
Over the past year, an abundance of clinical evidence has emerged addressing the conceptually appealing and theoretically justified concept of dropping aspirin, rather than the P2Y12 antagonist, as part of antiplatelet regimens after percutaneous coronary intervention (PCI)1,2. In anticoagulated patients, a meta-analysis including 10,000 patients overall from WOEST, PIONEER-AF PCI, RE-DUAL PCI and AUGUSTUS indicated that omission of aspirin early after PCI allows reduction in bleeding with no significant trade-off regarding ischaemic protection3. After the publication of GLOBAL LEADERS in 2018, the year 2019 brought the results of STOPDAPT-2, SMART-CHOICE, and TWILIGHT (A Randomized Trial of Ticagrelor Monotherapy vs Ticagrelor Plus Aspirin Beginning at 3 Months in High-Risk Patients Undergoing PCI) presented by Roxana Mehran at Transcatheter Cardiovascular Therapeutics (TCT), San Francisco, USA, 2019, moving interventional cardiologists closer towards the answers to such practical questions as “When is it best to stop aspirin in patients without oral anticoagulant therapy?” and “Who could ultimately benefit from aspirin-free antiplatelet strategy after PCI?”.
Discontinuation of aspirin, rather than the P2Y12 antagonist, following PCI, has been suggested as a means of reducing the risks of adverse events specifically related to the use of aspirin, including gastrointestinal bleeding and intracranial haemorrhage4, as well as avoiding its potentially detrimental effects related to inhibition of the formation of prostacyclin (prostaglandin I2)5,6. The benefits of aspirin in secondary prevention have been strongly documented in the past7, but this past evidence may no longer be valid in the context of the substantial progress that has occurred regarding optimised stent implantation techniques, improved stent designs, and aggressive reduction of atherosclerosis risk factors, including prevalent use of effective hypolipidaemic agents. In primary prevention “the best strategy for the use of aspirin nowadays could simply be to prescribe a statin instead”, as stated by Paul M. Ridker in an editorial following three publications in the New England Journal of Medicine on primary and secondary prevention with aspirin8. In addition, increasing clinical dissemination of more potent P2Y12 antagonists – prasugrel and ticagrelor – further underscores the theoretical rationale for single antiplatelet therapy after PCI, abandoning aspirin rather than P2Y12 antagonists.
GLOBAL LEADERS, STOPDAPT-2, SMARTCHOICE, TWILIGHT: which patient may benefit from aspirin withdrawal after PCI?
In the GLOBAL LEADERS study, ticagrelor in combination with aspirin for one month followed by ticagrelor monotherapy for 23 months was not superior to 12 months of dual antiplatelet therapy (DAPT) followed by 12 months of aspirin alone in the prevention of all-cause mortality or new Q-wave myocardial infarction (MI) in a broad patient population undergoing PCI9. Nevertheless, the lower-risk clinical profile of patients enrolled in GLOBAL LEADERS, compared to PLATO, needs to be considered. Interestingly, post hoc secondary analyses collectively suggested the need for an additional selection process in an “all-comers” population, to identify those individuals who could derive net clinical benefit by having further decreased ischaemic event rates at minimised expense of bleeding10,11 (Figure 1).
