
Abstract
Aims: Our aim was to report one-year outcomes of Absorb bioresorbable scaffold implantation under real-world conditions in an all-comers population of patients with high proportions of complex lesions.
Methods and results: Patients undergoing Absorb 1.1 implantation were included in a single-centre, prospective, all-comers registry. The primary outcome was target lesion failure (TLF), defined as the combination of cardiac death, target vessel myocardial infarction (MI), or clinically driven target lesion revascularisation (TLR). A total of 319 patients received 604 Absorb BVS in 406 lesions. Of note, 24.8% of patients had diabetes and 49.5% presented with an acute coronary syndrome. A total of 51% of lesions were type B2/C. The reference vessel diameter and lesion length were 2.9±0.5 and 21.2±16.8 mm, respectively. The one-year cumulative rate of TLF was 4.9%. Rates of cardiac death, target vessel MI and TLR were 0.9%, 1.3% and 4.2%, respectively. The cumulative one-year rate of definite/probable scaffold thrombosis was 1.3%, with all events occurring within 30 days.
Conclusions: These data suggest that twelve-month clinical outcomes of Absorb use in “real-world” unselected patients with high proportions of complex lesions are reasonably good.
Introduction
Bioresorbable scaffolds (BRS) have recently been introduced for coronary interventions. These novel devices degrade completely over 12-36 months after implantation, enabling transient luminal support, while preventing long-term metallic “caging” of coronaries, promising to overcome the limitations of permanent drug-eluting stents (DES)1. Indeed, BRS implantation has been associated with vessel lumen enlargement, plaque/media reduction, vasomotion restoration, expansive remodelling (which may reduce angina), and new media formation, potentially reducing mechanisms underlying late events, such as inflammation and neoatherosclerosis2. In addition to these biological effects, the complete bioresorption eliminates malapposed, fractured or non-endothelialised struts, and late polymer/metal reactions, with the potential of reducing late thrombotic events triggered by these latter mechanisms. Additional effects of BRS may be important in high-risk settings, in which mechanisms underlying late thrombotic events are more pronounced. However, BRS have specific features, including larger crossing profile, thicker struts and expansion capability limitations, which may impact on their clinical performance, in complex lesions.
Among BRS, the everolimus-eluting scaffold (Absorb; Abbott Vascular, Santa Clara, CA, USA) has been the most extensively investigated in clinical studies. The use of the Absorb appears to provide a similar degree of safety and efficacy compared with metallic DES in the treatment of relatively simple lesions treated under trial conditions3,4. Clinical reports on the use of these novel devices in the more complex settings commonly encountered in routine practice have shown promising results overall5-9, although non-negligible rates of early scaffold thrombosis (ST) were observed in some registries5-8. Nevertheless, real-world data on BRS in unselected populations at more advanced stages of follow-up and with the use of a standardised implantation technique are still limited10.
The aim of this single-centre study was to report one-year clinical outcomes of Absorb implantation under real-world conditions in an unselected population with high proportions of complex coronary lesions.
Methods
PATIENT POPULATION
The GHOST (Gauging coronary Healing with biOresorbable Scaffolding plaTforms) is an ongoing prospective non-randomised, single-centre registry conducted at Ferrarotto Hospital, University of Catania, Italy, from March 2013. The registry includes patients undergoing single-vessel or multivessel percutaneous coronary intervention (PCI) with the Absorb (revision 1.1). Concurrent implantation of DES or bare metal stents was allowed at the operator’s discretion.
Lesions suitable for stenting with a reference vessel diameter of ≥2.0 mm and ≤3.8 mm were eligible for treatment with the Absorb. If these criteria were present and the correct scaffold size was in stock, all angiographic lesions were considered eligible, with the choice between Absorb and permanent metallic stents left to the discretion of the operator. Exclusion criteria for Absorb treatment included: contraindication to prolonged dual antiplatelet therapy (DAPT); high likelihood of poor DAPT compliance; indication for oral anticoagulation; high bleeding risk; planned surgery within 12 months; cardiogenic shock; Killip class III or IV; unstable arrhythmias; cancer or comorbidities with limited expected survival.
