.
In the present document, thrombotic risk is defined on the basis of four factors (Table 1): I) type of implanted stent (BMS vs. DES)76-82, II) time from PCI to surgery83, III) angiographic features of coronary lesions9,84-86, IV) clinical characteristics4,6,38,39,87.

Of note, second-generation DES have been developed with an improved design that may help to overcome the current limitations of the first-generation DES81. Improved stent designs with thinner struts and more biocompatible polymers may enhance endothelial coverage and functional recovery81-83. Due to their safer profile, as demonstrated by previous studies, second-generation DES may confer a lower thrombotic risk as compared to first-generation DES, thus allowing an earlier discontinuation (beyond six months) of dual antiplatelet therapy, when necessary79-83.
A large retrospective study from Hawn et al has recently challenged the concept that the timing of surgery from PCI and antiplatelet discontinuation are potential triggers for cardiac events at the time of surgery88. MACE within 30 days were associated with emergency surgery and advanced cardiac disease but were not associated with stent type or timing of surgery beyond six months after stent implantation. Moreover, there was no significant relationship between perioperative antiplatelet cessation and 30-day MACE (odds ratio 0.86, 95% confidence interval 0.57-1.29). Although the authors concluded that the guideline emphasis on stent type and surgical timing for both DES and BMS should be re-evaluated, their findings should be judged with caution because they arise from an observational study with potential for residual confounding, where the surgical population was heterogeneous (e.g., the procedures ranged from minor out-patient to emergent in-patient operations) and clinical decision-making factors that influenced stent selection were largely unavailable or limited to administrative data. Moreover, the study was underpowered to detect a true association between perioperative antiplatelet cessation and 30-day MACE.
DEFINITION OF BLEEDING RISK
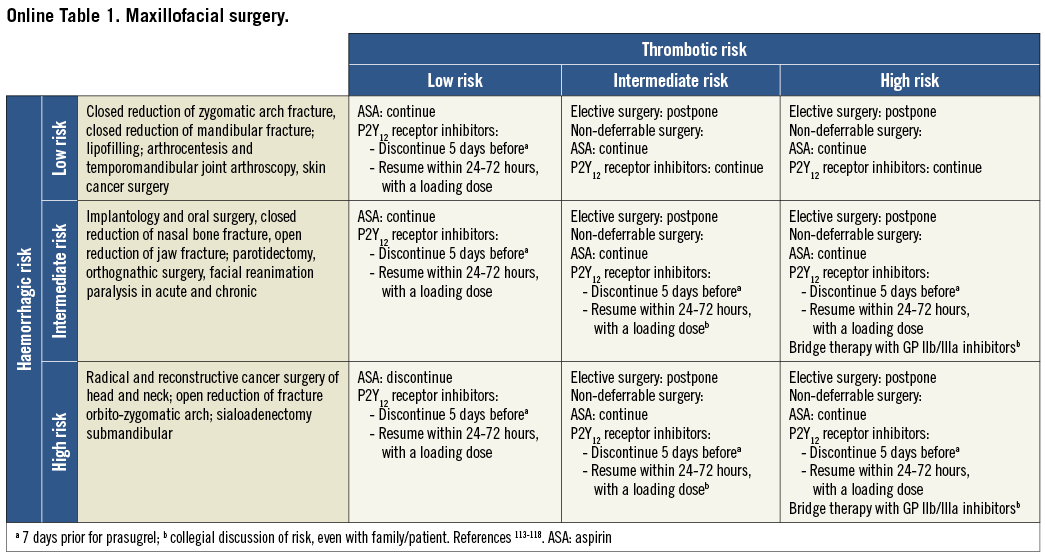
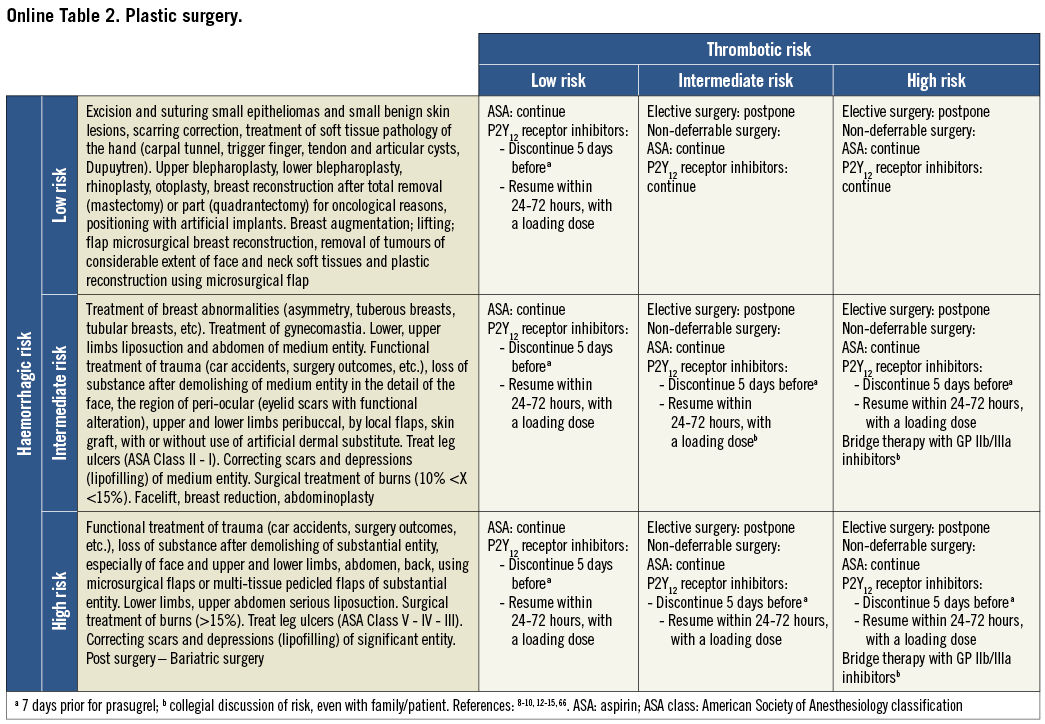
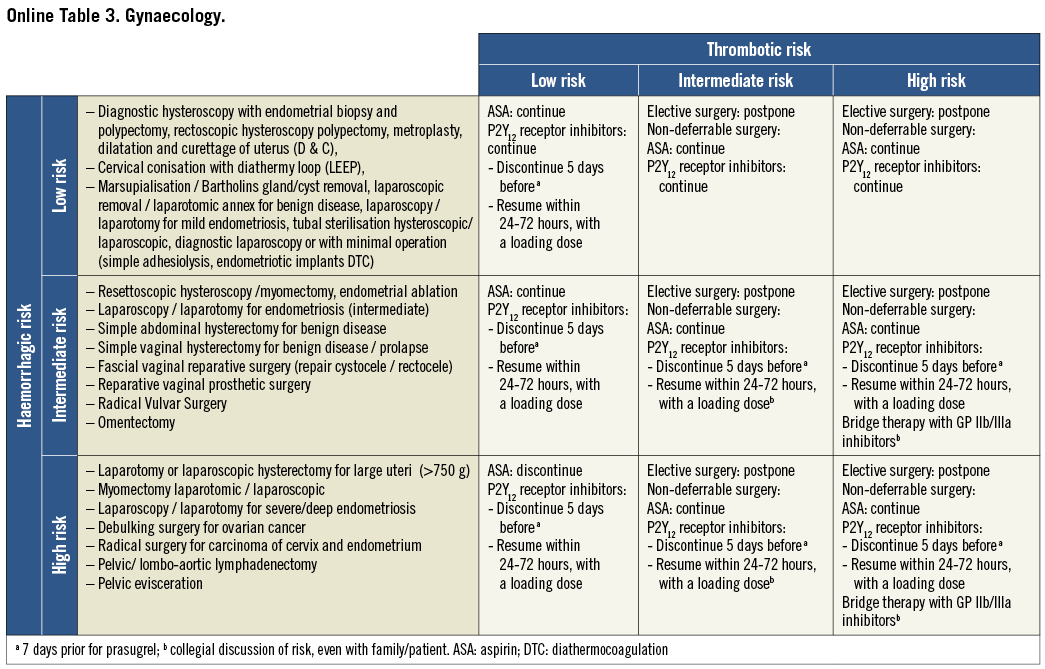
