Abstract
Aims: To evaluate the impact of ad hoc percutaneous coronary intervention (PCI) which combines coronary angioplasty and PCI in the same procedure in the era of drug-eluting stents (DES).
Methods and results: From the IRIS-DES registry, 4,738 angina patients treated using PCI with DES were enrolled. The 18-month outcomes were compared between ad hoc and non-ad hoc groups after adjustment using inverse-probability-of-treatment weighting. Ad hoc PCI was performed in 3,562 (75.2%) patients. The ad hoc PCI group had less extensive coronary disease and received fewer stents. The incidence of major adverse cardiac or cerebrovascular events, consisting of death, myocardial infarction (MI), stroke, and repeat revascularisation, did not differ between the ad hoc and the non-ad hoc groups (8.3% vs. 7.6%; adjusted hazard ratio [aHR] of ad hoc PCI, 1.22; 95% confidence interval [CI]: 0.91 to 1.63; p=0.18). The individual endpoints of death (2.0% vs. 1.9%; aHR, 1.57; 95% CI: 0.86- 2.88; p=0.14), MI (0.8% vs. 1.0%; aHR, 0.62; 95% CI: 0.29 - 1.33; p=0.22), stroke (1.0% vs. 0.9%; aHR, 1.25; 95% CI: 0.58-2.69; p=0.57), and repeat revascularisation (4.4% vs. 4.0%; aHR, 1.23; 95% CI: 0.86-1.77; p=0.25) also did not differ between the groups.
Conclusions: Ad hoc PCI using DES appears to be feasible for angina patients at a relatively low risk of procedure. This approach may reasonably be performed with evaluation of objective ischaemia using non-invasive or invasive tests.
Introduction
Ad hoc percutaneous coronary intervention (PCI) combines angiography and PCI in the same procedure. In contrast, non-ad hoc PCI is a staged procedure performed during two different sessions. With the recent advancement in devices and techniques, ad hoc PCI has now been widely used in elective and urgent situations1. This strategy can reduce access problems, hospital stay, and contrast-induced nephropathy, which are potentially related to repeated procedures. However, this was also countered by the lack of a long enough pause for physicians to consider the appropriate treatment strategy thoughtfully2. The patient may not be provided with the full information about the course of disease and the benefit of alternative treatments. In addition, ad hoc PCI may cause potentially rushed doctors not to comply with standard recommendations due to the “oculo-stenotic” reflex3. Angiography-guided revascularisation may lead to overutilisation of devices and poor long-term prognosis4,5.
Despite their importance and clinical implication, the acute and long-term outcomes of ad hoc PCI compared to non-ad hoc PCI have not been fully evaluated. In particular, there is a lack of data in the era of drug-eluting stents (DES). The benefit of DES, which have decreased the need for repeat revascularisation together with the cost of high incidence of stent thrombosis, may alter the impact of ad hoc PCI compared to PCI previously using bare metal stents. Therefore, our study aimed at comparing the long-term outcomes of ad hoc PCI with non-ad hoc PCI in a large multicentre registry which prospectively enrolled consecutive patients receiving DES.
Methods
PATIENTS
The study population was part of the IRIS-DES (Interventional Cardiology Research In-cooperation Society-Drug-Eluting Stents) registry and included 4,738 patients. The IRIS-DES registry was a prospective, multicentre recruitment of all consecutive consenting patients undergoing PCI with DES from 42 academic and community hospitals in Korea between April 1st, 2008, and June 30th, 2010, and for whom complete follow-up data were available for at least one year and up to three years6. During the enrolment period, a DES was the default device for PCI. Patients who were treated with everolimus-eluting stents (XIENCE V®; Abbott Vascular, Santa Clara, CA, USA) or sirolimus-eluting stents (Cypher Select; Cordis, Johnson & Johnson, Warren, NJ, USA) were included in this study. Exclusion criteria were minimal. Patients with cardiogenic shock, acute myocardial infarction (MI), malignant disease, or other comorbid conditions with life expectancy less than 12 months, those treated with a mixture of different types of DES, and those with planned surgery necessitating interruption of antiplatelet drugs within six months after the procedure were excluded from the study. After DES implantation, dual antiplatelet therapy with aspirin and clopidogrel was recommended for at least one year. The institutional review board of our hospitals approved the use of clinical data for this study, and all patients provided written informed consent for enrolment into our registry.