The registry population also encompassed patients with clinical and angiographic characteristics that were among the exclusion criteria of the ABSORB II trial11, reflecting a broader “real-world” use. Outcomes were compared between two subgroups stratified according to the entry and exit ABSORB II trial criteria.
Only patients with at least one-year follow-up eligibility were evaluated in the present analysis. The first 209 patients of our registry were part of the larger multicentre GHOST-EU registry, including patients from 10 European centres and reporting six-month outcomes6.
The local ethics committee approved the use of aggregated clinical data for this analysis, and written informed consent was obtained from all patients.
INTERVENTIONAL PROCEDURE
All procedures were performed according to current PCI standards. Lesion preparation by predilatation with non-compliant balloons 0.5 mm smaller than or equal to the scaffold device diameter was mandatory. The recommended pressure for scaffold implantation was at least 10 atm. Post-dilatation at high pressure with non-compliant balloons 0.5 mm larger than or equal to the scaffold device diameter was strongly recommended regardless of the angiographic results. The use of on-line quantitative coronary angiography (QCA) to assess appropriate device size and the use of intravascular imaging techniques were left to the discretion of the operator.
During the procedure, patients received appropriate anticoagulation according to standard hospital practice. Glycoprotein IIb/IIIa inhibitors were used at the physician’s discretion. A loading dose of aspirin 250-500 mg was given before PCI, followed by 75-100 mg oral daily indefinitely thereafter. A loading dose of a P2Y12 inhibitor was administered before or immediately after PCI. Dual antiplatelet therapy (DAPT) was recommended for at least 12 months (with a strict minimum of six months) after Absorb implantation.
ANGIOGRAPHIC ANALYSIS
The QCA was performed with a dedicated software (Cardiovascular Angiographic Analysis System II; Pie Medical Imaging, Maastricht, The Netherlands) using an automated detection algorithm. The interpolated and proximal reference vessel diameters (RVD) in the treated segment were assessed. The acute gain was calculated as minimal lumen diameter (MLD) post scaffold implantation minus MLD pre scaffold implantation.
Technical failure was defined as residual in-scaffold diameter stenosis >30% by QCA.
Scaffold undersizing, correct sizing and oversizing were defined as the ratios between the nominal scaffold size and the proximal RVD ≤0.9, >0.9 and <1.1, and ≥1.1, respectively.
FOLLOW-UP DATA
Clinical follow-up information on medical therapy and the clinical status of patients was prospectively collected through scheduled outpatient clinic evaluations and/or phone contact. Additional information, if necessary, was obtained from referring cardiologists and general practitioners. In case of inability to reach the patient and the referring doctor, the vital status was verified at the registry office. Clinical follow-up was scheduled at one, six and 12 months. There was no independent or external monitoring of data entry.
OUTCOMES AND DEFINITIONS
The primary outcome of interest was a device-oriented composite endpoint (target lesion failure [TLF]), defined as the combination of cardiac death, target vessel myocardial infarction (MI), or clinically driven target lesion revascularisation (TLR), either percutaneous or surgical12. Secondary outcomes of interest included the components of TLF, target vessel failure (TVF), defined as the composite of cardiac death, target vessel MI, or clinically driven target vessel revascularisation (TVR), and ST. Deaths that could not be attributed to another cause were regarded as cardiac deaths. Recurrent MI was defined according to the universal definition13. ST was classified according to the Academic Research Consortium criteria12.
STATISTICAL ANALYSIS
Continuous variables are presented as mean±standard deviations and were compared using a Student’s unpaired t-test for comparisons. Categorical variables are presented as counts and percentages, and were compared using chi-square or Fisher’s exact tests, as appropriate. Kaplan-Meier methods were used to derive the event rates at follow-up. Time-to-event curves were compared between groups using the log-rank test. All reported probability values are two-sided, and a probability value <0.05 was considered significant. All data were processed using the Statistical Package for Social Sciences, Version 20 (IBM Corp., Armonk, NY, USA).