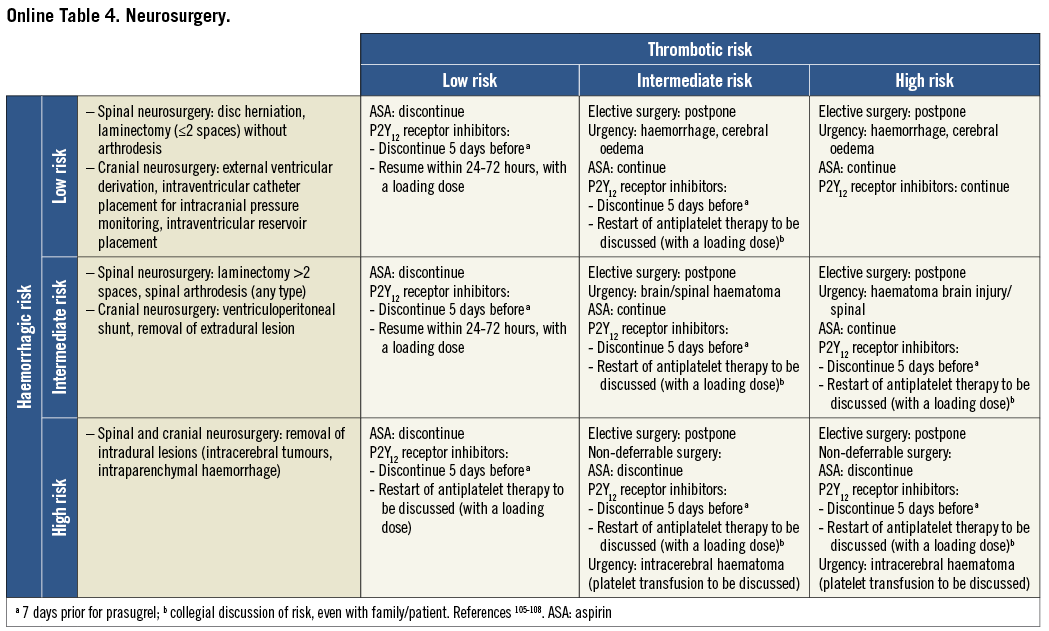
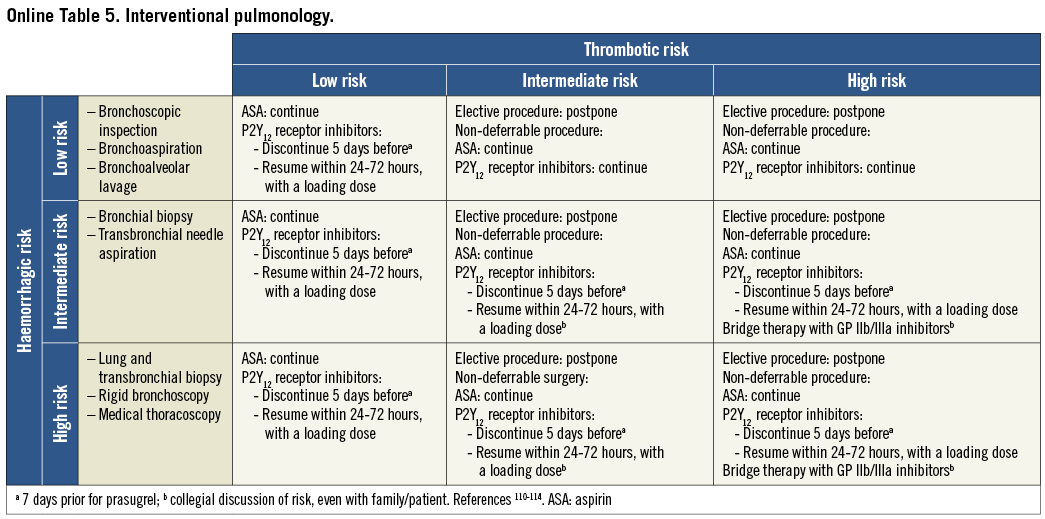
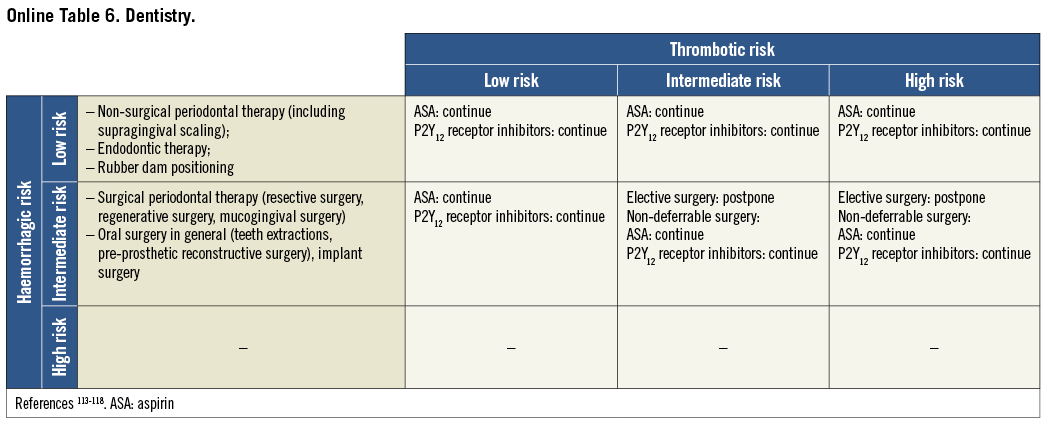
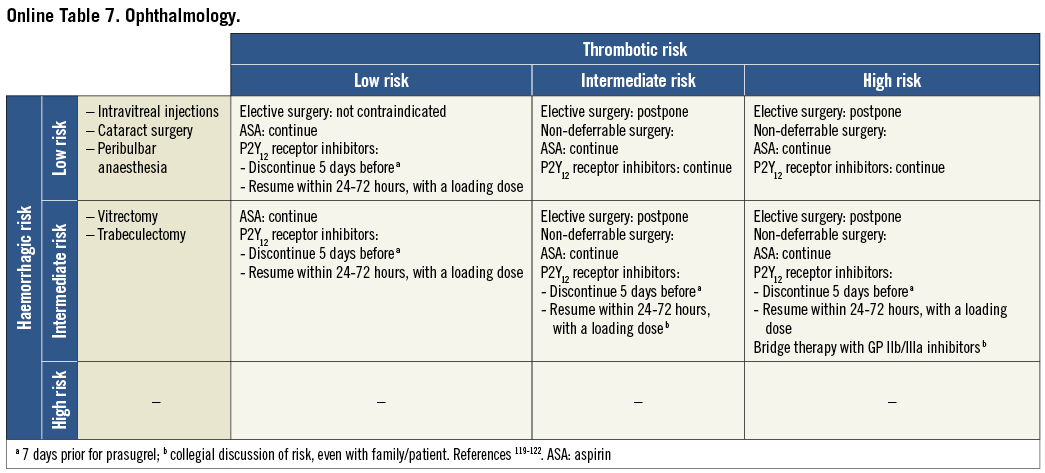
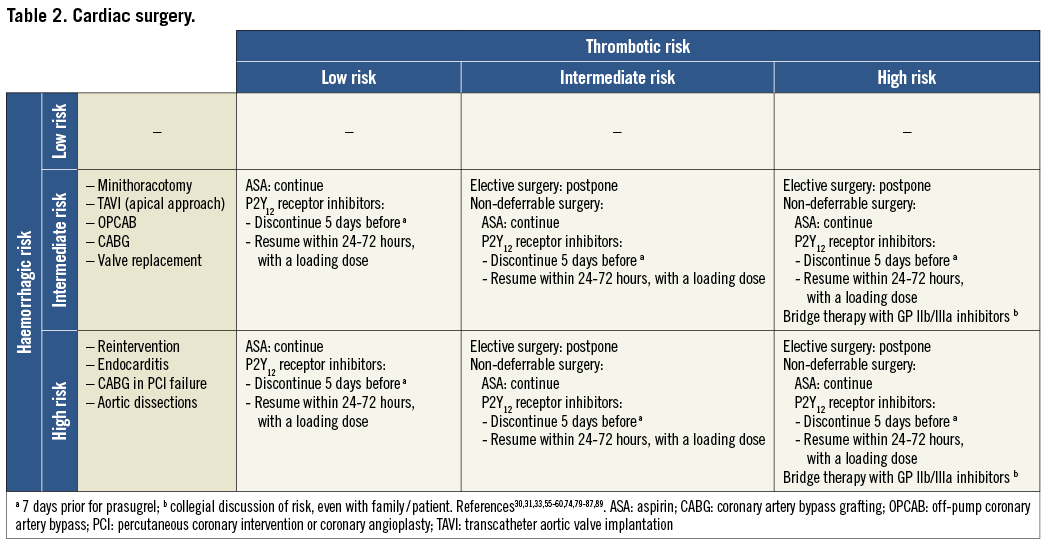
On the basis of the haemorrhagic risk, the main surgical interventions have been classified into three groups: high, medium, and low risk (Table 2-Table 8, Online Table 1-Online Table 7). The definition was mostly derived both from previous published studies, whenever available, and from the experts’ opinion9,11,13,54-59,61-66,89-127. Table 2-Table 8 and Online Table 1-Online Table 7 include general, practical recommendations, while they do not consider clinical characteristics on an individual basis. Of