ENDPOINTS AND FOLLOW-UP
The primary endpoint of this study was the rate of major adverse cardiac and cerebrovascular events (MACCE), consisting of death, MI, stroke, and repeat revascularisation. Secondary endpoints included the individual endpoints of MACCE and the composite of death, MI, and stroke. Deaths were considered cardiac unless an unequivocal, non-cardiac cause was established. MI as a complication was defined as either at index admission (defined as new Q-wave after index treatment) or at follow-up (defined as any CK-MB or troponin increase above the upper range limit with or without the development of Q-waves), as described7. Repeat revascularisation included target vessel revascularisation, regardless of whether the procedure was clinically or angiographically driven, and non-target vessel revascularisation. Stroke, as indicated by neurologic deficits, was confirmed by a neurologist based on imaging modalities. Definite stent thrombosis was captured according to the Academic Research Consortium classification8.
Clinical, angiographic, procedural and outcome data were prospectively recorded in a dedicated, electronic case report form by independent research personnel. Patients were clinically followed up at one, six, and 12 months, and then every six months thereafter, via office visit or telephone contact. Monitoring and verification of registry data have been periodically performed in participating hospitals by members of the academic coordinating centre. All outcomes of interest were confirmed by source documentation collected at each hospital and were centrally adjudicated by an independent clinical events committee.
STATISTICAL ANALYSIS
Differences in baseline clinical and angiographic characteristics and procedural findings were compared using the t-test for continuous variables and χ2 or Fisher’s exact tests for categorical variables, as appropriate. Survival curves were constructed using the Kaplan-Meier method and compared using the log-rank test. Patients were censored at 18 months (540 days) or when events occurred.
For the primary and secondary endpoints, differences between the ad hoc and non-ad hoc groups in unadjusted long-term rates of outcomes were assessed using Cox proportional hazards regression analysis. The proportional hazards assumption was confirmed by testing of partial (Schoenfeld) residuals9, and no relevant violations were identified. In addition, we adjusted for differences in patient baseline characteristics by using weighted Cox proportional hazards regression models with inverse-probability-of-treatment weighting10. Adjustments were performed in all patients using the clinical covariates of age, sex, body mass index, diabetes mellitus, hypertension, current smoking, hyperlipidaemia, left ventricular ejection fraction, history of MI, cerebrovascular disease, chronic lung disease, peripheral vascular disease, congestive heart failure, prior coronary angioplasty, unstable angina, multivessel disease, and number of diseased lesions. Subgroups of patients based on various clinical and angiographic characteristics, including age ≥70 years old, stent type, sex, diabetes mellitus, renal failure, decreased EF of <50%, and multivessel disease, were analysed after adjustment using the multivariable Cox model with clinical factors as covariates. The frailty Cox model was used to account for the effect of hospitals. Interactions between factors associated with ad hoc PCI and subgroups were tested by incorporation of formal interaction terms in the multivariable Cox model. All reported p-values are two-sided, and p-values less than 0.05 were considered statistically significant. SAS software, version 9.1 (SAS Institute Inc., Cary, NC, USA) and R programming language were used for statistical analyses.
