Abstract
Aims: The FOCUS registry is a prospective, multicentre, web-based programme designed to collect clinical outcome data from real-world patients receiving the second-generation cobalt-chromium sirolimus-eluting stent (CoCr-SES).
Methods and results: From March 2009 to February 2010, a total of 5,084 patients from 83 centres who were eligible to receive CoCr-SES were enrolled in the FOCUS registry. The primary endpoint was 12-month major adverse cardiac events (MACE, defined as the composite of cardiac death, myocardial infarction [MI], and target vessel revascularisation [TVR]). One-year data were available for 5,013 (98.6%) of the 5,084 patients enrolled. The primary endpoint occurred in 174 (3.47%) of 5,013 patients, consisting of 43 (0.86%) cardiac deaths, 132 (2.63%) MI, and 46 (0.92%) TVR. According to the Academic Research Consortium definition, definite and probable stent thrombosis (ST) occurred in 0.52% (26/5,013) of patients, including 19 cases of early ST and 7 of late ST. The 12-month MACE rates were 3.73% and 2.60% for extended-use and standard-use patients, respectively (p=0.065).
Conclusions: The second-generation CoCr-SES was associated with low rates of 12-month MACE and ST in a broad spectrum of patients, thereby confirming the clinical safety and efficacy of this stent in a real-world setting.
Introduction
The introduction of the drug-eluting stent (DES) has led to a dramatic reduction of restenosis, which was a major limitation of percutaneous coronary intervention (PCI) for coronary artery disease in the bare metal stent (BMS) era1–6. The first-generation sirolimus-eluting or paclitaxel-eluting stents have a polymer release of antiproliferative drugs from a stainless steel stent platform. More recently, second-generation DESs have been developed, mainly to improve long-term DES safety but also to facilitate the procedure by using a cobalt-chromium stent platform7-9. Cobalt-chromium is stronger and more radiopaque than stainless steel, and thus allows strut thickness and total stent volume to be reduced while maintaining radial strength leading to more flexible and deliverable stent platforms10-13.
The Firebird2® stent (MicroPort Medical, Shanghai, China) is based on the cobalt-chromium alloy metal platform, coated with a two-layer styrene-butylene-styrene (SBS) polymer that elutes sirolimus14-16. With the stent receiving approval for use in China and Southeast Asia, a prospective, non-randomised, multicentre registry (Firebird2 cObalt-Chromium alloy sirolimus-elUting Stent [FOCUS] registry, ClinicalTrials.gov Identifier: NCT00868829) has been designed and conducted to evaluate the safety and efficacy of this second-generation cobalt-chromium sirolimus-eluting stent (CoCr-SES) in routine treatment of patients with coronary artery stenosis, including patients with clinical characteristics or lesion types that are often excluded from randomised controlled trials. This report describes the primary endpoint, 12-month clinical data from the FOCUS registry for all-comers, and further examines the outcomes among patients with clinical and lesion characteristics similar to those enrolled in randomised clinical trials (standard-use group) compared with patients with more complex clinical and lesion characteristics (extended-use group).
Methods
PATIENT POPULATION
The FOCUS registry was designed to be a prospective, non-randomised, observational, web-based programme conducted at 83 medical centres in three countries. Between March 2009 and February 2010, a total of 5,083 consecutive and eligible patients for whom implantation with a Firebird2 stent was intended were enrolled at the time of stent introduction into the guiding catheter. Patient inclusion and exclusion criteria were not mandated specifically, except those who presented with acute myocardial infarction (MI) within seven days, received a mixture of different brands of stents, or refused to provide informed consent, according to the inclusion and exclusion criteria. Neither the number of stents nor the number of lesions was limited. Thus, the registry population included a large number of patients with clinical and lesion characteristics that did not fit the standard-use criteria of previous clinical trials. The extended-use group was defined as patients with left main lesions, chronic total occlusions, bypass graft lesions, in-stent restenosis, bifurcated or ostial lesions, severe tortuosity, multivessel stenting, severe calcification, reference vessel diameter ≤2.5 mm, lesion length >28 mm, or moderate or severe renal impairment. All other patients were classified as standard-use. The registry protocol was approved by the ethics committee of each participating medical centre. All enrolled patients granted their oral consent to participate in the registry before the stent implantation and signed the written consent form either by themselves immediately after the index procedure or through their representatives (their family members) before or after the index procedure.