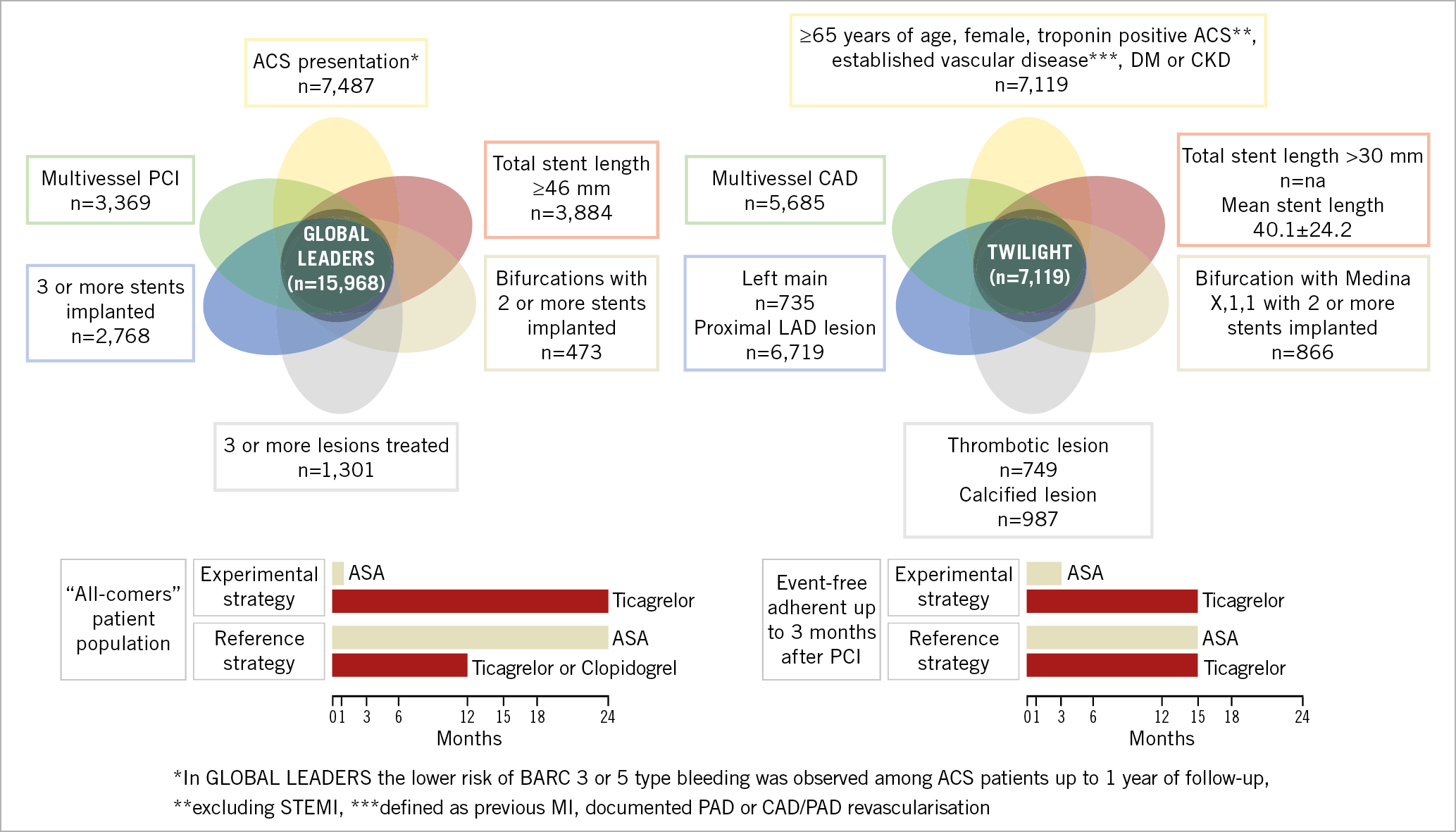
Figure 1. Venn diagram presenting the clinical and angiographic profile of patients likely to benefit from aspirin-free antiplatelet regimens after PCI. High risk of both bleeding and ischaemic events appears a prerequisite for achieving net clinical benefit from more potent P2Y12 monotherapy.
Non-pre-specified analysis in relation to PCI complexity, based on the risk criteria described in the European Society of Cardiology guidelines on myocardial revascularisation (at least one of the following characteristics: multivessel PCI, ≥3 stents implanted, ≥3 lesions treated, bifurcation PCI with ≥2 stents, or total stent length >60 mm)12, indicated that ticagrelor monotherapy, after one-month DAPT, was associated with a significantly lower risk of the primary endpoint of all-cause death or new Q-wave MI and the patient-oriented composite endpoint (POCE: all-cause death, any stroke, MI, revascularisation) while maintaining a similar risk of bleeding, thereby resulting in a net clinical benefit at two years10. Interestingly, a subgroup with individual features of complex PCI, such as patients undergoing multivessel PCI (22.4% of GLOBAL LEADERS population) or PCI with a high total stent length implanted (>46 mm)11, could also demonstrate the potential of the experimental strategy in reducing the rates of the primary endpoint, POCE and net adverse clinical events (NACE) at two years.
