The Academic Medical Center received unrestricted research grant support from OrbusNeich Medical BV. R. J. de Winter receives grant and research support from OrbusNeich Medical BV. All other authors have no conflicts of interest to declare. The e-HEALING Registry was funded by OrbusNeich Medical BV, The Netherlands.
This paper also includes accompanying supplementary data published at the following website: www.eurointervention.org.
Abstract
Aims: e-HEALING is a worldwide, internet-based registry designed to capture post marketing clinical data on the use of the Genous™ EPC capturing R stent™. Rapid restoration of a healthy endothelial layer after stent placement by capturing circulating endothelial progenitor cells may reduce both stent thrombosis (ST) and in-stent-restenosis.
Methods and results: We planned a 5,000 patient registry with ≥1 lesion suitable for stenting. The 12-month primary outcome was target vessel failure (TVF), defined as target vessel-related cardiac death or myocardial infarction (MI) and target vessel revascularisation. Secondary outcomes were the composite of cardiac death, MI or target lesion revascularisation (TLR), and individual outcomes including ST.
A total of 4,939 patients received ≥1 Genous stent between 2005 and 2007. Baseline characteristics showed a median age of 63 years, 79% males, 25% diabetics, and 37% with prior MI. A total of 49% of lesions treated were ACC/AHA type B2 or C; 1.1 stents per lesion were used. At 12 months, TVF occurred in 8.4% and the composite of cardiac death, MI or TLR in 7.9%. Twelve-month TLR and ST were 5.7% and 1.1%, respectively.
Conclusions: Coronary stenting with the Genous results in good clinical outcomes, and low incidences of repeat revascularisation and ST.
Introduction
Percutaneous coronary intervention (PCI) and stent placement is effective and safe in the treatment of patients with symptomatic ischaemic coronary disease; however in-stent-restenosis remains a problem1. Drug-eluting stents (DES) have been shown to reduce the incidence of in-stent-restenosis, but the use of antiproliferative drugs has been associated with delayed or inhibited endothelialisation of the stent struts, which is associated with abnormal vessel homeostasis and the occurrence of late stent thrombosis (ST). The incidence of late ST associated with DES has been estimated to be 0.6% per year up to four years after stent implantation2. Although earlier reports were alarming, the long-term outcomes of randomised trials and large registries comparing DES with bare-metal stents have not been able to show an increase in the incidence of death or myocardial infarction (MI) associated with DES3. Technologies that together with stent placement promote endothelialisation and inhibit smooth muscle cell growth are an attractive alternative to DES.
Recently, the bio-engineered Genous endothelial progenitor cell (EPC) capturing stent, a novel stent technology with a “pro-healing” approach, became available. This stent is coated with a biocompatible matrix to which murine, monoclonal, antihuman CD34+ antibodies are covalently attached. The antibody is specific to the surface antigens present on circulating EPCs, creating an immunoaffinity surface for preferentially capturing these circulating cells. Animal studies showed that within 48 hours of stent implantation the surface exhibited a rich endothelial cell population both visualised by immunohistochemistry and scanning electron microscope and no sign of mural thrombus formation was seen4. In humans, the safety and efficacy of the EPC capturing stent was demonstrated in the HEALING studies with only one month of dual anti-platelet therapy (DAPT) prescribed5-7. e-HEALING is a worldwide multicentre, prospective, post-approval registry aimed at collecting data on approximately 5,000 patients treated with the Genous capturing stent during routine non-urgent coronary intervention. We report the incidence of adverse ischaemic events, clinically-driven repeat intervention and stent thrombosis at 12 months.
Methods
Study design
The e-HEALING (Healthy Endothelial Accelerated Lining Inhibits Neointimal Growth) was a worldwide, multicentre post marketing registry. Approximately 5,000 patients were included between October 2005 and October 2007 from 144 centres in Europe, Asia/Pacific, Middle East, Africa and Latin America (for the participating centres, refer to the online appendices at www.eurointervention.org ). The objective of the e-HEALING registry was to assess clinical outcomes up to 12 months after placement of the Genous stent in a “real world” subject population with a non-urgent PCI. This study was conducted according to the Declaration of Helsinki. The local medical ethics committees approved the study protocol at sites at which such approval was required. If determined necessary, written informed consent was obtained.