Results
PATIENT CHARACTERISTICS
Investigating centres enrolled a median number of 82 patients (range, 12~1,529; interquartile range, 40~107) in this study. Ad hoc PCI was performed in 3,562 (75.2%) patients. From the total, 908 (77.2%) of patients receiving non-ad hoc PCI were treated in a hospital, in which 1,592 (32.3%) of all patients were enrolled. Figure 1 shows the prevalence of ad hoc PCI in each hospital. From 42 centres, the mean prevalence of ad hoc PCI was 90±16% and more than 50% of the patients received ad hoc PCI in 40 (95%) centres. Ad hoc PCI patients were more likely to be female and have hypertension and cerebrovascular disease (Table 1). However, a history of hyperlipidaemia and prior coronary angioplasty were more prevalent in non-ad hoc PCI patients. In addition, non-ad hoc PCI patients had lower left ventricular ejection fraction, more extensive coronary artery disease, and received more stents.
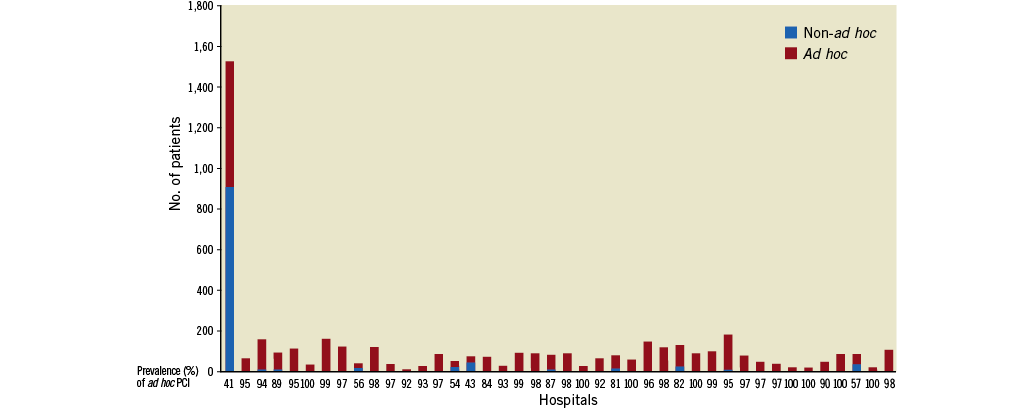
Figure 1. Prevalence of ad hoc percutaneous coronary intervention (PCI) in investigating centres.
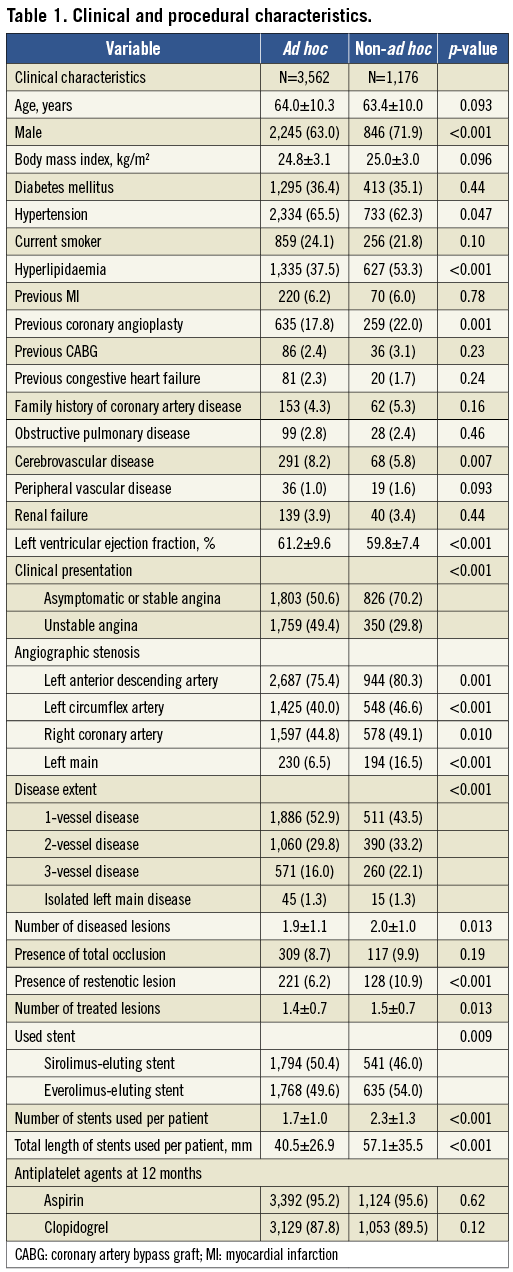
UNADJUSTED AND ADJUSTED OUTCOMES
Table 2 and Figure 2 present the crude incidence of events over 18 months in the ad hoc and non-ad hoc PCI groups. The incidences of MACCE or individual endpoints did not differ between the two groups. When the outcomes were adjusted using inverse-probability-of-treatment weighting, the risks of any individual or composite endpoints were not associated with the use of ad hoc PCI. The significance of the p-value was not changed with the frailty Cox model.