The benefits of the experimental treatment were mainly derived from the first year of treatment10,11. Indeed, a dilutive effect of the neutral head-to-head comparison between ticagrelor and aspirin from 12 to 24 months, mandated by the study protocol, has been identified among potential factors affecting the neutral results of the overall study9. This consideration has been addressed in the recent post hoc landmark analysis examining the clinical outcomes occurring between 31 and 365 days after randomisation specifically in patients with acute coronary syndromes (ACS) who, within this time frame, were assigned to receive either ticagrelor alone or ticagrelor and aspirin according to the GLOBAL LEADERS protocol13. Continuation of aspirin on top of ticagrelor beyond one month and up to one year after PCI was associated with an excess in bleeding risk and appeared not to be associated with a reduced risk of ischaemic complications13. These observations may suggest that dropping aspirin at one month after an ACS is safe in terms of ischaemic events and could yield a net clinical benefit due to less risk of bleeding. In general, an increase in bleeding risk also needs to be considered with its negative impact on treatment adherence, potentially leading to higher rates of ischaemic events and mortality. Nevertheless, this analysis was underpowered to detect differences in ischaemic events, and thus it has to be viewed strictly as hypothesis-generating.
Two randomised clinical trials presented at the American Congress of Cardiology (ACC), New Orleans, USA, 2019, STOPDAPT-2 and SMARTCHOICE, compared 12-month DAPT with one- or three-month DAPT followed by P2Y12 inhibitor monotherapy14,15. In STOPDAPT-2, the rates of major secondary cardiovascular events (cardiovascular death, MI, ischaemic or haemorrhagic stroke, and definite stent thrombosis) at 12 months were 1.96% in the one-month DAPT group and 2.51% in the 12-month DAPT group14. In SMARTCHOICE, the rates of major adverse cardiovascular events (all-cause death, MI, and stroke) at 12 months were 2.9% in the monotherapy group and 2.5% in the DAPT group15. The high frequency of intravascular imaging use, study design with net benefit primary endpoint – despite the differential effect of DAPT on ischaemic and bleeding events – and the limited statistical power of these investigations have been put forward among factors leaving some uncertainties and affecting the generalisability of the results to populations other than those in Japan and Korea14,15. Still, despite different designs and primary endpoints, these two randomised clinical trials resulted in similar conclusions: shortening the duration of DAPT and continuing with P2Y12 monotherapy reduced bleeding complications (STOPDAPT-2: Thrombolysis In Myocardial Infarction [TIMI] major or minor bleeding 0.4% vs 1.5%; SMARTCHOICE: BARC 2-5 type bleeding 2.0% vs 3.4%) without increasing the risk of death or ischaemic events in “non-complex” populations (Table 1).
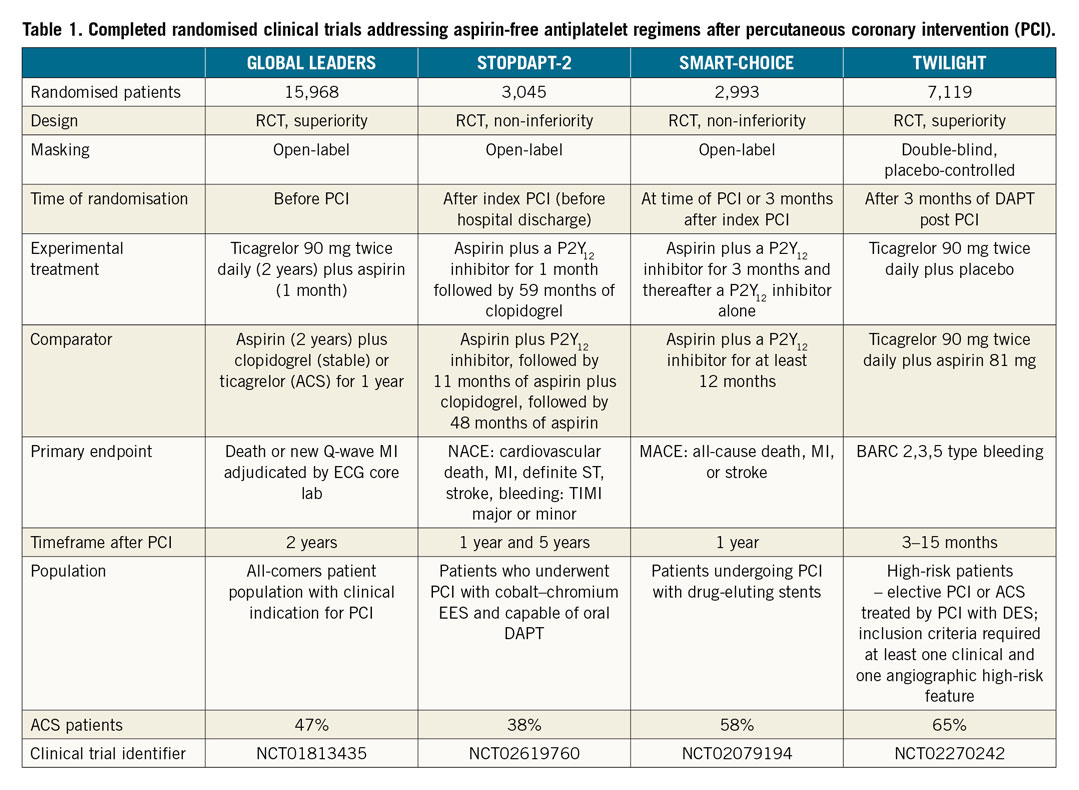
The results of the TWILIGHT trial dotted the i’s on the role and safety of aspirin omission after PCI in complex patients, addressing a relatively similar hypothesis to that in GLOBAL LEADERS, with aspirin withdrawal on a background of ticagrelor at three months after PCI in high-risk, event-free patients16. TWILIGHT, a randomised, double-blind, placebo-controlled trial, demonstrated a significant reduction of the composite primary endpoint of BARC 2, 3 or 5 type bleeding (hazard ratio [HR] 0.56, 95% confidence interval [CI]: 0.45-0.68, pfor