Device description
The Genous stent comprises a polysaccharide matrix coating with murine, monoclonal anti-human CD34+ antibodies covalently bonded to the surface of a 316L stainless steel stent (GenousTM Bio-engineered R stentTM; OrbusNeich Medical Technologies, Fort Lauderdale, FL, USA). The Genous stent received CE mark approval on August 11, 2005 for the treatment of patients with symptomatic ischaemic heart disease eligible for stenting de novo and/or restenotic coronary artery lesions.
Patients and procedures
Patients undergoing PCI with at least one lesion suitable for stenting with a Genous stent (diameter 2.50-4.00 mm, length 9-33 mm) in accordance with the instructions for use were eligible for the e-HEALING registry. The indication for PCI was left at the discretion of the operator. Patients were recommended to receive at least two weeks of statin therapy prior to PCI6, DAPT for at least one month post-procedure and aspirin indefinitely. The use of concomitant medication was left at the discretion of each participating centre. In case of multiple lesions, all lesions were preferably treated with a Genous stent but not mandatory per protocol.
Outcomes
The primary outcome of the e-HEALING registry was target vessel failure (TVF) at 12-month follow-up, defined as the composite of cardiac death or MI unless unequivocally attributable to a non-target vessel or target vessel revascularisation (TVR). Secondary outcomes were the composite of cardiac death, MI and clinically-indicated target lesion revascularisation (TLR), and the individual outcomes all-cause death, cardiac death, MI (non-Q-wave or Q-wave) according to protocol, TLR, TVR, stent thrombosis according to the definitions of the Academic Research Consortium (ARC)8 or according to protocol, major and minor bleeding and stroke. According to recommendations of the ARC, a patient-oriented and device-oriented composite outcome was included8. A non-Q-wave MI was defined as an elevation of post-procedure CK levels above two times the upper limit of normal (ULN) in the absence of pathological Q-waves. A Q-wave MI was defined as the development of new, pathological Q-waves in two or more continuous leads with an elevation of CK-MB above the ULN. TLR was defined as any repeat-PCI of the target lesion or coronary artery bypass grafting (CABG) of the target vessel. A revascularisation was indicated clinically if the stenosis of the treated lesion was at least 50% of the lumen diameter based on quantitative coronary angiography with one of the following: a positive history of recurrent angina pectoris, objective signs of ischaemia at rest (ECG changes) or a positive ischaemia-detection test, or abnormal results of any invasive functional diagnostic test. A revascularisation of a stenosis of at least 70% of the lumen diameter by angiographic assessment in the absence of the above mentioned ischaemic signs or symptoms was also considered a TLR. TVR was defined as the repeat revascularisation of any segment of the index major coronary artery treated at the index procedure. ST was defined in the protocol as an in-segment TIMI flow grade 0 or TIMI flow grade 1, 2, or 3 in the presence of a thrombus. Both should be accompanied by acute ischaemic symptoms or ECG changes. Finally, bleeding was considered major when it led to death or permanent disability, suspected or proven intracranial, produced a fall in haemoglobin >3 mmol/l, led to transfusion of two or more units of whole blood of packed cells, or led to peripheral vascular surgery. All other bleeding was considered as minor.
Data collection and management
Baseline patient and lesion, procedure-related and angiographic characteristics were collected and stored in a central internet-based electronic data capture system (Eventa; KIKA Medical, Paris, France) with built-in queries to improve accuracy maintained by a contract research organisation (CRO) (Cardialysis, Rotterdam, The Netherlands). Angiographic variables were obtained by visual estimation.
All outcome events were assessed at discharge of initial hospitalisation, at 30 days, at six months and at 12 months. The following events were adjudicated by an independent Clinical Event Committee (CEC) whose members did not participate in the study: death, MI, TVR, TLR, and ST. The CEC was managed independently by Cardialysis.
Quality control
All site personnel were trained on the protocol, device and internet-based database prior to the initiation of the registry at each respective centre. Trained and qualified clinical research associates of Cardialysis monitored the registry throughout its duration remotely through the internet-based database. Ten percent of the sites were selected randomly for on-site monitoring including full source data verification. A comprehensive, integrated data management plan, including on-line queries and remote data cleaning was implemented to insure the integrity of the data. All changes to the database were tracked by an audit trail.