note, the overall risk derives from the interaction between procedural and individual features. The present document focuses mostly on perioperative bleeding risk related to surgical procedures rather than to a patient’s haemorrhagic profile. Each table on surgical bleeding risk is given to provide the reader with a standard frame that might be adapted depending on individual patients’ characteristics. Once the surgical haemorrhagic risk has been defined, it is advisable to evaluate carefully each patient’s risk on an individual basis, which might be taken into account by using ad hoc bleeding risk scores. Several practical bleeding risk scores are available and are mostly based on sex, renal function, and comorbidities128-130. Therefore, when applying these recommendations to daily clinical practice, each single case should be carefully evaluated in terms of ischaemic and bleeding risk.
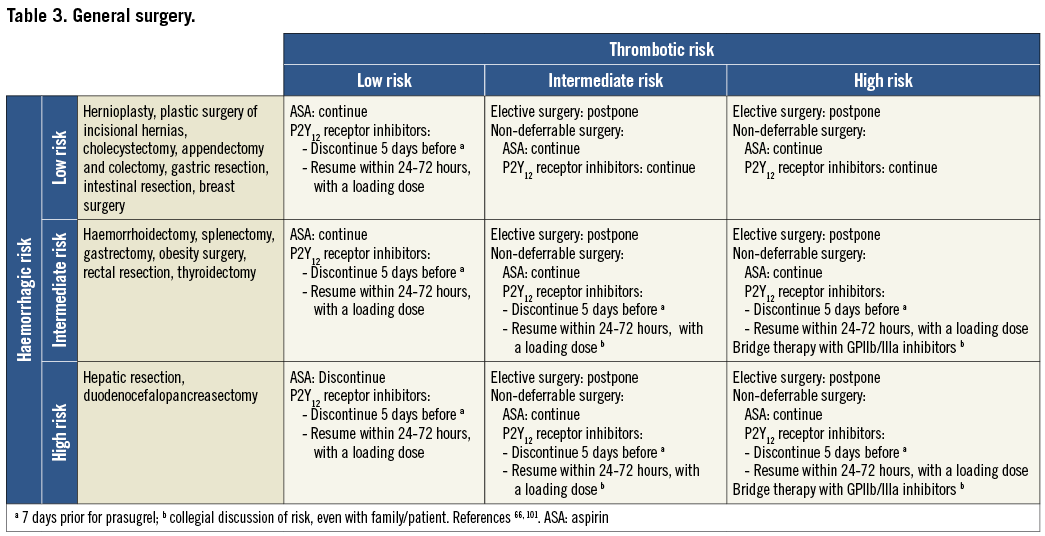
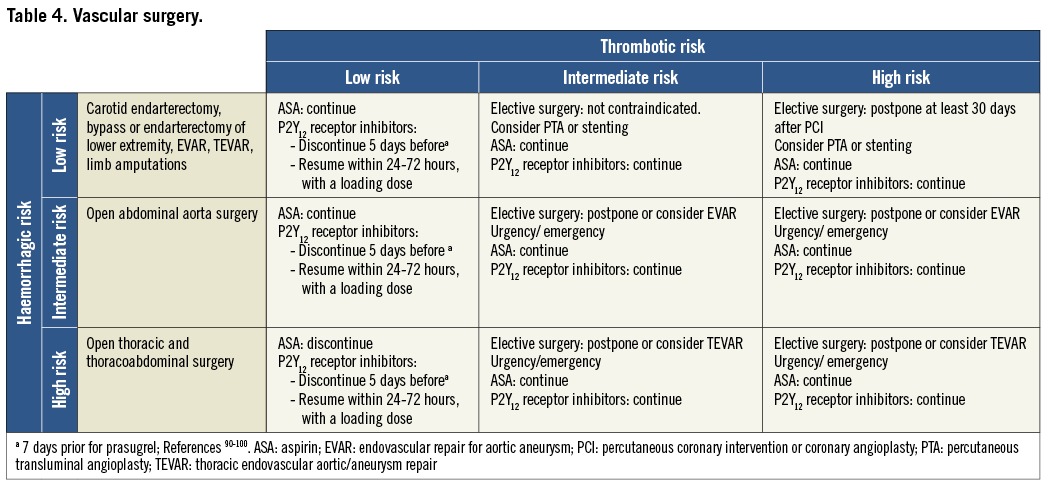
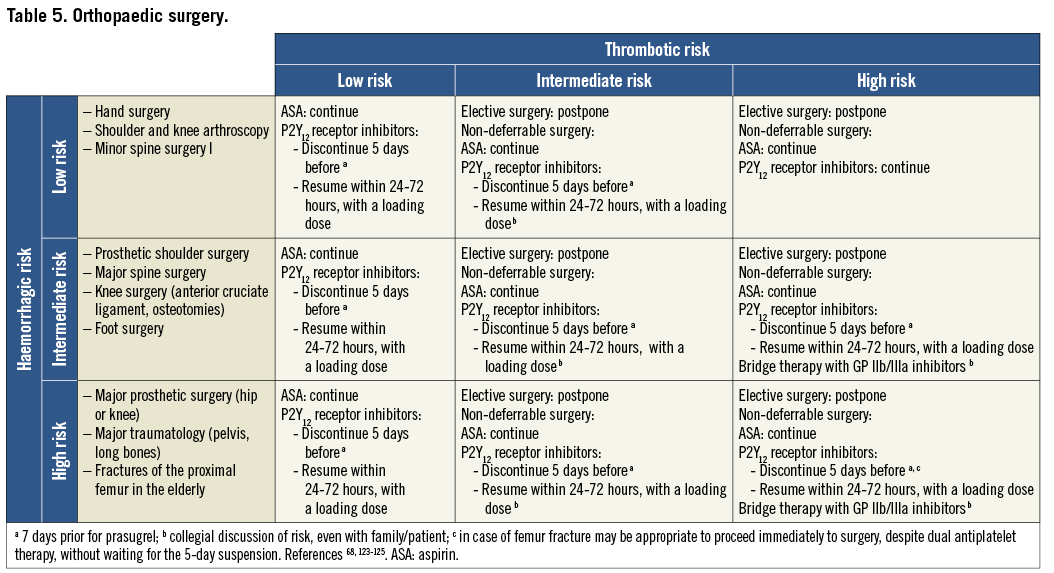
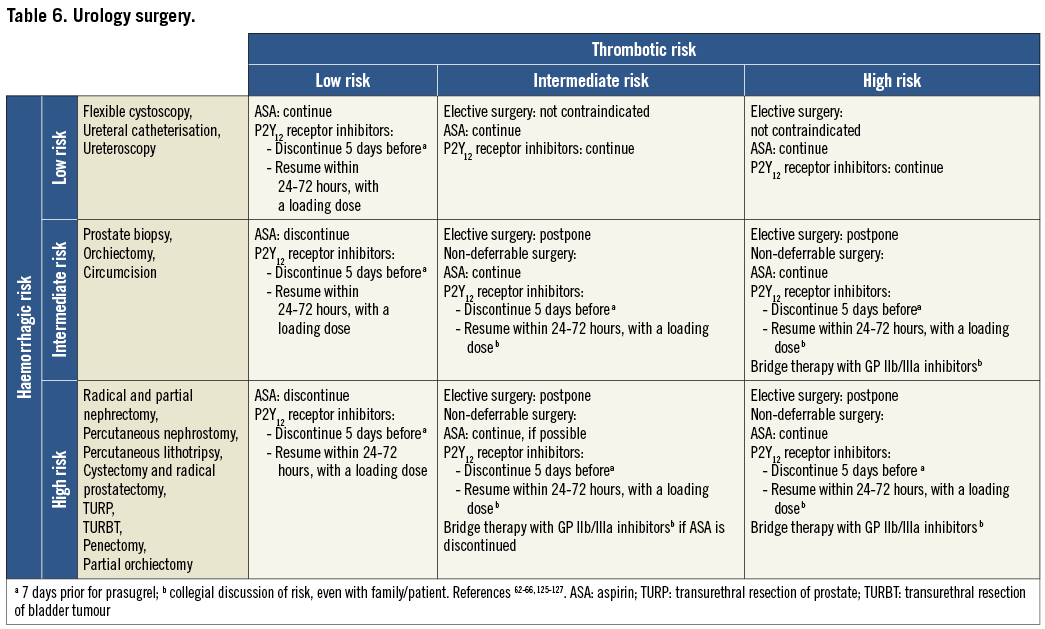
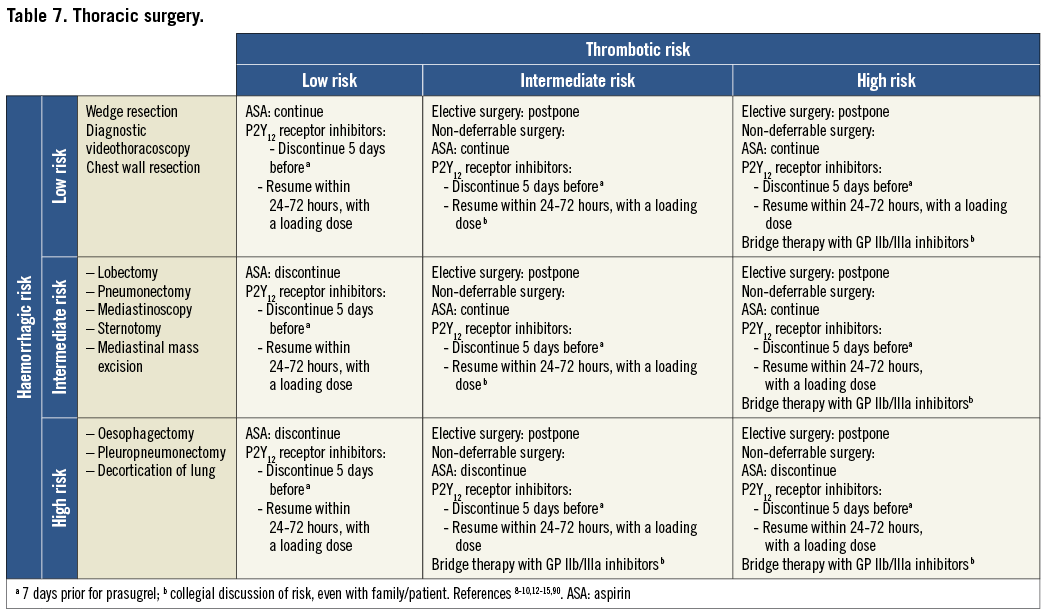
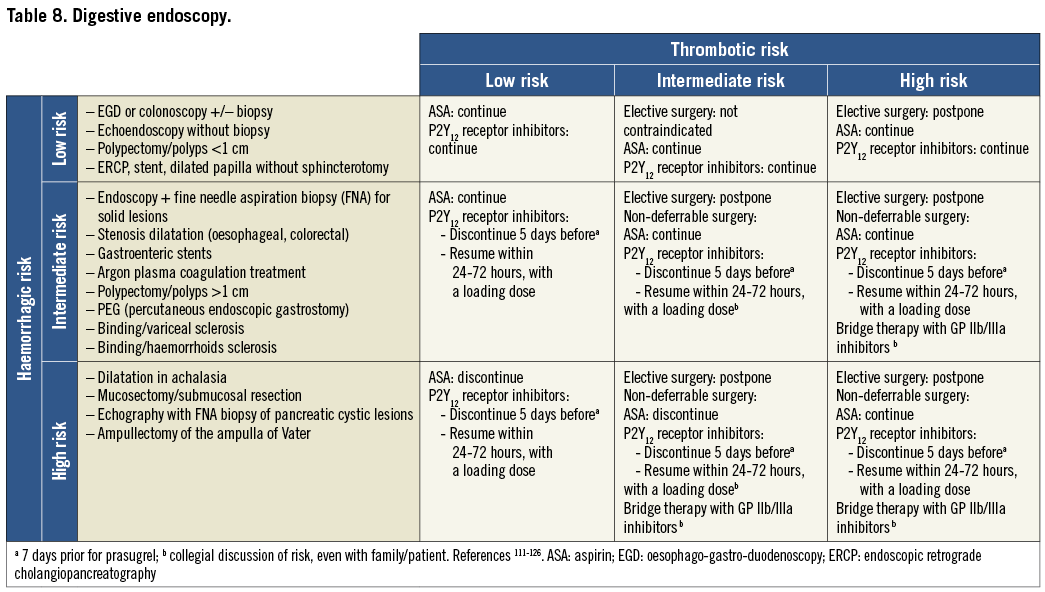
Resumption of antiplatelet drugs after surgery may be deferred in case of clinically relevant bleeding complications. It could be recommended that high-risk patients be referred to centres where the most minimally invasive therapies such as pure laparoscopic, robotic-assisted procedures and new-generation lasers are available.