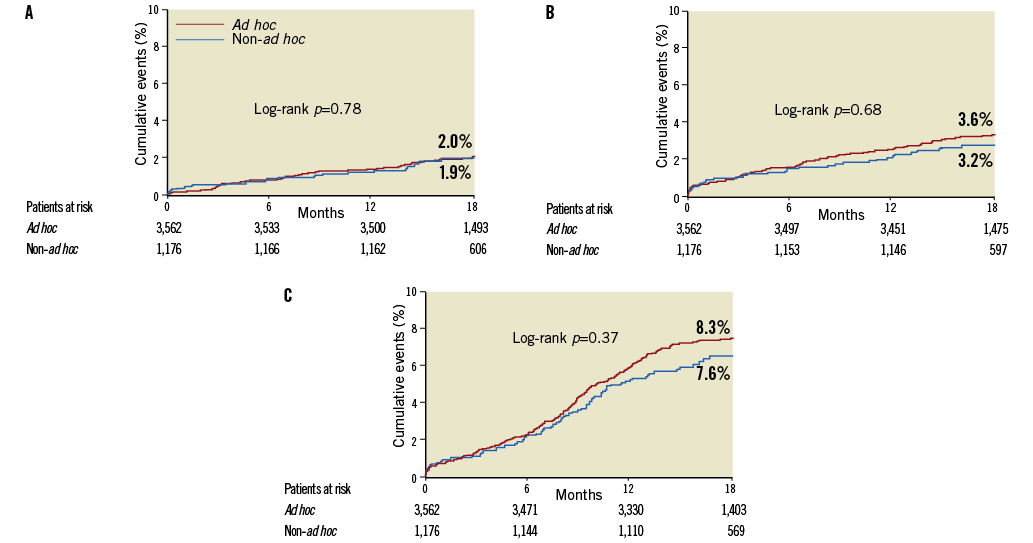
Figure 2. Unadjusted 18-month event curves. Kaplan-Meier event curves for: A) all-cause death; B) composite of death, myocardial infarction (MI), and stroke; and C) major adverse cardiac or cerebrovascular events.
Figure 3 shows the adjusted hazard ratios of ad hoc PCI for the primary endpoint of MACCE over 18 months in diverse subgroups. In most of the subgroups, ad hoc PCI was not associated with the risk of MACCE. However, in subgroups stratified by stent type, ad hoc PCI increased the risk of MACCE in the everolimus-eluting stent subgroup, but not the sirolimus-eluting stent subgroup without a significant interaction.