Results
PATIENT POPULATION
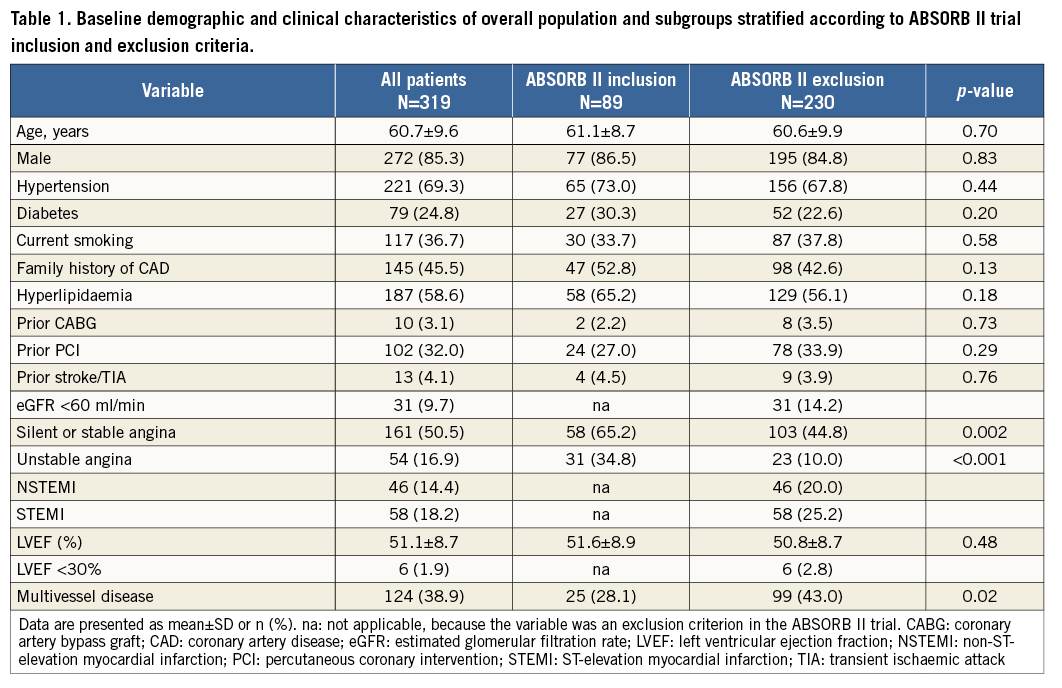
Between March 2013 and June 2014, 319 patients underwent PCI with one or more Absorb in a total of 406 lesions. These Absorb-treated patients represent 19.8% of the overall number of patients (n=1,610) undergoing PCI in the same period. Baseline clinical and angiographic characteristics of the overall population and in subgroups stratified by entry and exit criteria of ABSORB II are reported in Table 1 and Table 2. The mean age was 60.7±9.6 years and 85% were male. Of note, 24.8% of patients had diabetes and 49.5% presented with an acute coronary syndrome (ACS). A total of 51% of lesions were type B2/C. The means of RVD and lesion length were 2.9±0.5 and 21.2±16.8 mm, respectively. The two subgroups did not differ for most demographic and clinical characteristics, except for acute MI (ABSORB II exclusion criteria) and for multivessel disease, which were significantly more common among patients with ABSORB II exclusion criteria. Compared with those fitting the trial entry criteria, these latter patients had higher proportions of type B2/C and longer lesions. Among patients with ABSORB exclusion criteria there were considerable proportions of complex lesions (i.e., bifurcation 18.9%, chronic total occlusion [CTO] 11.5%).