BRIDGE THERAPY
Even if controlled clinical studies are lacking, guidelines and expert reviews recommend the use of short half-life GPI in the perioperative phase in patients at high thrombotic and bleeding risk13,14,17,18. “Bridge” therapy with iv GPI is reserved to patients at high risk of stent thrombosis for whom the perioperative discontinuation of antiplatelet drugs is required because of an unacceptably high bleeding risk1,2. Savonitto et al22,131 carried out a prospective study on 60 patients with DES considered at high risk for stent thrombosis, and candidates for major surgery. All patients received GPI therapy with tirofiban in the perioperative phase. No cardiac ischaemic event was observed. The rates of bleeding and transfusion were low, in relation to the types of surgery, and no bleeding complications requiring new surgery were observed. Similar studies on more limited patient populations have been performed with eptifibatide132-134. Based on the results of these studies, in highly selected patients, a bridge therapy with iv tirofiban or eptifibatide can reasonably be recommended. GPI infusion, at the dose reported in the summary of product characteristics (decreased by 50% in patients with renal failure and increased pre-/post-surgery bleeding risk) should start three days prior to surgical intervention, whereas clopidogrel and ticagrelor should be discontinued five days prior to surgery (seven days with prasugrel). GPI infusion should be stopped at least four hours prior to surgery (eight hours in patients with creatinine clearance <30 ml/min). P2Y12 inhibitors should be resumed within 24-48 hours after the intervention, with a loading dose (300 mg for clopidogrel, 60 mg for prasugrel and 180 mg for ticagrelor). In selected cases (especially in abdominal surgery, if gastrointestinal function has not yet recovered), infusion with tirofiban or eptifibatide can be restarted (with loading dose) a few hours after the end of the intervention, after a careful evaluation of the bleeding risk. After complete intestinal recanalisation, therapy with P2Y12 inhibitors can be resumed with a loading dose, and, after two hours, the infusion of tirofiban or eptifibatide can be stopped. Of note, GPI are potent antiplatelet agents and might be associated with an increased risk of bleeding during their infusion. Afterwards, they might be contraindicated in patients with an active, clinically relevant bleeding (i.e., macrohaematuria). This therapy should be prescribed by cardiologists and administered in a cardiology ward. GPI administration is currently off-label as a “bridge therapy” in the perioperative period29. The perioperative maintenance of aspirin therapy, which might be administered iv, is strongly recommended in the vast majority of interventions. As ischaemic complications occur most frequently soon after surgery, a close clinical and electrocardiographic monitoring of the patient is strongly recommended. Antithrombotic therapy with unfractionated or low molecular weight heparin is not recommended, unless administered as prophylaxis for venous thromboembolism.
Cangrelor is a new potent antiplatelet agent that inhibits the P2Y12 receptor competitively. On the basis of the BRIDGE trial results, it might be used in future as a “bridge” therapy in patients undergoing surgery, in whom the perioperative discontinuation of oral antiplatelet drugs is necessary135.
Limitations
The present consensus document derives mostly from experts’ opinions rather than from the results of randomised trials, which represents its main limitation. Moreover, many procedures require a more urgent management according to the severity of the clinical presentation, and often the distinction between “deferrable” and “un-deferrable” surgery is not a clear issue and can be misleading both for the surgeon and for the cardiologist. Finally, the haemorrhagic risk is determined not only by the type of surgical intervention, but also by the patient’s clinical characteristics, which have not been considered in the bleeding risk assessment.
Acknowledgements
This manuscript and supplementary data have been adapted with permission from Rossini et al19.
Conflict of interest statement
R. Rossini received payment as an individual for consulting fees or honoraria from Eli Lilly and Co., and Daiichi Sankyo, Inc and Astra Zeneca. L.O. Visconti received payment as an individual for consulting fees or honoraria from Eli Lilly, and Daiichi Sankyo, Astra Zeneca, Menarini, Bayer, Pfizer, BMS and Boehringer. D. Angiolillo reports receiving payment as an individual for: a) consulting fees or honoraria from Bristol Myers Squibb, Sanofi-Aventis, Eli Lilly, Daiichi Sankyo, The Medicines Company, AstraZeneca, Merck, Evolva, Abbott Vascular and PLx Pharma; b) participation in review activities from Johnson & Johnson, St. Jude, and Sunovion. He also reports receiving institutional payments for grants from Bristol Myers Squibb, Sanofi-Aventis, Glaxo Smith Kline, Otsuka, Eli Lilly, Daiichi Sankyo, The Medicines Company, AstraZeneca, Evolva; and having other financial relationships with the James and Esther King Biomedical Research Program. D. Capodanno reports receiving honoraria for lectures/consulting from Eli Lilly and Co., The Medicines Company, and AstraZeneca. G. Guagliumi reports receiving consulting fees from Boston Scientific, St. Jude Medical and AstraZeneca and receiving grant support from St. Jude Medical, Medtronic Vascular, Boston Scientific and Abbott Vascular. M. Lettino reports speaker’s fees and being an advisory board member for AZ, Bayer, Boehringer, Daiichi Sankyo, Eli Lilly, The Medicines Company, BMS, MSD, Pfizer. G. Musumeci reports receiving honoraria for lectures from Eli Lilly and Co., Daiichi Sankyo, AstraZeneca, St. Jude Medical and Abbott Vascular. L. Francetti reports receiving payment as an individual for consulting from Valeas spa. S. Savonitto reports receiving research grants from Eli Lilly, Novartis, and Iroko. B. Castiglioni reports receiving payment as an individual for speaker fees from CID. G. Staurenghi reports receiving payment as an individual for consulting fees or honoraria from Heidelberg Engineering, OD-OS, Optos, Ocular Instruments, Quentel Medical, Carl Zeiss Meditec, Alcon, Allergan, Bayer, Boheringer, Genentech, GSK, QLT, Novartis and Roche. All the other authors have no conflicts of interest to report.