PERIPROCEDURAL TREATMENT
Coronary angioplasty and DES implantation were performed according to standard practice. The lesions could be pre-treated with any technique or device, such as balloon angioplasty, cutting balloon, or atherectomy, although implantation of the Firebird2 stent in each target lesion during the index procedure was mandatory. Antiplatelet therapy with 300 mg of clopidogrel was administered orally within 24 hours before the procedure, unless the patient was already taking clopidogrel. At the start of each procedure, all patients received an intra-arterial bolus of unfractionated heparin (100 IU/kg) to achieve an activated clotting time ≥250 sec. The administration of procedural glycoprotein IIb/IIIa inhibitors was used according to the operator’s discretion. The postprocedural antiplatelet regimen included indefinite aspirin therapy and 75 mg/day of clopidogrel orally for 12 months at least.
DEVICE DESCRIPTION
The Firebird2 stent is composed of a cobalt-chromium alloy stent platform with an open-cell geometry coated with a 6-µm-thick two-layer durable SBS polymer containing the antiproliferative drug sirolimus. The dosage of sirolimus was 9 µg/mm stent length, and in vitro testing indicated that about 50% of the drug would be released within one week and 90% within one month. The Firebird2 stent was available in diameters of 2.5-4.0 mm and in lengths of 13-33 mm.
DATA COLLECTION AND MANAGEMENT
The data collected by the FOCUS registry included demographic information, cardiovascular history, comorbidity, lesion and procedure characteristics, and antithrombotic regimens. Clinical follow-up was scheduled by telephone communication or office visit at 30 days, six, 12, 24 and 36 months after the index procedure. Angiographic follow-up was not mandatory in this registry.
For this registry, study data was collected via electronic data capture using web-based case report forms. For quality control purposes, all participating centres were randomly monitored to detect and correct any inaccuracies in the data reported and to check for under-reporting of events. Approximately 10% of recorded data in each centre was source-verified against the patient’s medical records and other applicable source documentation. All monitoring activities were conducted for all sites by an independent clinical research organisation (China Cardiovascular Research Foundation, CCRF, Beijing, China). All events relating to endpoints were reported to and adjudicated by an independent clinical endpoints committee, which consisted of cardiologists not taking part in the study.
STUDY ENDPOINTS AND DEFINITIONS
The primary endpoint of the study was the rate of major adverse cardiac events (MACE) relating to the Firebird2 stent at 12 months. Other adjudicated event data included all death, cardiac death, myocardial infarction (MI), target vessel revascularisation (TVR), target lesion revascularisation (TLR), target lesion failure (TVF) and stent thrombosis.
MACE was defined as the composite endpoint of cardiac death, MI or TVR. MI was classified as: Q-wave, when new pathological Q-waves in two or more contiguous leads of the surface electrocardiogram were accompanied by a rise in creatine kinase-myocardial band isoenzyme (CK-MB) level >3 times the upper limit of normal (ULN); or non-Q-wave, when the CK-MB concentrations were greater than three times the ULN, in the absence of new pathological Q-waves (cardiac enzymes quantification post percutaneous intervention was recommended but not mandatory per protocol). TVR was defined as any PCI or coronary artery bypass grafting (CABG) performed on the index target vessel at any time after the index procedure. TLR was defined as any repeat percutaneous or surgical treatment of the target lesion. TLF was defined as the composite of TLR, cardiac death or MI related to the target vessel. Stent thrombosis was classified according to the Academic Research Consortium (ARC) definition17.
STATISTICAL ANALYSIS
Both quantitative and qualitative data were summarised using descriptive statistics. The primary analytical population consisted of all enrolled patients in whom a Firebird2 stent was attempted and/or implanted. A secondary analysis compared outcomes in the extended-use and standard-use patient subgroups. Continuous variables are expressed as means ±standard deviations (SD) and categorical variables as percentages. The Fisher’s exact test was used for comparisons between proportions and the two-sample t-test was used for comparisons of mean values. The time to MACE event and stent thrombosis were summarised and displayed with cumulative incidence curves by the Kaplan-Meier method. Statistical significance was set at p<0.05.