PROCEDURAL DETAILS AND MEDICATIONS
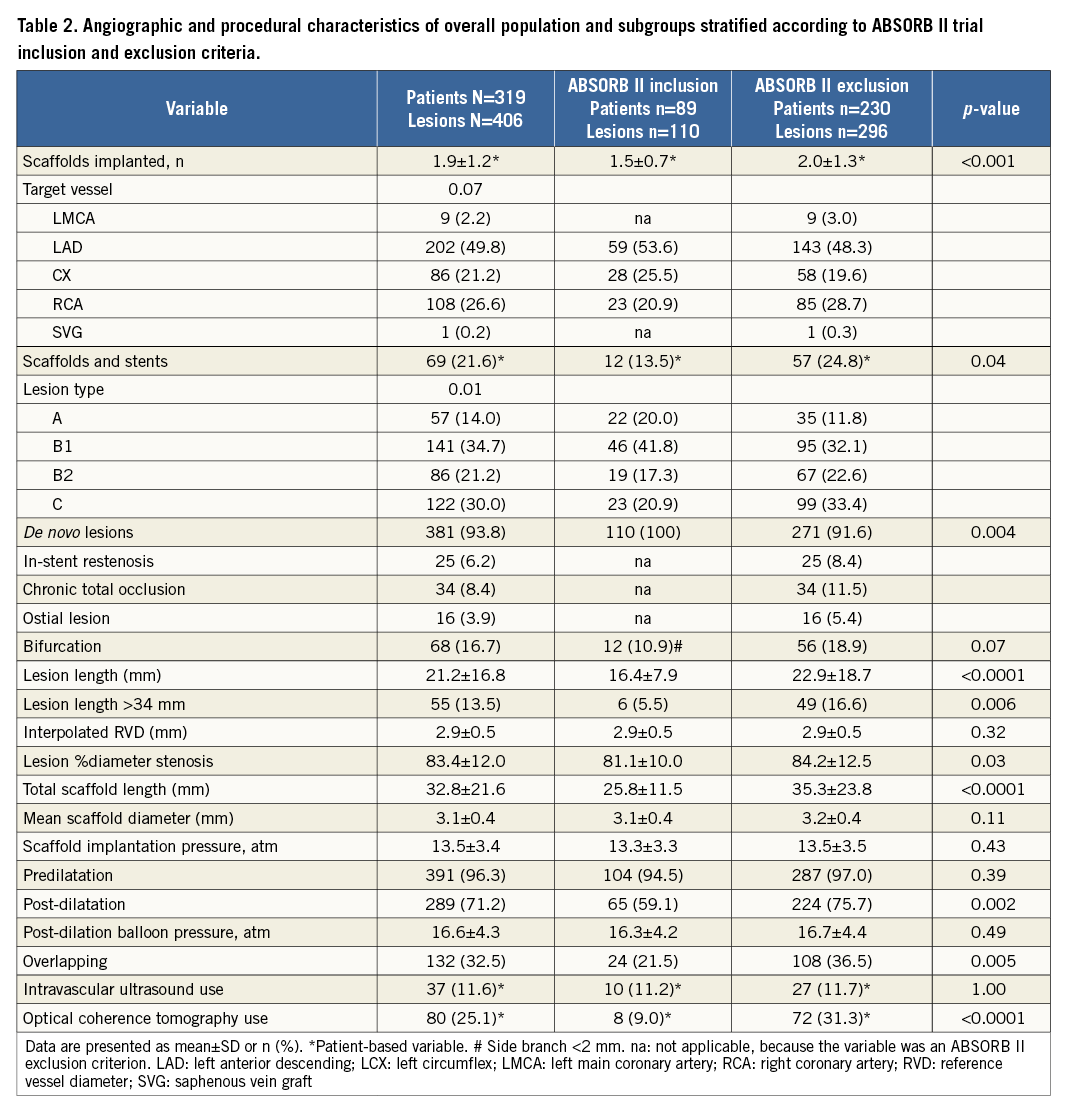
A total of 604 Absorb of 3.1±0.4 mm mean scaffold diameter were implanted at a mean of 13.5±3.4 atm, with a mean number of scaffolds implanted per patient of 1.9±1.2 (Table 2). Predilation was performed in 96.3% of lesions. Mean scaffold length per lesion was 32.8±21 mm and placement of overlapping scaffolds was required in 32.5% of lesions. Post-dilation, at a mean pressure of 16.6±4.3 atm, was performed in 71.2% of lesions. Manual thrombectomy was performed in 59% of STEMI patients. Compared with those fitting the trial entry criteria, patients with ABSORB II exclusion criteria had received higher means of number of scaffolds per patient and of scaffold length per lesion, and underwent post-dilatation, overlapping and intravascular imaging more frequently.