superiority<0.001) in the experimental versus the reference group 15 months after PCI (12 months after randomisation)17. The trial also showed non-inferiority of the experimental treatment regarding the composite secondary endpoint of ischaemic events (all-cause death, non-fatal MI, or stroke) (HR 0.99, 95% CI: 0.78-1.25, pfor non-inferiority<0.001), with the caveat of a higher than anticipated drop-out in the first three months after the index procedure, resulting in a lower rate of this endpoint and potential bias of the results towards null17.
Notably, in TWILIGHT, the “high-risk” definition involved not only bleeding risk but also ischaemic risk features17. Specifically, at least one of the following clinical inclusion criteria was required at enrolment: ≥65 years of age, female gender, troponin positive ACS, established vascular disease defined, diabetes mellitus treated with medications (oral therapy or subcutaneous insulin), chronic kidney disease (CKD) in addition to at least one of the following angiographic characteristics: multivessel coronary artery disease, target lesion requiring total stent length >30 mm, thrombotic target lesion, bifurcation lesions with Medina X,1,1 classification requiring at least two stents, left main stem (≥50%) or proximal left anterior descending (≥70%) lesion, or calcified target lesion requiring atherectomy16,17.
Interestingly, these “high-risk” features, such as prior coronary revascularisation, bifurcation lesions and stent length >30 mm, have previously been associated with high risk of thrombosis18,19 and correspond well with the observations from post hoc analyses of GLOBAL LEADERS10,11,13. A significant interaction between treatment effects and clinical presentation, favouring ticagrelor monotherapy in ACS, is also a common observation in both studies9,13,17.
Taking the findings from TWILIGHT and GLOBAL LEADERS together, the patients with ACS or other high-risk clinical and/or procedural profiles, including complex PCI, multivessel PCI or long stenting, might represent the target population in which abandoning aspirin after PCI, while continuing with a potent P2Y12 antagonist, will be favourable. High risk of both bleeding and ischaemic events appears to be a prerequisite for achieving net clinical benefit from an aspirin-free antiplatelet strategy, with aspirin omission – to decrease bleeding – and potent P2Y12 inhibitor monotherapy – to reduce the thrombosis risk (Figure 1).
Preclinical and clinical evidence: when is it best to stop aspirin after PCI?
Low cost and the benefit attributed to aspirin resulted in all other antiplatelet medications being tested on top of aspirin, with the assumption of additive and even synergistic effects of both agents. Nevertheless, in the context of the central role of the P2Y12 signalling pathway on platelet activation and amplification processes, more potent P2Y12 blockade was also shown to lead to downregulation of other markers of platelet reactivity, including arachidonic acid- and collagen-induced aggregation2,20,21. Aspirin was hypothesised to add little additional inhibition of platelet aggregation in the presence of potent P2Y12 inhibitors2,5,6. Nevertheless, it still yields substantial additive inhibition of arachidonic acid- and collagen-induced platelet aggregation, even in the presence of potent P2Y12 inhibitors, supporting the rationale for the additive antithrombotic effect of aspirin in preventing thrombosis induced by subendothelial collagen exposure as well as explaining the adverse additive effect on haemostasis22,23.