Appendix
Supplement to: Perioperative management of antiplatelet therapy in patients with coronary stents undergoing cardiac and non-cardiac surgery: a consensus document from Italian cardiological, surgical and anaesthesiological societies
Acknowledgments
Piersilvio Gerometta1, Enrico Guffanti2, Giada Beltramini3, Luca Devalle4, Sergiomaria Gaini5, Stefano Corbella6, Antonio Castelli7, Emanuela Menozzi7, Alessandro Locatelli8, Lorenzo Mantovani9, Nicolina Russo10, Gennaro Savoia11
1. U.O. di Cardiochirurgia, Humanitas Gavazzeni, Bergamo, Italy; 2. Chirurgia II, Ospedale di Circolo, Varese, Italy; 3. U.O. di Chirurgia Maxillo-Facciale, Ospedale San Paolo, Milan, Italy; 4. U.S.C. di Chirurgia Plastica, AO Papa Giovanni XXIII, Bergamo, Italy; 5. U.O. di Neurochirurgia, Ospedale Maggiore Policlinico, Milan, Italy; 6. Dipartimento di Tecnologie per la Salute, Università degli Studi di Milano, Clinica Odontoiatrica, IRCCS Istituto Ortopedico Galeazzi, Milan, Italy; 7. U.O. di Anestesia e Rianimazione, Ospedale L. Sacco, Milan, Italy; 8. Dipartimento Cardiovascolare, Ospedale Santa Croce, Cuneo, Italy; 9. Dipartimento di Anestesia, AO Papa Giovanni XXIII, Bergamo, Italy. 10. Dipartimento Cardiovascolare, AO Papa Giovanni XXIII, Bergamo, Italy; 11. UOSC Terapia Intensiva AORN A. Cardarelli, Naples, Italy.
Società Italiana di Cardiologia Invasiva (Alberto Cremonesi, Dipartimento Cardiovascolare, GVM Care and Research - Maria Cecilia Hospital, Cotignola, Italy)
Associazione Nazionale Medici Cardiologi Ospedalieri (Francesco Bovenzi, U.O. di Cardiologia, Ospedale Campo di Marte, Lucca, Italy)
Società Italiana di Chirurgia (Gianluigi Melotti, Chirurgia Generale, Ospedale di Baggiovara [NOCSAE], USL Modena, Italy)
Associazione Chirurghi Ospedalieri Italiani (Stefano Bartoli, Chirurgia Vascolare ASL RM C; Luigi Presenti, U.O. Chirurgia Generale, Ospedale Giovanni Paolo II, Olbia, Italy; Mauro Longoni, U.O.C. di Chirurgia Generale I, P.O. di Sesto San Giovanni A.O. di Vimercate, Italy)
Società Lombarda di Chirurgia (Giampietro Creperio, Chirurgia Generale, Ospedale Erba-Renaldi di Menaggio, Italy)
Società Italiana di Chirurgia Cardiaca (Lorenzo Menicanti, Department of Cardiac Surgery, IRCCS Policlinico San Donato, Milan; Italy)
Società Italiana di Anestesia, Analgesia, Rianimazione e Terapia Intensiva (Massimo Antonelli, Terapia Intensiva e Anestesia, Università Cattolica-Policlinico Universitario A. Gemelli, Rome, Italy)
Società Italiana Chirurgia Maxillo-facciale (Giuseppe Ferronato, Chirurgia Maxillofacciale, Azienda Ospedaliera - Università di Padova, Padua, Italy)
Società Italiana di Chirurgia Plastica ed Estetica (Enrico Robotti, USC Chirurgia Plastica, AO Papa Giovanni XXIII, Bergamo, Italy)
Società Italiana di Chirurgia Toracica (Davide Dell’Amore, U.O. Chirurgia Toracica “Antonio Vio” Ospedale G.B. Morgagni-L. Pierantoni, Forlì, Italy)
Società Italiana di Chirurgia Vascolare ed Endovascolare (Carlo Setacci, Chirurgia vascolare ed endovascolare, Azienda Ospedaliera Universitaria Senese, Siena, Italy)
Società Italiana di Ortopedia e Traumatologia (Paolo Cherubino, Dip. Scienze Ortopediche e Traumatologiche “M. Boni”, Università degli Studi dell’Insubria, Ospedale di Circolo, Varese, Italy)
Federazione Italiana delle Società scientifiche delle Malattie dell’Apparato Digerente (Marco Soncini, Giancarlo Spinzi, Maurizio Vecchi)
Associazione Ostetrici Ginecologi Ospedalieri Italiani Lombardia (Claudio Crescini, U.O. Ostetricia Ginecologia Ospedale di Treviglio (BG), Italy)
Società Oftalmologica Lombarda (Giovanni Staurenghi, Divisione di Oculistica, Ospedale L. Sacco, Milan, Italy)
Società Italiana di Parodontologia (Luca Francetti, Servizio di Odontostomatologia, Dipartimento di Tecnologie per la Salute, Università degli Studi di Milano, Istituto Ortopedico Galeazzi, Milan, Italy)
Società Italiana di Urologia (Francesco Rocco, UO Urologia, Ospedale Maggiore, Policlinico Milan, Italy)
Società Italiana di Neurochirurgia (Franco Servadei, Azienda Ospedaliera Universitaria di Parma, Italy)