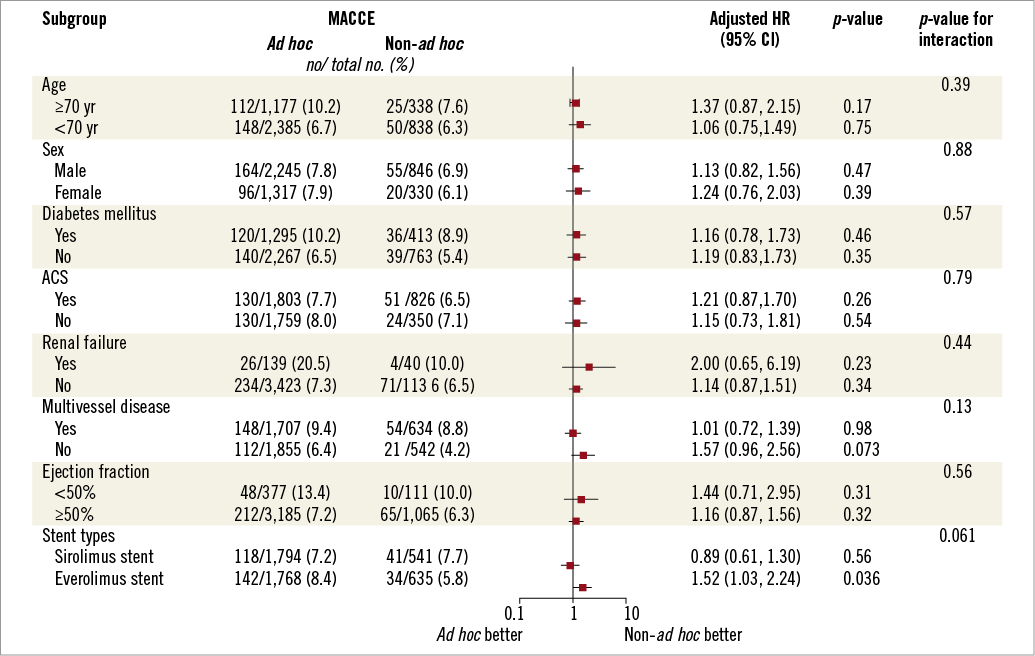
Figure 3. Adjusted hazard ratios (HR) of ad hoc percutaneous coronary intervention (PCI) for major adverse cardiac or cerebrovascular events (MACCE) in different subgroups. ACS: acute coronary syndrome
Discussion
The major findings of our study were: (1) ad hoc PCI is frequently performed for patients with stable angina, but the utilisation rate is diverse according to physician preference, and (2) long-term outcomes of PCI with DES appear to be similar whether or not ad hoc PCI is performed. However, in patients receiving everolimus-eluting stents, the MACCE rate was higher after ad hoc PCI than after non-ad hoc PCI.
With the improvement of devices and medications, ad hoc PCI is frequently performed1. The prevalence of ad hoc PCI ranged from 40% to 80% in the literature and seems to be more widely used in current practices1,11-13. This procedure may reduce the cost and risk of complications related to the second procedure. In particular, it was preferred when patients had fewer comorbidities, such as renal failure, chronic lung disease, and extensive coronary disease14. In the New York State registry, which was a large registry evaluating hospital-level outcomes after cardiac surgery or PCI in New York State, the prevalence increased from 62% between 1995 and 199814 to 83% between 2003 and 20051. In our study, ad hoc PCI was also commonly performed in three quarters of all PCIs using DES for angina patients. In spite of the variations in utilisation rates, most centres utilised ad hoc PCI in more than half of the patients.
A few studies reported the prevalence and long-term outcomes of ad hoc PCI compared with non-ad hoc PCI1,11,14-16. The investigators involved in the New York State registry reported a series of studies addressing the issue of ad hoc PCI. In the bare metal stent era, ad hoc PCI did not affect the rate of in-hospital mortality14,15. However, in the DES era DES, ad hoc PCI was reported to decrease the risk of three-year mortality, but to increase the risk of repeat revascularisation1. In spite of several limitations related to its non-randomised observational nature, this study has been used as supporting evidence for the rapid spread of ad hoc PCI in current practice. However, extensive use of ad hoc PCI was recently criticised by studies which showed no benefit of the prompt revascularisation strategy using PCI in stable angina patients2,17,18. Since ad hoc PCI provides less opportunity for thoughtful decision making when considering medical treatment versus revascularisation strategy or PCI versus coronary artery bypass graft, some authors recommended a “pause” after angiography to consider the risk and benefit of PCI2,19.