Results
PATIENT DEMOGRAPHIC CHARACTERISTICS
From March 2009 to February 2010, a total of 5,084 patients were recruited to the FOCUS registry at 83 clinical centres in China, Thailand and Indonesia. As shown in Table 1, the mean age was 62.9±10.7 years, and 71.3% of patients were male. The incidence of diabetes was 22.8%, prior myocardial infarction occurred in 26.1% of patients, and previous PCI was reported in 11.9% of patients.

LESION AND PROCEDURAL CHARACTERISTICS
Lesion and procedural characteristics are reported in Table 2. In total, 7,454 lesions of 5,084 patients were treated. Among them, 62.6% were American College of Cardiology (ACC) and American Heart Association (AHA) B2/C lesions, 16.4% were bifurcation lesions, 2.9% were left main coronary stenoses, and 10.5% were total occlusions (Table 2). The average lesion length was 27.9±16.7 mm. On average, the number of stents implanted per lesion was 1.33±0.59. The overall lesion success rate was 99.8%, and the overall procedure success rate was 99.7%.

ANTIPLATELET THERAPY
At baseline, during the periprocedural period, 100% of patients were receiving dual antiplatelet therapy (99.2% were taking aspirin plus clopidogrel, and 0.8% were taking cilostazol plus clopidogrel). The percentage of patients receiving dual antiplatelet therapy was 99.3% at 30 days, 98.1% at six months, and 76.6% at 12 months.
Clinical outcomes
OVERALL RESULTS
One-year data were available for 5,013 (98.6%) of the 5,084 patients enrolled. The primary endpoint, a composite of MACE at one-year follow-up, occurred in 174 (3.47%) of 5,013 patients, consisting of 43 (0.86%) cardiac deaths, 132 (2.63%) MI, and 46 (0.92%) TVR. The 12-month rate of TLF for all patients in the registry was 2.47% (124/5,013). The 12-month adverse clinical events are shown in Table 3, and the 12-month MACE and TVF curves are illustrated in Figure 1 and Figure 2, respectively.
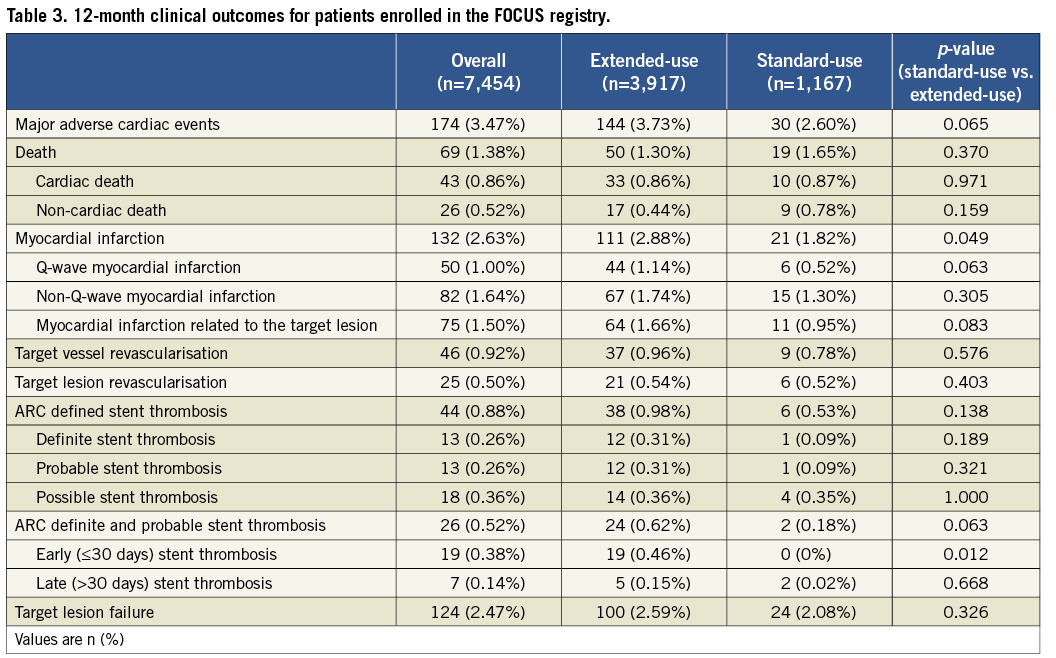
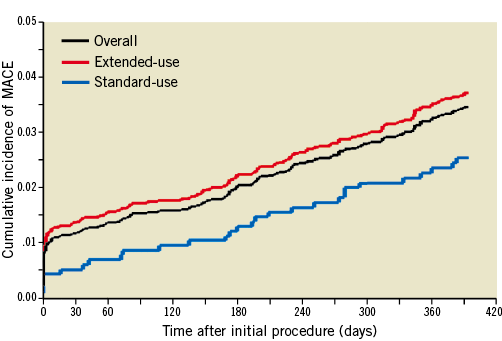
Figure 1. Cumulative incidence of MACE at 12 months.