Given these data, the study designers and safety monitoring boards of all of the discussed studies secured the early and most vulnerable phase after PCI with the established benefit of DAPT directly after the procedure. The first-to-date attempt of complete aspirin discontinuation from the day after PCI has been evaluated among stable coronary artery disease (CAD) or stabilised ACS patients in the Acetyl-Salicylic acid Elimination (ASET) trial24 – a first-in-man, proof-of-concept, exploratory trial, also presented at TCT 2019, showing the potential feasibility and safety of prasugrel monotherapy directly after PCI, at least in stable patients.
The positive results of the TWILIGHT trial –with aspirin abandoned after a three-month event-free period following PCI– represent practice-changing evidence and will impact on the upcoming recommendations on DAPT regimen. The remaining challenge now is the appropriate translation of the observation from the specific TWILIGHT patient phenotype to the “real-life” patients encountered in daily practice. Clearly, the results from TWILIGHT cannot be generalised to lower-risk patients, patients on other P2Y12 antagonists and subjects with less than the three-month DAPT course after PCI. In TWILIGHT, all analysed patients had already “passed” the initial bleeding and adherence “test” prior to randomisation as only event-free, adherent subjects were randomised three months post PCI, in contrast to GLOBAL LEADERS in which randomisation was carried out at the time of PCI17. Distinct from GLOBAL LEADERS, TWILIGHT excluded patients with ST-segment elevation MI and a history of stroke17. Furthermore, the results of TWILIGHT do not provide sufficiently robust evidence to overturn the IIa class of recommendation of the 2019 ESC Chronic Coronary Syndromes guidelines that a dual antithrombotic therapy approach, consisting of aspirin with a P2Y12 antagonist or very low-dose rivaroxaban, should be considered in a carefully selected cohort of patients in sinus rhythm with both (i) diffuse multivessel disease combined with diabetes mellitus requiring medication, recurrent MI, pulmonary artery disease (PAD), or CKD with eGFR 15-59 mL/min/1.73 m2, and (ii) no condition associated with a high risk of bleeding25. Of note in TWILIGHT, the point estimates for the key secondary endpoint of death, MI or stroke were in favour of a dual-therapy approach in those with multivessel disease or CKD, acknowledging that there was no significant interaction between subgroups with or without these characteristics17. It may be reasonably speculated that improvements in stent technology mean that the long-term antithrombotic strategy should now be determined by the burden of atherothrombotic risk beyond the stented segments as well as the risk of bleeding.
The hypothesis-generating evidence from studies preceding TWILIGHT warrants randomised investigations with aspirin discontinuation at an earlier phase after PCI; with aspirin discontinuation at one month after intervention already evaluated in GLOBAL LEADERS, further studies abandoning aspirin at an even earlier time point after PCI are anticipated in patients at low risk of stent thrombosis. A randomised comparison of different long-term antiplatelet monotherapies – beyond the follow-up time frames after PCI analysed to date – is also of clinical interest, since the vast majority of individuals participating in contemporary trials probably returned to aspirin upon study completion.
Conflict of interest statement
M. Tomaniak reports lecture fees from AstraZeneca, outside the submitted work. R. Storey reports grants and personal fees from PlaqueTec, personal fees from Bristol-Myers Squibb/Pfizer, Amgen, Bayer, Novartis, Idorsia, Thromboserin, Haemonetics, GlyCardial Diagnostics; and grants, personal fees and other support from AstraZeneca, outside the submitted work; in addition, R. Storey has a patent PCT/GB2017/050692 pending. P.W. Serruys reports personal fees from Abbott Laboratories, AstraZeneca, Biotronik, Cardialysis, GLG Research, Medtronic, Sino Medical Sciences Technology, Société Europa Digital & Publishing, Stentys France, Svelte Medical Systems, Philips/Volcano, St. Jude Medical, Qualimed and Xeltis, outside the submitted work.