Statistical analysis
Categorical variables were reported with counts and percentages, and continuous variables were reported with the means and standard deviations (SD) or median and interquartile ranges (IQR). Cumulative event rates were estimated using the Kaplan-Meier method and compared with the log-rank test. Follow-up was censored at the last known date of follow-up or at 12 months, whichever came first. A pre-specified subgroup analysis included 12-month outcomes according to the presence of diabetes. Statistical analyses were performed at the Academic Medical Centre and independently verified by Cardialysis.
Results
Patients
A flowchart of patients included in the registry is shown in Figure 1.
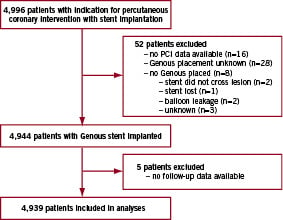
Figure 1. Trial profile.
Of the 4,996 patients entered in the e-HEALING registry, 52 patients were excluded because of missing procedure-related data (n=16), no Genous stent was placed or Genous placement was unknown (n=36). Five patients were excluded due to missing follow-up data. The baseline characteristics of the remaining 4,939 patients are shown in Table 1.
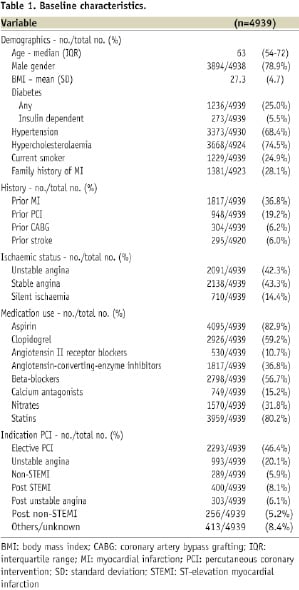
The median age of the study patients was 63 years, 79% were male, and 25% were diabetics. The median time of follow-up was 365 days (IQR 358-365 days). The completeness of follow-up for clinical events was 98.9% at 30 days (±1 week), 97.1% at six months (±2 weeks), and 92.3% at 12 months (±4 weeks).
Angiographic and procedural characteristics
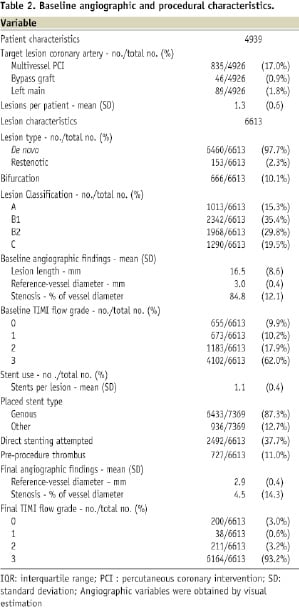
On average, 1.3 lesions per patient were treated. A de novo lesion was treated in 97.7%, compared with 2.3% restenotic lesions. Of all lesions, 10% were bifurcations. A total of 49% of lesions treated were ACC/AHA type B2 or C, mean lesion length was 16.5±8.6 mm, and the reference vessel diameter was 3.0±0.4 mm by visual estimation. A mean of 1.1 stents per lesion was used. Of a total of 7,369 stents, 87.3% were Genous stents compared with 12.7% other stents. Detailed angiographic and procedural characteristics are shown in Table 2.
Dual antiplatelet therapy
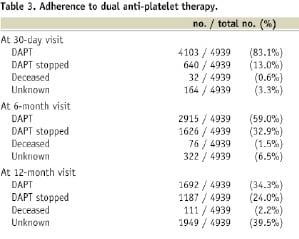
At the 30-day visit, 83% of the patients were on DAPT. These rates were 59% at the 6-month visit and 34% at the 12-month visit (Table 3).
Outcomes
The 12-month clinical outcomes are summarised in Table 4.
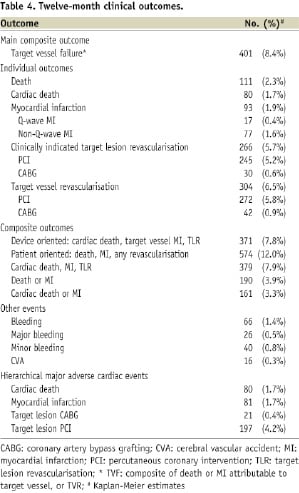
At 12-month follow-up, the cumulative event rate of TVF was 8.4%. TVF was 1.7% and 5.7% at 30-day and 6-month follow-up, respectively. The 12-month composite of cardiac death, MI or TLR occurred in 7.9% of all patients. These event rates were 1.9% and 5.5% at 30-day and 6-month follow-up. Kaplan-Meier curves of TVF and the composite of death, MI, or TLR are shown in Figure 2.