Cumulative incidence curves of major adverse cardiac events (MACE) for all patients and patients in the standard-use and extended-use groups at 12 months.
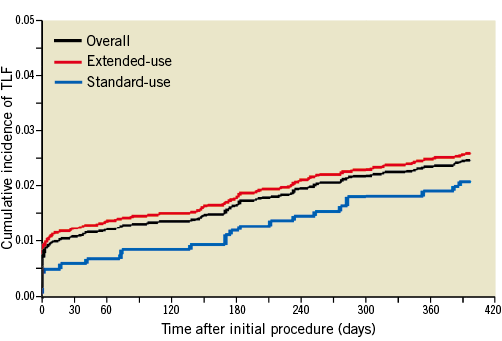
Figure 2. Cumulative incidence of TLF at 12 months.
Cumulative incidence curves of target lesion failure (TLF) for all patients and patients in the standard-use and extended-use groups at 12 months.
The 12-month overall incidence of all ARC-defined stent thrombosis was 0.88%. Among them, the ARC definite, probable and possible stent thrombosis occurred in 13 (0.26%), 13 (0.26%) and 18 (0.36%) patients, respectively. At 12 months, the ARC definite and probable stent thrombosis rate was 0.52%, consisting of 19 (0.38%) cases of early and 7 (0.14%) of late stent thrombotic events (Figure 3).
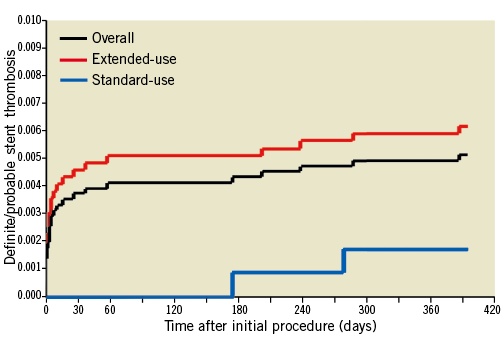
Figure 3. Cumulative incidence of ARC definite/probable stent thrombosis at 12 months. Cumulative incidence curves of Academic Research Consortium (ARC) definite and probable stent thrombosis for all patients and patients in the standard-use and extended-use groups at 12 months.
RESULTS FOR STANDARD-USE VERSUS EXTENDED-USE
According to the pre-specified definition, the extended-use group comprised 3,917 (77.1%) patients in this registry. As shown in Table 1 and Table 2, patients with extended use of the Firebird2 stent more often presented with high-risk features such as acute coronary syndrome, diabetes, previous MI, prior PCI or CABG, and had more complex coronary anatomy (all p<0.05). When compared to the standard-use group, the incidence of MACE (3.73% vs. 2.60%, p=0.065), TLF (2.59% vs. 2.08%, p=0.326), and ARC-defined stent thrombosis (0.98% vs. 0.53%, p=0.138) was numerically increased in the extended-use group; however, none of these differences in event rates reached statistical significance.
Discussion
The present report describes 12-month data from the large-scale real-world FOCUS registry of the second-generation Firebird2 CoCr-SES. These results, obtained from 83 medical centres in China and Southeast Asia, provide compelling evidence for the safe and effective use of the Firebird2 CoCr-SES in routine clinical practice. Despite the high proportion of patients with high-risk characteristics and complex lesions that are usually excluded from randomised trials, the Firebird2 stent was associated with a very low incidence of MACE and stent thrombosis during a mid-term follow-up.