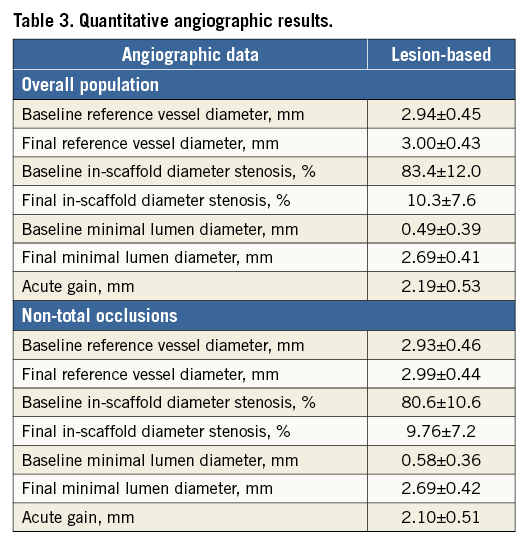
QCA data are shown in Table 3: the acute gain was 2.2±0.5 mm and 2.1±0.5 mm in the overall population and in patients without baseline total occlusions, respectively. Technical failure was observed in seven patients (2.2%), six of whom had a CTO treated.
At discharge, DAPT was prescribed for 12 months in 97.2% of patients. Clopidogrel, prasugrel and ticagrelor were prescribed in 50.8%, 23.2% and 26.0% of patients, respectively.
CLINICAL OUTCOME
Complete clinical twelve-month follow-up information was available in 310 patients (97.2%). The vital status at one year was known in all patients, except one who had moved from Italy.
Over one year after Absorb implantation, TLF was recorded in 15 patients, occurring between 0-30 days in four (26.7%) patients, 30-180 days in four (26.7%) patients, and after 180 days in seven (46.6%) patients. The Kaplan-Meier cumulative incidences of TLF were 2.6% at six months and 4.9% at one year (Table 4). Cumulative one-year TLF rates were 2.4% and 5.8% (p=0.19) in the subgroups with ABSORB II trial inclusion and exclusion criteria, respectively (Table 5, Figure 1). Patients with one-year TLF had significantly lower (1.91±0.38 mm) acute gain than patients without TLF (2.16±0.52 mm). In addition, one-year TLF occurred at rates of 9%, 5% and 3% among patients with scaffold undersizing (n=22), correct sizing (n=201) and oversizing (n=96), respectively.
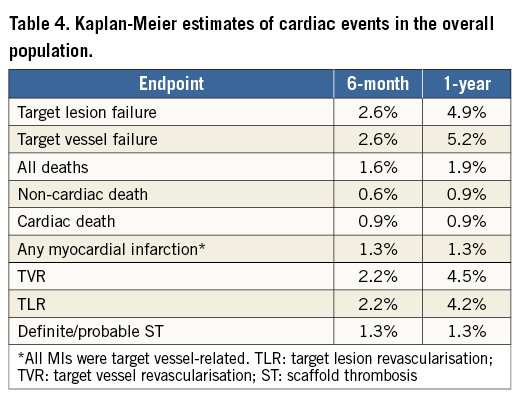
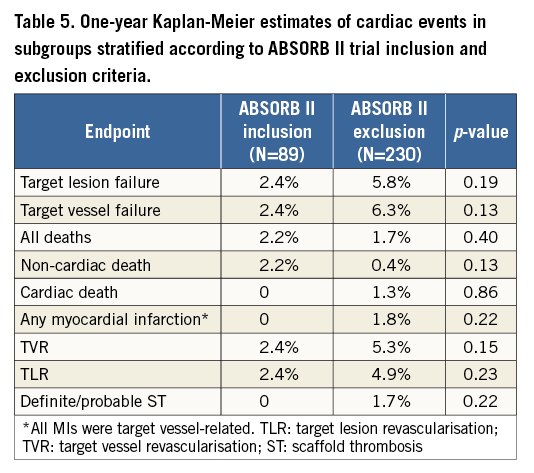
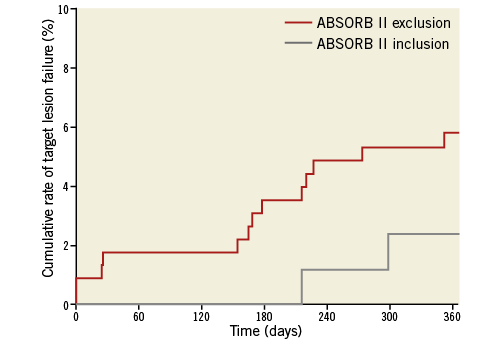
Figure 1. One-year cumulative rates of target lesion failure in subgroups stratified according to ABSORB II trial inclusion and exclusion criteria.