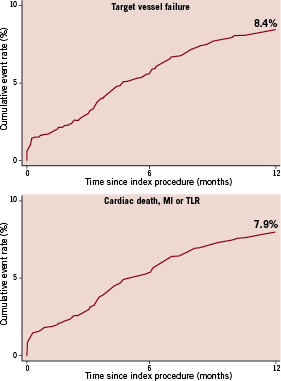
Figure 2. Twelve-month outcomes. Upper panel: TVF (composite of cardiac death or myocardial infarction unless unequivocally attributable to a non-target vessel and target vessel revascularisation), lower panel: composite of cardiac death, MI, or TLR.
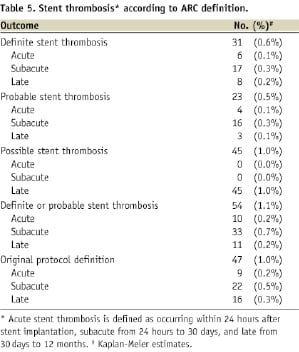
The 12-month cumulative event rates of the device-oriented (cardiac death, target vessel MI, TLR) and patient-oriented (death, MI, any revascularisation) composite outcomes were 7.8% and 12.0%, respectively. The 12-month cumulative event rates of the individual outcomes cardiac death, MI, and TLR were 1.7%, 1.9%, and 5.7%, respectively. Acute definite or probable ST, according to the ARC criteria, occurred in 10 patients (0.2%). Subacute or late definite or probable ST occurred in 33 (0.7%) and 11 (0.2%) patients, respectively.
In an exploratory analysis, we evaluated the outcomes TVF, TLR, definite ST and definite or probable ST in the 4,242 patients treated with only Genous stents. The event rates were 8.2%, 5.7%, 0.6% and 1.1%, respectively.
Diabetics
In diabetic patients (n=1,236), the 12-month TVF rate was 10.0% compared with 7.9% in non-diabetics (P=0.03). The composite of cardiac death, MI, or TLR occurred in 9.3% of all diabetic patients. The 12-month cumulative event rates of individual outcomes cardiac death, MI, and TLR were 3.0%, 2.1%, and 6.4%, respectively. Although cardiac death and MI occurred significantly more often in diabetics, we did not observe a significant difference in TLR compared with non-diabetics (5.4%, P=0.23).
Discussion
Our study provides information from a large, multicentre registry encompassing real-world, data of patients undergoing non-urgent PCI with the Genous stent. We found that overall the incidence of TVF and the composite of cardiac death, MI, or TLR at 12 months were low. The Kaplan-Meier curves are smooth: no “oculostenotic reflex” is observed owing to the absence of routine angiographic follow-up9. The 12-month incidence of definite or probable ST was 1.1%. Importantly, and in contrast to the use of DES, these results were obtained without the need for DAPT for 12 months after the procedure.
Comparison with DES registries
The 5.7% incidence of clinically indicated TLR at 12 months in this large patient population is remarkable and compares well with the TLR rate in the ARRIVE 1 registry (paclitaxel-eluting stent) (5.6%) or the E-Five registry (zotarolimus-eluting stent) (4.5%)10,11. The incidence of repeat revascularisation in registries evaluating the sirolimus-eluting stents was slightly lower; 3.1% in the e-Cypher registry and 4.4% in the EVENT registry12,13. A recent study from Milan by Latib et al reported a 7.9% incidence of clinically driven repeat revascularisation at 12 months with the everolimus-eluting XienceV™ stent in a single centre registry comprising a contemporary cohort of real-world data14. The incidence of the composite of cardiac death, MI or clinically driven TLR in our study was 7.9% and comparable to that observed in the e-Cypher (5.8%) or ARRIVE 1 (7.2%) and numerically better than that observed in the study by Latib et al (10.8%). Although comparisons between registries are hampered by all the differences in patient characteristics and practice patterns, our results were obtained in a patient population from everyday clinical practice, including 25% of patients with diabetes mellitus, 37% with prior MI and 25% of patients with previous revascularisation procedures. In addition, nearly 50% of lesions were considered complex lesions; either ACC/AHA type B2 or type C lesions. Long lesions and small vessels were infrequent, resulting in a mean reference vessel diameter of 3.0 mm and mean lesion length of 16.5 mm.