PCI has been a mainstay in the management of coronary artery disease since its introduction in the late 1970s. More recently, the first-generation DESs, such as sirolimus-eluting CYPHER® (Cordis, Johnson & Johnson, Warren, NJ, USA) and paclitaxel-eluting TAXUS™ Boston Scientific, Natick, MA, USA) stents, have further improved results of PCI by reducing the risk of restenosis. However, there is currently debate on the long-term safety of these first-generation DES, especially concerning the potential risk of late stent thrombosis as well as late restenosis18-20. The second-generation DESs, such as zotarolimus-eluting Endeavor® (Medtronic, Minneapolis, MN, USA) or Resolute® (Medtronic), everolimus-eluting XIENCE V® (Abbott Vascular, Redwood City, CA, USA) or PROMUS™ (Boston Scientific), and sirolimus-eluting Firebird2® stents, have recently become available in the international market14,21,22. It is important to note that all of these next-generation DESs are based on cobalt-chromium alloy stent platforms, whose main characteristics are low strut thickness, high flexibility and deliverability, acceptable compliance, recoil and risk of plaque prolapse, and good overall radiopacity14,21,22. A pilot study has already suggested the sustained superiority of the Firebird2 CoCr-SES in selected patients, as compared to a bare cobalt-chromium metal stent in terms of prevention of restenosis and TVR, without significant untoward events14,15. Nonetheless, the strict inclusion and exclusion criteria, the small number of patients and the relatively short follow-up duration are still too limited to enable definitive conclusions.
In the present FOCUS registry, clinical data were available for 5,013 of the 5,084 patients. This represents a 98.6% one-year follow-up rate, which compares favourably with other DES registries23,24. Additionally, all events related to endpoints were reported to and adjudicated by an independent clinical endpoints committee to assure accuracy of the data. For quality control purposes, moreover, all participating sites were randomly monitored to detect and correct any inaccuracies in the data recorded and to check for under-reporting of events. All the above mentioned issues support the high quality of the FOCUS registry and further strengthen the validity of the data reported.
In this study, the majority of patients had an off-label lesion treated with a Firebird2 CoCr-SES. However, despite this increased complexity of the patients and lesions treated, the cumulative 12-month MACE was only 3.47%. Overall outcomes reported for the zotarolimus-eluting stent (ZES) and everolimus-eluting stent (EES) in recent registry reports suggest that the Firebird2 CoCr-SES is non-inferior in performance compared to other second-generation DES22,23. The cumulative rate of MACE at 12 months was reported to be 7.5% for the ZES in the E-Five registry and 5.1% for the EES in the SPIRIT V registry23,24. With regard to the hard endpoints, the rates of 12-month death (1.38%), cardiac death (0.86%) and MI (2.63%) in this study were also similar to those in the E-Five (2.4%, 1.7% and 1,6%, respectively) and SPIRIT V registry (1.7%, 1.1% and 3.5%, respectively)23,24. It is noteworthy that the overall incidence of 12-month TVR (0.92%) and TLR (0.50%) in this registry were dramatically low, indicating the powerful efficacy of this new-generation DES in preventing restenosis.
At one-year follow-up, the incidence of ARC definite and probable stent thrombosis was 0.52% overall, with 0.38% occurring early (0 to 30 days) and 0.14% occurring late (31 to 365 days) in the present study. This value is similar to the cumulative 12-month data from the SPIRIT V registry which reported a 0.66% incidence of ARC definite/probable stent thrombosis overall with a rate of late stent thrombosis of 0.23%24, and even numerically lower than the cumulative 12-month data from the E-Five registry which reported a 1.1% incidence of ARC definite/probable stent thrombosis with a rate of late stent thrombosis of 0.4%23. In this registry, the percentages of patients receiving dual antiplatelet therapy at six months and 12 months respectively reached 98.1% and 76.6%, higher than those in the E-Five registry (85% and 61%)23. The satisfactory stent thrombosis outcomes in this study may partly be linked to prolonged combined antiplatelet therapy.
Of course, some factors may, at least in part, have contributed to the good efficacy and safety results in the present study. Although the registry enrolled almost all patients with high-risk characteristics and complex lesions, a high-risk subset of patients with AMI was excluded from this study population. Moreover, the percentages of some baseline high-risk parameters (renal failure, LVEF below 30%, prior CABG, saphenous vein graft target lesion, etc.) were very low in our PCI population as compared to those in Western countries. Because not all written consent forms were signed before the index procedure, the procedural complications and acute events might be under-reported if the patients or their representatives changed their mind, especially when some unexpected event occurred during the procedure. Additionally, because all events were adjudicated but not subject to on-site monitoring, the reporting of events might be dependent upon the goodwill of the investigators.