At one year, in the overall population the rate of cardiac death was 0.9%, target vessel MI was 1.3%, TLR was 4.2%, TVR was 4.5% and TVF was 5.2% (Table 4). All cases of cardiac death, MI and ST occurred among patients with ABSORB II trial exclusion criteria (Table 5).
Four ST were observed: two acute definite, and two subacute, of which one was definite (at 25 days) and one was probable (at 26 days). No cases of ST were observed after 30 days. The Kaplan-Meier cumulative ST incidence was 1.3% at one year. The first case of acute ST could probably be explained by residual distal edge dissection and on-treatment high platelet reactivity (HPR), as assessed by platelet function testing. The second case of acute ST occurred about one hour after PCI in a patient who had received the clopidogrel loading dose after the procedure, and who, at the time of ST, still had HPR. The subacute definite ST could be explained by DAPT cessation five days prior to ST. In the subacute probable ST, a scaffold underexpansion could be identified.
Discussion
One-year safety and efficacy data of BRS implantation in a real-world setting for the treatment of unselected populations with high proportions of complex coronary lesions typically encountered in routine clinical practice are still limited10. Indeed, the few one-year reports on Absorb outcomes have mostly focused on ACS patients7,8 or have selective inclusion criteria14. We reported on the one-year clinical outcomes of patients enrolled in a prospective all-comers registry of Absorb from a single high-volume centre. Our data showed that Absorb were associated with reasonably low rates of TLF at one year (4.9%), particularly when considering the complexity of patients and lesions included. Four cases of definite/probable early (within one month) ST were observed, but no cases of late (>1 month) thrombosis occurred, resulting in an overall cumulative incidence of 1.3% at one year, quite similar to the incidence reported in contemporary all-comers trials and registries of second-generation DES15,16. For instance, in the ESTROFA-2 registry, the cumulative one-year incidences of definite/probable thrombosis were 1.3% and 1.4% for zotarolimus- and everolimus-eluting stents (EES), respectively16. Finally, the acute gain achieved in the present study was high and similar to that reported for DES, possibly explaining our favourable results.
Currently, the one-year outcomes of extended Absorb use in a real-world setting, including all-comers patients with a worse health status and higher proportion of complex lesions, while waiving the obligatory intravascular imaging guidance used in clinical trials, have been reported only by the ASSURE registry10. In this registry, very low rates (<3.0%) of device-related events were observed after Absorb implantation in 183 patients and 198 lesions. One-year rates of TLR and TVR were 2.8% and 2.2%, respectively. Moreover, there were no ST cases. The authors stated that high scaffold expansion pressure and slight oversizing seem to be the key factors in achieving good results. Despite similar procedural features, twofold higher incidences of device-related events were reported in our registry, most likely due to the more complex population treated compared with the ASSURE registry (i.e., longer lesions, higher proportions of bifurcations and CTO and inclusion of acute MI in our registry). Of note, our one-year results were similar to those reported in the first 512 patients of the ABSORB EXTEND registry, which reported a 4.9% TVF rate in a relatively selected population14.
Recently, the results from the ABSORB II randomised trial comparing Absorb (n=335) versus XIENCE (Abbott Vascular) (n=166) have shown similar one-year rates of the device-oriented events (composite secondary endpoint) between the two devices (5% vs. 3%, respectively)5. Of note, patients of the present study had similar one-year outcomes to those included in the ABSORB II trial, who had relatively simpler lesions (4.9% vs. 5.0% of TLF, respectively). Interestingly, only 28% of patients included in the present registry had ABSORB II trial inclusion criteria. As expected, the one-year TLF rate was twofold higher within the group with ABSORB II exclusion criteria (5.8%) compared with the group with trial inclusion criteria, although this rate is similar to that reported for second-generation DES15.