In contrast to the New York State registry, our study showed similar long-term outcomes of ad hoc PCI compared with non-ad hoc PCI for angina patients. Although patients receiving ad hoc PCI had less complex clinical and angiographic features than those in the New York State registry, the unadjusted and adjusted risks of 18-month mortality, MI, stroke and repeat revascularisation were comparable between ad hoc and non-ad hoc PCI. The pattern was identical regardless of the presence of traditional PCI risks, such as old age, female sex, diabetes mellitus, multivessel disease or renal failure. The difference between our study and the New York State registry may be explained by several potential reasons1. First, our study might have included patients with more unstable conditions than the NY registry. Therefore, the benefit of ad hoc PCI might have been diminished due to the unstable patients in our study. Second, the prevalence of ad hoc PCI was diverse across the investigating centres. Therefore, different procedural patterns across hospitals might have influenced the results. Third, because non-ad hoc PCI was intentionally selected for patients at high procedural risk, patients receiving a non-ad hoc PCI might have been treated with greater care. Fourth, as indicated in the adjusted results, the strategy of ad hoc or non-ad hoc PCI may not have altered outcomes for patients with stable coronary disease. This hypothesis is supported by previous randomised studies showing that prompt revascularisation with PCI for patients with stable angina did not influence the risk of death or any hard clinical events compared with provisional revascularisation17,20. Nonetheless, it is of note that ad hoc PCI, which decreases hospital stay and problems related to a staged procedure, may lead to feasible outcomes in stable patients at relatively low procedural risk.
In spite of its clinical feasibility, however, ad hoc PCI should be performed with caution. In fact, ad hoc PCI increased the risk of MACCE for patients receiving everolimus-eluting stents in this study. In spite of unclear mechanism, it should be noted that ad hoc PCI may potentially inflate unnecessary procedures due to the “oculo-stenotic”’ reflex. In our study, a substantial proportion of patients received ad hoc PCI although the indication for revascularisation was not favourable to PCI19,21. For instance, 47% of patients with left main or multivessel disease were treated without a pause after angiography. Previous studies have already shown that angiographic complete revascularisation may not improve clinical outcomes in stable patients with multivessel coronary disease4. On-site evaluation of objective ischaemia using fractional flow reserve may prevent the potential risk of overtreatment due to angiography-guided procedure during ad hoc PCI5,22.
Study limitations
Our study has limitations. First, although patients were recruited in a prospective cohort, this analysis was performed retrospectively. Therefore, important factors in the selection between ad hoc versus non-ad hoc procedures were not considered appropriately. For instance, the prevalence and clinical impact of non-invasive assessment of myocardial ischaemia could not be analysed in this study. Second, because of a non-randomised study design, our observation is exploratory and cannot exclude the impact of selection bias in spite of rigorous statistical adjustment. However, our study provides important information on healthcare policy because this issue cannot be confirmed by a randomised clinical study. In fact, without enough data, the current guidelines recommend that ad hoc PCI should not be performed for high-risk patients for whom the superiority of PCI compared with other strategies is not clear19,21. Third, our study did not analyse the cost-effectiveness of ad hoc PCI due to the lack of financial information. This issue needs to be investigated in future studies. Finally, the appropriateness of PCI was not counted in this registry due to a lack of baseline information. Therefore, the impact of appropriate PCI between ad hoc and non-ad hoc PCI could not be analysed.
Conclusion
In conclusion, our study demonstrates that ad hoc PCI is widely used and leads to similar long-term clinical outcomes compared with non-ad hoc PCI for patients at a relatively low risk of procedures. However, it should be performed with evaluation of objective ischaemia using non-invasive or invasive tests to decrease the potential risk of unnecessary procedures. Staged PCI should also be considered for patients at high risk for PCI, to allow for appropriate selection of the revascularisation strategy and to enhance patient understanding of treatment risks and benefits.
Funding
This study was supported by funds from the Korea Healthcare Technology Research and Development Project, Ministry of Health and Welfare (A120711), the CardioVascular Research Foundation, Seoul, Korea, and by Abbott and Cordis.