In our FOCUS registry, a second-generation CoCr-SES was very often used for extended indications. Approximately 77.1% of the patients were treated with CoCr-SES in extended situations. This is in accordance with data from the E-Five registry (extended-use of DES accounted for 74%)23. Extended indications represent a high-risk group of patients. As expected, clinical, angiographic and procedural characteristics differed significantly between the two groups. In concordance with previous observational studies, patients receiving DES for off-label indications more likely had a history of diabetes, prior myocardial infarction and previous PCI/CABG. Furthermore, coronary atherosclerosis and lesion characteristics were more severe among patients receiving CoCr-SES for off-label indications. At one-year follow-up, use of CoCr-SES for unapproved reasons was associated with a numerical increase of MACE, TLF and stent thrombosis rates. However, none of these differences in event rates reach statistical significance, and were most likely related to clinical or specific procedural characteristics that predispose a patient to adverse outcomes.
Study limitations
As with other registry studies, there was no control group in the FOCUS registry for simultaneous comparisons. Since the collected data are observational, any findings should be confirmed by a prospective, sufficiently powered clinical trial. Despite these limitations, the FOCUS study is one of the largest prospective, new-generation DES registries and provides valuable insight into patient characteristics and usage patterns in routine interventional cardiology practice.
Conclusions
These 12-month clinical outcomes from the FOCUS registry clearly provide evidence for the safe and effective use of the Firebird2® CoCr-SES in unrestricted daily practice. Despite the high proportion of patients with extended use of this stent, the occurrence of early and late stent thrombosis was very low. Rates of MACE, cardiac death, MI and TVR were similar to those observed with other second-generation DES registries.
Acknowledgements
This registry is funded in part by MicroPort Medical (Shanghai), Ltd. This work was also supported by the National Natural Science Foundation (No. 81101133), the Young Scientific “Phosphor” (Tracking) Foundation from the Shanghai Science and Technology Development (No. 11QH1400500), and the Shanghai Outstanding Young Scientist Foundation from the Shanghai Municipal Health Bureau (No. XYQ2011001).
Conflict of interest statement
The authors have no conflicts of interest to declare.
Online data supplement
Appendix. Participating centres and principal investigators of the FOCUS registry.
APPENDIX
Participating centres and principal investigators of the FOCUS registry were as follows (sequence according to the contributed case numbers):
Zhongshan Hospital of Fudan University (Dr Junbo Ge), Fuwai Cardiovascular Hospital (Dr Yuejin Yang), Beijing Anzhen Hospital (Dr Fang Chen, Xiaoling Zhu, Shuzheng Lv, Zhizhong Li), Xi’an Xijing Hospital (Dr Haichang Wang), Tianjin Chest Hospital (Dr Yin Liu), Shanghai Tenth People’s Hospital (Dr Yawei Xu), The People’s Hospital of Liaoning Province (Dr Zhanquan Li), Putuo District Central Hospital of Shanghai (Dr Huigen Jin), Shanghai Chest Hospital (Dr Weiyi Fang), The First Affiliated Hospital of Dalian Medical University (Dr Xuchen Zhou), Guangdong General Hospital (Dr Lijun Jing), People’s Liberation Army General Hospital (Dr Yundai Chen), The Affiliated Hospital of Medical College of Qingdao University (Dr Changyong Zhou, Zhexun Lian, Yi An), Chengdu Military General Hospital (Dr Yongjian Yang), Meizhou People’s Hospital (Dr Zhixiong Zhong), The First Affiliated Hospital of Medical College of Xi’an Jiaotong University (Dr Zuyi Yuan), The First People’s Hospital of Jining City (Dr Xiaofei Sun), Jiangxi