Unexpectedly high six-month incidences of definite/probable ST, 2.1% and 3%, have been reported among all-comers complex patients treated with Absorb in the multicentre GHOST-EU (1,189 patients) and in the single-centre Academic Medical Center (AMC, 135 patients) registries, respectively6,7. Importantly, in both registries, 70-75% of the ST cases occurred within 30 days after implantation, suggesting that procedural issues (i.e., residual dissection, stent malapposition or underexpansion) may be the underlying factors triggering most cases. In two out of four cases of the AMC registry, a residual distal edge dissection and an incomplete expansion of the distal scaffold edge were found. Similarly, in two of our four cases a residual distal edge dissection and underexpansion of the scaffold implanted on a severely calcified plaque were documented. Indeed, underexpansion has been identified as an important mechanism underlying ST17. These observations prompt the need for a more accurate lesion selection and for key implantation technique refinements, including a more accurate sizing, the systematic use of high-pressure post-dilatation with non-compliant balloons, and the more liberal use of intravascular imaging, especially in complex lesions. Of note, we observed a lower six-month ST rate (1.3%) compared with the GHOST-EU and AMC registries. This difference may be in part attributed to several technical differences, including greater post-dilatation rate, higher post-dilatation pressure, a more frequent use of intravascular imaging, especially among those with more complex lesions, and a slight scaffold oversizing in our registry. For instance, in the GHOST-EU registry, post-dilatation was performed in 49% of overall lesions versus 71.2% in our registry, and its use decreased as more experience was accumulated. By contrast, our post-dilation rate increased from around 60% in the first enrolment period (the cohort included in the GHOST-EU) to >95% thereafter.
In the blood flow, the presence of thick struts creates flow-dynamic alteration, resulting in high shear stress on top of the strut and low shear stress behind the strut, which may impact on vessel wall healing and trigger platelet aggregation18. In addition, in ex vivo studies thick-strut BRS showed higher acute thrombogenicity than thin-strut biodegradable polymer metallic EES19. Therefore, an adequate platelet inhibition is key to prevent ST. In the AMC registry, two out of four cases of ST occurred in patients discontinuing DAPT. In our registry, DAPT-related issues were present in three out of four cases of ST. Whether the use of more potent antiplatelet agents (prasugrel or ticagrelor) after Absorb implantation might decrease ST is plausible but is not known and warrants dedicated investigations.
Study limitations
Our study suffers from the obvious limitations of an observational real-world registry, including patient selection bias and no independent event adjudication. Patients who received metallic stents together with Absorb represented about 22% of the overall population. To account for the potential influence of these patients on outcomes, the device-oriented composite endpoint was reported to represent the clinical performance of a new device12. Furthermore, the study lacks a control group. Finally, for the present general analysis we have not used a dedicated bifurcation QCA algorithm, which has recently been recommended20.
Conclusions
The GHOST registry has shown that one-year “real-world” safety and efficacy outcomes of Absorb use for the treatment of unselected patients with high proportions of complex lesions are, overall, acceptable and comparable to those reported in the literature for second-generation DES. Several implantation technique features are key to achieve good angiographic and clinical results with Absorb, especially in more complex settings. Nevertheless, longer follow-up and randomised comparisons versus best-in-class DES are needed to confirm the promising results.
| Impact on daily practice The present study provides favourable six-month and one-year safety and efficacy outcomes associated with the implantation of bioresorbable scaffolds (BRS) in an unselected population with a high proportion of complex lesions. These results provide additional supportive evidence for the use of BRS in daily practice under real-world conditions. In particular, the findings of the present study suggest a key positive association between good clinical results and an optimal implantation technique, which should be pursued in daily practice. |
Conflict of interest statement
C. Tamburino has received honoraria/lecture fees from Medtronic, Abbott Vascular and Edwards Lifesciences. D. Capodanno has served on advisory boards of Eli Lilly/Daiichi Sankyo, AstraZeneca, The Medicines Company and Abbott Vascular. The other authors have no conflicts of interest to declare.