Conflict of interest statement
Y-H. Kim has received lecture fees from AstraZeneca, MSD, and Medtronic. S-J. Park has received research grants and lecture fees from Abbott Vascular, Boston Scientific, Cordis, and Medtronic. The other authors have no conflicts of interest to declare.
Acknowledgements
The authors thank Sung Ho Her, MD (The Catholic University of Korea, Daejeon St. Mary’s Hospital, Daejeon), Seung Ho Hur, MD (Keimyung University Dongsan Medical Center, Daegu), Jin Sik Park, MD (Sejong General Hospital, Bucheon), Myeong-Kon Kim, MD (Kyunghee University Medical Center, Seoul), Yun Seok Choi, MD (The Catholic University of Korea Yeido St. Mary’s Hospital, Seoul), Hyun Sook Kim, MD (Hallym University Sacred Heart Hospital, Anyang), Jang-Hyun Cho, MD (St. Carollo Hospital, Suncheon), Sang Gon Lee, MD (Ulsan University Hospital, Ulsan), Yong Whi Park, MD (Gyeongsang National University Hospital, Jinju), Myung-Ho Jeong, MD (Chonnam National University Hospital, Gwanju), Bong Ki Lee, MD (Kangwon National University Hospital, Chuncheon), Nae-Hee Lee, MD (Soon Chun Hyang University Hospital, Bucheon), Do-Sun Lim, MD (Korea University Anam Hospital, Seoul), Junghan Yoon, MD (Wonju Christian Hospital, Wonju), Ki Bae Seung, MD (The Catholic University of Korea Seoul St. Mary’s Hospital, Seoul), Won-Yong Shin, MD (Soon Chun Hyang University Hospital Cheonan, Cheonan), Seung-Woon Rha, MD (Korea University Guro Hospital, Seoul), Kee-Sik Kim, MD (Daegu Catholic University Medical Center, Daegu), Seung-Jea Tahk, MD (Ajou University Hospital, Suwon), Byoung Eun Park, MD (Dankook University Hospital, Cheonan), Taehoon Ahn, MD (Gachon University Gil Hospital, Incheon), Joo-Young Yang, MD (National Health Insurance Corporation Ilsan Hospital, Seoul), Yong Seok Jeong, MD (Good Samaritan Hospital, Pohang), Jay-Hyun Rhew, MD (Presbyterian Medical Center, Jeonju), Jong-Seon Park, MD (Yeungnam University Medical Center, Daegu), Keun Lee, MD (Seoul Veterans Hospital, Seoul), Keon Woong Moon, MD (The Catholic University of Korea St. Vincent’s Hospital, Suwon), Keum Soo Park, MD (Inha University Hospital, Incheon), Joo Hyeon Oh, MD (Samsung Chanwon Medical Center, Chanwon), Seung Uk Lee, MD (Kwangju Christian Hospital, Kwanju), Yong-Mo Yang, MD (Cheongju St. Mary’s Hospital, Cehonju), Jang Ho Bae, MD (Konyang University Hospital, Daejeon), Woo-Young Chung, MD (Boramae Medical Center, Seoul), Nam Ho Lee, MD (Kangnam Sacred Heart Hospital, Seoul), Kyoo-Rok Han, MD (Kangdong Sacred Heart Hospital, Seoul), Kook Jin Chun, MD (Pusan National University Yangsan Hospital, Pusan), Moo Hyun Kim, MD (Dong-A Medical Center, Pusan), Kyoung-Ha Park, MD (Hangang Sacred Heart Hospital, Seoul), Jin Ok Jeong, MD (Chungnam National University Hospital, Daejeon), Si-Hoon Park, MD (Ewha Womans University Mokdong Hospital, Seoul), and Sung Yun Lee, MD (Inje University Ilsan Paik Hospital, Goyang) in Korea for their outstanding contribution to patient enrolment.