Provincial People’s Hospital (Dr Guotai Sheng), General Hospital of Fushun Mining Group (Dr Zhenming Yan, RenKe Yi), Shanxi Provincial Institute of Cardiovascular Disease (Dr Jian An), The Second Hospital of Jilin University (Dr. Bin Liu), Cangzhou Central Hospital (Dr Zesheng Xu), Xiangya Hospital of Zhongnan University (Dr Xiaoqun Pu), Shanghai Changhai Hospital (Dr Yongwen Qin), Xingtai Cardiovascular Disease Hospital (Dr Shuangying Feng), Sir Run Run Shaw Hospital of Zhejiang University (Dr Guosheng Fu), The People’s Hospital of Shanxi Province (Dr Chunlin Lai), The Second Clinical College of Harbin Medical University (Dr Bo Yu), Armed Police Medical College Hospital (Dr Tiemin Jiang), Beijing Chaoyang Hospital (Dr Lefeng Wang), People’s Hospital of Peking University (Dr Weiming Wang), Beijing Shijitan Hospital (Dr Jianjun Peng), Daqing Oil Field General Hospital (Dr Hui Li), Shengjing Hospital of China Medical University (Dr Wenyue Pang), Nanjing Drum Tower Hospital (Dr Biao Xu), Huashan Hospital of Fudan University (Dr Haiming Shi), Shanghai Sixth People’s Hospital (Dr Meng Wei), Shanghai Xinhua Hospital (Dr Changqian Wang), The Second Affiliated Hospital of Zhejiang University (Dr Ying Zhu), Hangzhou First People’s Hospital (Dr Ningfu Wang), Nanjing First Hospital (Dr Shaoliang Chen), Xuzhou Centre Hospital (Dr Qiang Fu), Affiliated Hospital of Guangdong Medical College (Dr Shian Huang, Keng Wu), Weifang Yidu Zhongxin Hospital (Dr Yutian Tong), The First Affiliated Hospital of Lanzhou University (Dr Zheng Zhang), The Second Affiliated Hospital of Kunming Medical College (Dr Ge Zhang), Wuhan Tongji Hospital of Huazhong University of Science and Technology (Dr Hesong Zeng), Wuhan University Renmin Hospital (Dr Hong Jiang), Xiangfan City Central Hospital (Dr Wenwei Liu), Wuhan Asia Heart Hospital (Dr Guoying Zhu), First People’s Hospital of Yulin City (Dr Ping Li), Beijing Luhe Hospital (Dr Xuekun Zhang), The First Affiliated Hospital of Sun Yat-sen University (Dr Zhimin Du), Zhujiang Hospital (Dr Yingfeng Liu), The First Clinical College of Harbin Medical University (Dr Weimin Li), The People’s Hospital of Guangxi Zhuang Autonomous Region (Dr Ling Liu), Shanghai Renji Hospital (Dr Ben He), The Third Hospital of Peking University (Dr Wei Gao), The First Affiliated Hospital of China Medical University (Dr Dalin Jia), Affiliated Hospital of Jiangsu University (Dr Jinchuan Yan), Yantai Yuhuangding Hospital (Dr Shaorong Liu, Chuanhuan Zhang, Yimin Fang, Zhigang Tao), Qilu Hospital of Shandong University (Dr Jifu Li), Xiangtan Central Hospital (Dr He Huang), Shanghai Huadong Hospital (Dr Xingui Guo), Shandong Provincial Hospital (Dr Xinghua Zhang, Tongbao Liu, Lianqun Cui), The First Affiliated Hospital of Kunming Medical College (Dr Jianming Xiao), Fujian Medical University Union Hospital (Dr Lianglong Chen), Tongji Hospital of Tongji University (Dr Jinfa Jiang), Tianjin First Central Hospital (Dr Chenzhi Lu), The Fourth Clinical College of Harbin Medical University (Dr Xueqi Li), The Second Affiliated Hospital of Dalian Medical University (Dr Peng Qu), Zhongshan Hospital of Xiamen University (Dr Yan Wang), The First People’s Hospital of Ningbo City (Dr Honghua Ye), Affiliated Hospital of Weifang Medical College (Dr Jingtian Li), Guangzhou Nanfang Hospital, Southern Medical University (Dr Yuqing Hou), Wuhan Union Hospital (Dr Qiutang Zeng), The Fourth Affiliated Hospital of China Medical University (Dr Yuanze Jin), Dalian 210 Hospital (Dr Dongju Jiang), The First Affiliated Hospital of Chongqing Medical University (Dr Kanghua Ma), Shanghai Minhang District Central Hospital (Dr Dadong Zhang), Siriraj Hospital of Mahidol University, Bangkok, Thailand (Dr Suwatchai Pornratanarangsi), Bina Waluya Cardiac Hospital, Indonesia (Dr Muhamad Munawar), Medistra Hospital, Indonesia (Dr Teguh Santoso).