Stent thrombosis
The incidence of definite or probable stent thrombosis was 0.2% within 24 hrs (acute ST), 0.7% between 24 hrs and 30 days (subacute ST) and 0.2% between 30 days and 12 months (late ST), with a 1.1% incidence overall at 12 months. Acute and subacute ST are often the result of procedural factors such as dissections, stent malapposition, geographic miss or tissue protrusion through the stent struts in the lumen, which may be invisible on the coronary angiogram. It is unlikely that stent technology aimed at rapid endothelialisation of the stented segment could completely prevent ST due to such mechanical causes. Late ST was very low in this study. These are important results, keeping in mind the recommendation for only one month of DAPT after the procedure.
Previous clinical studies using the Genous stent
The first clinical trial to evaluate the efficacy of the Genous stent in patients carrying noncomplex coronary artery lesions was the HEALING-First-In-Man study which included 16 patients5. At 9-months clinical follow-up, the composite of cardiac death, stroke, MI, and TVR was 6.3% and no cases of stent thrombosis were reported. In the non-randomised HEALING II study, a total of 63 patients with non-complex lesions were enrolled7. At 18-month follow-up, no ST was observed and the composite of cardiac death, MI and TLR was 7.9%, mainly attributed to a relatively low clinically-driven TLR rate of 6.3%. Both these studies enrolled selected patients with relatively low-risk lesions. Recently Miglionico et al reported a single centre, non-randomised registry including 80 patients with complex lesions treated with the EPC capturing stent15. At 14-month follow-up, the composite of cardiac death, MI and TVR was 16%, and the TLR rate was 13%. In addition, Beijk et al recently reported the first randomised study comparing the Genous stent with the Taxus Liberté stent in patients with a high risk of restenosis including long lesions, small vessels and patients with diabetes16. In this small study, TLR at 12 months was 12.2% in Genous treated patients compared to 8.4% in Taxus treated patients. Stent thrombosis occurred in 4.2% with Taxus compared to 0% with Genous. No cardiac death occurred in both groups, and the spontaneous MI rates were not significantly different (0% in Genous versus 3.2% in Taxus group). The TVF rate was markedly different from that observed in e-HEALING (17.3% versus 8.4%). There are two potential explanations for the higher TVF rate in the randomised study. First, only patients at a high risk of restenosis were eligible for the trial. Second, higher repeat revascularisations can be expected due to the performance of angiographic follow-up between six and 12 months in 53 of the 98 Genous treated patients.
Endothelial progenitor cells
Bone-marrow derived, circulating EPCs were first described by Asahara et al in 199717. They showed that EPCs have regenerative capacities and play an important role in vessel wall homeostasis. Animal studies have shown that EPCs beneficially influence the repair of endothelium after injury and the progression of atherosclerosis, yet the role of EPCs in humans is less well clear18,19.
In subjects with cardiovascular risk factors, such as hypertension and diabetes mellitus, studies have shown that the number of circulating EPCs is reduced, and their function adversely affected20,21. In contrast, elevated EPC levels were seen in patients that suffered an acute MI22 and patients that underwent a PCI23. Unfortunately, studies reporting on the number of circulating EPCs in patients with coronary artery disease (CAD) fail to show agreement. Some studies report that the EPC number is reduced in patients with atherosclerotic disease24,25, whereas other studies report that EPC levels are indeed increased in CAD patients26,27. There is accumulating evidence that a reduced number of EPCs is associated with the occurrence of ischaemic cardiovascular events in patients with angiographically documented CAD28,29.
Limitations
The potential underreporting of adverse events and patient selection are potential important shortcomings of all large registries. The present study was organised with a comprehensive data-management plan that included frequent monitoring of all participating sites and full event verification designed to minimise the effects of event underreporting. In addition, 12 months of follow-up duration may be too short to accurately assess the risk of very late stent thrombosis. Finally, angiographic variables were obtained by visual estimation.
Conclusion
Coronary stenting with the Genous stent results in good clinical outcomes, a low incidence of repeat revascularisation and a low incidence of ST.
Acknowledgements
We thank all centres and investigators participating in the e-HEALING registry. Most importantly, we thank all participating patients.